Abstract
The importance of coexpression of myeloid antigens in childhood acute lymphoblastic leukemia (ALL) has long been debated; results are conflicting. We studied children with ALL treated at Italian Association for Pediatric Hematology-Oncology (AIEOP) institutions over 6 years with Berlin-Frankfurt-Muenster (BFM)-based protocols and have analyzed the incidence of coexpression of six MyAg (CD11b, CD13, CD14, CD15, CD33, CD65w) to determine its prognostic impact. Criteria for MyAg coexpression (MyAg+ALL) included positivity to one or more MyAg on at least 20% of blasts and confirmation of coexpression at double-fluorescence analysis. A total of 291 of 908 cases were MyAg+ALL (32%). Incidence was similar in B-ALL and T-ALL; among common, pre-B, and pre-pre–B-ALL. CD13 and CD33 were most common. Patients with MyAg+ALL had presenting features similar to MyAg−ALL. They entered standard or intermediate risk protocols more frequently and had better prednisone response, but similar complete remission rates. Six-year event-free survival (EFS) was 69.0% in 291 MyAg+ALL cases and 65.3% in 617 MyAg−ALL cases, without significant difference. Cases expressing two or more MyAg presented similar clinical features and treatment response. MyAg+ALL had worse EFS only in infants (0% v47%) (P = .01). Therefore, in this series of homogeneously diagnosed and treated ALL, coexpression of MyAg was not associated with prognostic significance, without relevance for clinical purposes or for patient stratification, except for infants.
© 1998 by The American Society of Hematology.
RECOGNIZABLE IMMUNOLOGIC patterns usually allow to attribute childhood acute lymphoblastic leukemia (ALL) to T or B lineages.1,2 Based on experience of a large series of patients homogeneously treated, such information has been associated with some clinical and biologic features and with different prognosis in some cases.3-5
Abnormal expression of antigens of different lineages on leukemic cells has driven much interest because it suggests aberrant hematopoietic differentiation.6 Moreover, its correlation with leukemias with peculiar clinical and biological features [eg, ALL with Ph1 chromosome or translocation t(4;11)] has long been studied as a sign of neoplastic involvement of very immature progenitors. In fact, these leukemias often present “hybrid” features as the rearrangement of different genes or the response to various growth factors. Some of them often present an aggressive clinical course, with poor response to standard therapy.7-9 The above observations have suggested we should consider the expression of myeloid antigens (MyAg) in ALL as a possible indicator of abnormal biological behavior and possibly of different prognosis.6,10 However, results have been controversial in different series and prognostic data are still awaiting a conclusive confirmation. In fact, while many adult series have shown poorer outcome for ALL with myeloid markers,10-17 this significance has not yet been firmly established for pediatric patients, about whom opinions in the literature are rather conflicting.16-25 However, differences may be due to the consistency of series, as well as to technical details about recognition of coexpression. The current widespread use of monoclonal antibodies (MoAbs) and of fluorescence activated cytometry has extended the possibility of precise immunophenotyping, thereby allowing easy confirmation of the coexpression of different markers on the surface of blasts by multiparametric analysis.
In this study, we have analyzed the pattern of expression of the most common MyAg in a large group of childhood ALL (908 cases), all homogeneously diagnosed and treated according to two cooperative studies of the Italian Association for Pediatric Hematology-Oncology (AIEOP). Three myeloid markers (CD13, CD33, and CDw65) have been analyzed as part of an obligatory panel, according to the Italian-Berlin-Frankfurt-Muenster Study Group (I-BFM-SG) recommendations.26 Three additional markers (CD11b, CD14, and CD15) have also been evaluated to extend the analysis on the most widely studied antigens in the literature. The association with the most important clinical parameters and the prognostic significance of the coexpression of MyAg are also analyzed and discussed in this report.
MATERIALS AND METHODS
Patients.
A total of 1,811 children with newly diagnosed ALL have been reported by the AIEOP institutions from January 1988 to April 1995. Of them, 1,612 (89%) were eligible for the two subsequent trials, AIEOP ALL 88 and 91, respectively. For this study, a group of 908 patients (56.3%) was analyzed, of whom the main prognostic indices and a complete immunophenotype were known. The remaining patients were not used for analysis because a full set of their records was not available.
Treatment schedule.
Patient stratification was based on tumor burden estimated as BFM Risk Factor (RF).3 They were treated using three different risk-directed regimens according to the criteria of BFM and AIEOP groups.27-29 Details of the treatment schedule have been provided elsewhere for both the AIEOP-ALL 8828 and the AIEOP-ALL 91 study.29 Treatment consisted of a classical BFM backbone3 with minor modifications. Patients in the high-risk (HR) group of the study 91 received a block-type chemotherapy, derived from the BFM experience in relapsed ALL.30 Cranial irradiation was only given to HR patients older than 1 year of age or with central nervous system (CNS) involvement. The remaining patients had extended intrathecal chemotherapy for CNS prophylaxis.
Diagnostic criteria and immunophenotype.
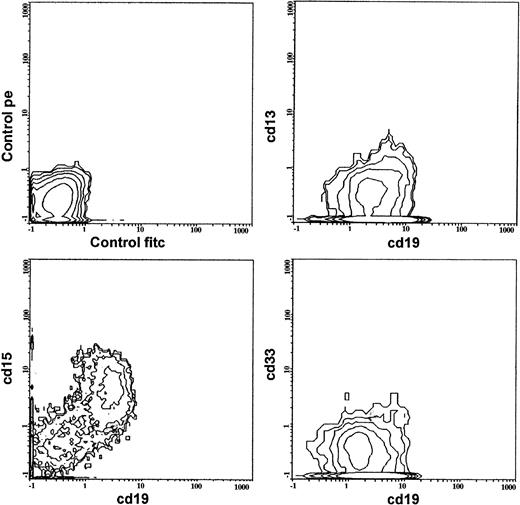
All cases were diagnosed as ALL according to the French-American-British (FAB) morphological and cytochemical criteria.31 Immunophenotypic studies were performed on bone marrow samples at diagnosis by indirect immunofluorescence on flow cytometry.32 Analyses were centrally performed using shipped samples at the reference Laboratory for Hemato-Oncology (Department of Pediatrics, University of Padova, Padova, Italy). All results were carefully revised and accepted by standard procedures. A base-line panel of leukocyte antigens was used in all cases, comprising B-cell antigens (CD19, CD10, CD20; CD24; surface [SIg] and cytoplasmic [CyIg] immunoglobulins), T-cell antigens (CD2, CD7, CD1a, surface and cytoplasmic CD3, CD5), myeloid antigens (CD13, CD14, CD33, CD65w, CD11b, CD15), and lineage-nonspecific markers (CD34, HLA-DR, TdT). Surface markers were considered positive when present on more than 20% of the blast cells. Antigen coexpression was always confirmed by double fluorescence tests using directly conjugated reagents (fluorescein isothiocyanate [FITC] and phycoerythrin [PE]) (Fig 1). Analyses were performed on an Epics XL (Coulter, Miami, FL) cytometer. Isotypic controls were always added. Nonspecific binding was prevented by incubation with AB human serum.
Different immunological groups were identified by positivity to different markers, and the criteria recommended by the I-BFM-SG Biological Cooperative Group were used for classification, thus recognizing the following categories26: pre-pre–B, common ALL, pre-B ALL, T ALL. Mature B-ALL were not eligible for these studies. We defined MyAg+ALLs as the cases coexpressing at least one MyAg among all six evaluated markers in any immunophenotypic group. Hybrid leukemias26 33 were the cases with coexpression of at least two MyAg (of CD13, CD33, CD65w) in common-ALL, pre-B-ALL, T-ALL, and of at least one MyAg in pre-pre–B-ALL.
Statistical methods.
Data were collected on protocol-specific forms. The information was stored, controlled, and analyzed by VENUS, an integrated system of software facilities manufactured by the North-East Italian Interuniversities Computing Center (CINECA, Bologna, Italy), running on an IBM mainframe. The following presenting features were evaluated34: age (cut-offs >1, 1-9, >9 years), sex, hepatomegaly, splenomegaly (below the umbilical line); CNS involvement, leukocyte count (cut-off point at 50,000/μL), hemoglobin (cut-off 8.0 g/dL), platelets (cut-off 50,000/μL), FAB morphology (L1 or L2), prednisone response (good for less than 1,000 blasts/μL after 7 days of steroid therapy)27 could be investigated only in the 816 cases (98.6%) for whom information was available. Cytogenetics and DNA index were excluded from analysis because they have become mandatory since 1995.
Difference in the distribution of single or multiple MyAg+coexpression among immunophenotypic subgroups was tested by χ2 test for heterogeneity.35 Event-free survival (EFS) was estimated by the Kaplan-Meyer method.36Time on study or time to terminal event was calculated from the day of diagnosis. Failure to achieve remission, relapse, or death from any cause were considered events, whichever occurred first. The follow-up was updated on May 31, 1996. The log-rank test37 was adopted to assess differences in univariate analysis. The Cox model was used to draw a multivariate regression.38
RESULTS
Clinical features.
The present study included 908 patients (56.3%) for whom a complete set of clinical and immunophenotypic information was available. Their presenting features are summarized in Table1. They were compared with the remaining 704 eligible patients for whom significant differences in presenting features were not found by Fisher and χ2 tests. However, cases in study had high white blood cell (WBC) counts more often than other patients (22% v19%) P = .05.
Characteristics of 908 Patients From Studies AIEOP 88 and 91 and Coexpression of MyAg
| Patient Characteristics . | All Cases . | MyAg+ALL . | MyAg−ALL . | |||
|---|---|---|---|---|---|---|
| No. . | % . | No. . | % . | No. . | % . | |
| Total | 908 | 100 | 291 | 32 | 617 | 68 |
| Sex | ||||||
| Males | 517 | 57 | 174 | 60 | 343 | 56 |
| Females | 391 | 43 | 117 | 40 | 274 | 44 |
| Age | ||||||
| <1 yr | 23 | 3 | 7 | 2 | 16 | 2 |
| 1-9 yrs | 726 | 80 | 238 | 82 | 488 | 79 |
| >10 yrs | 159 | 17 | 46 | 16 | 113 | 18 |
| Hepatomegaly-152 | 216 | 24 | 86 | 30 | 130 | 21 |
| Splenomegaly-152 | 253 | 28 | 81 | 28 | 172 | 28 |
| CNS disease | 35 | 4 | 6 | 2 | 29 | 5 |
| Hemoglobin >8 g/dL | 464 | 51 | 152 | 52 | 312 | 51 |
| WBC >50,000/μL | 203 | 22 | 60 | 21 | 143 | 23 |
| Platelets <50,000/μL | 403 | 44 | 123 | 42 | 280 | 45 |
| FAB L2 | 201 | 22 | 69 | 24 | 132 | 22 |
| Risk groups | ||||||
| Standard or intermediate | 630 | 69 | 227 | 78* | 403 | 65* |
| High | 278 | 31 | 64 | 22 | 214 | 35 |
| Prednisone good response | 732/816 | 90 | 242/260 | 93-151 | 490/556 | 88-151 |
| CR after induction therapy | 864 | 95 | 279 | 96 | 585 | 95 |
| Patient Characteristics . | All Cases . | MyAg+ALL . | MyAg−ALL . | |||
|---|---|---|---|---|---|---|
| No. . | % . | No. . | % . | No. . | % . | |
| Total | 908 | 100 | 291 | 32 | 617 | 68 |
| Sex | ||||||
| Males | 517 | 57 | 174 | 60 | 343 | 56 |
| Females | 391 | 43 | 117 | 40 | 274 | 44 |
| Age | ||||||
| <1 yr | 23 | 3 | 7 | 2 | 16 | 2 |
| 1-9 yrs | 726 | 80 | 238 | 82 | 488 | 79 |
| >10 yrs | 159 | 17 | 46 | 16 | 113 | 18 |
| Hepatomegaly-152 | 216 | 24 | 86 | 30 | 130 | 21 |
| Splenomegaly-152 | 253 | 28 | 81 | 28 | 172 | 28 |
| CNS disease | 35 | 4 | 6 | 2 | 29 | 5 |
| Hemoglobin >8 g/dL | 464 | 51 | 152 | 52 | 312 | 51 |
| WBC >50,000/μL | 203 | 22 | 60 | 21 | 143 | 23 |
| Platelets <50,000/μL | 403 | 44 | 123 | 42 | 280 | 45 |
| FAB L2 | 201 | 22 | 69 | 24 | 132 | 22 |
| Risk groups | ||||||
| Standard or intermediate | 630 | 69 | 227 | 78* | 403 | 65* |
| High | 278 | 31 | 64 | 22 | 214 | 35 |
| Prednisone good response | 732/816 | 90 | 242/260 | 93-151 | 490/556 | 88-151 |
| CR after induction therapy | 864 | 95 | 279 | 96 | 585 | 95 |
*P = .001.
P = .03.
Below umbilical line.
Immunophenotype.
A total of 572 cases (63%) were diagnosed as common ALL, 185 (20.7%) were pre-B ALL, 29 (3.2%) were pre-pre–B ALL, and 122 (13.5%) were T-ALL. In 291 cases (32%), one or more myeloid antigens (MyAg+ALL) were coexpressed; most of them (n = 256) were of B lineage and 35 were of T lineage; their frequency was similar in the two groups (32.5% and 28.7%, respectively, P = .3). Frequency among the three B-lineage subtypes was also similar; in particular, the pattern of myeloid positivity in the immature pre-pre–B type (38%) was comparable to that of C-ALL (31%) and pre-B ALL (36.2%) (Table 2). T-ALL expressing MyAg were present in all stages of cell maturation; intermediate stage T-ALL was more frequently associated with expression of MyAg (19 MyAg+ of the 43 intermediate T-ALL; 7 MyAg+ of the 24 early T-ALL; 9 MyAg+ of the 55 mature T-ALL;P = .005). Ninety-four cases (10.3%) were “hybrid” according to the above-mentioned definition26; 84 were B-lineage and 10 were T-ALL, without any difference in incidence either between these two lineages (10.6% B-ALL v 8% T-ALL) or for common-ALL versus pre-B ALL.
Distribution of Immunophenotype Among 908 Cases of Childhood ALL Studied for MyAg Coexpression and Reactivity to Each MyAg
| . | No. of Cases . | MyAg+ALL . | H-ALL . | CD15 . | CD65 . | CD33 . | CD13 . | CD14 . | CD11b . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. . | (%) . | No. . | (%) . | No. . | (%) . | No. . | (%) . | No. . | (%) . | No. . | (%) . | No. . | (%) . | No. . | (%) . | ||
| Pre-pre–B ALL | 29 | 11 | (38) | 8 | (27.6) | 6 | (20.6) | 6 | (20.6) | 3 | (10.3) | 2 | (6.8) | 2 | (6.8) | 3 | (10.3) |
| Common ALL | 572 | 178 | (31) | 50 | (8.7) | 27 | (4.7) | 34 | (5.9) | 84 | (14.6) | 88 | (15.3) | 31 | (5.4) | 37 | (6.4) |
| Pre-B–ALL | 185 | 67 | (36.2) | 26 | (14) | 20 | (10.8) | 23 | (12.4) | 31 | (16.7) | 37 | (20.0) | 11 | (5.9) | 12 | (6.4) |
| T-ALL | 122 | 35 | (28.6) | 10 | (8) | 8 | (6.5) | 13 | (10.6) | 12 | (9.8) | 15 | (12.2) | 5 | (4.0) | 12 | (9.8) |
| Total | 908 | 291 | (32) | 94 | (10.3) | 61 | (6.7) | 76 | (8.3) | 130 | (14.3) | 142 | (15.6) | 49 | (5.3) | 64 | (7.0) |
| . | No. of Cases . | MyAg+ALL . | H-ALL . | CD15 . | CD65 . | CD33 . | CD13 . | CD14 . | CD11b . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. . | (%) . | No. . | (%) . | No. . | (%) . | No. . | (%) . | No. . | (%) . | No. . | (%) . | No. . | (%) . | No. . | (%) . | ||
| Pre-pre–B ALL | 29 | 11 | (38) | 8 | (27.6) | 6 | (20.6) | 6 | (20.6) | 3 | (10.3) | 2 | (6.8) | 2 | (6.8) | 3 | (10.3) |
| Common ALL | 572 | 178 | (31) | 50 | (8.7) | 27 | (4.7) | 34 | (5.9) | 84 | (14.6) | 88 | (15.3) | 31 | (5.4) | 37 | (6.4) |
| Pre-B–ALL | 185 | 67 | (36.2) | 26 | (14) | 20 | (10.8) | 23 | (12.4) | 31 | (16.7) | 37 | (20.0) | 11 | (5.9) | 12 | (6.4) |
| T-ALL | 122 | 35 | (28.6) | 10 | (8) | 8 | (6.5) | 13 | (10.6) | 12 | (9.8) | 15 | (12.2) | 5 | (4.0) | 12 | (9.8) |
| Total | 908 | 291 | (32) | 94 | (10.3) | 61 | (6.7) | 76 | (8.3) | 130 | (14.3) | 142 | (15.6) | 49 | (5.3) | 64 | (7.0) |
The distribution of positivity to each MyAg in the four different immunological subtypes is also shown in Table 2. An example of a CD13−, CD33−, CD15+common ALL is shown in Fig 1. Of the analyzed MyAg, CD13 and CD33 were most frequently found: 142 cases were CD13+ and 130 were CD33+. They were mostly found in B precursor ALL positive to CD10 (common and pre-B) and in T-ALL (P, not significant). Eleven pre-pre–B ALL expressed MyAg; CD15 and CD65w were the most common ones (6 positive cases each), being the association of pre-pre–B phenotype with both antigens statistically significant (P = .003 and P = .008, respectively). Seven of 23 infant patients were MyAg+ALL: all markers were equally present twice in these cases. Among 291 MyAg+ALL, 158 cases (54.2%) showed positivity to one single MyAg. CD13 and CD33 were the most frequent (53 and 35 times, respectively). However, 44 ALL were only positive either to CD11b, CD14, or CD15. In 75 cases, two MyAg were positive and in 58 cases, three or more MyAg were present. The most common association was CD13+, CD33+ (28 cases). Three cases were positive to all six MyAg: their phenotypes were the following: one pre-B, one pre-pre–B, one T-ALL. Three more cases were positive only to “minor” MyAg (CD11b, CD14, and CD15) in various combinations.
Fluorescence-activated cell sorting (FACS) multigraph images of double-fluorescence analysis in a case of MYA+ALL. The patient had a B-lineage ALL with common phenotype. Blast cells showed positivity to CD19, CD11b, CD65w, and CD15 and negativity to CD13 and CD33. Fluorochrome used are FITC (for CD19) and PE (for MyAg).
Fluorescence-activated cell sorting (FACS) multigraph images of double-fluorescence analysis in a case of MYA+ALL. The patient had a B-lineage ALL with common phenotype. Blast cells showed positivity to CD19, CD11b, CD65w, and CD15 and negativity to CD13 and CD33. Fluorochrome used are FITC (for CD19) and PE (for MyAg).
Association with presenting features.
There were no significant associations between MyAg+ALL and the clinical presenting features; in particular, the distribution of age, initial WBC counts, T or B phenotype, and CD10 reactivity was more similar than in all other ALL. However, MyAg+ALL were more frequently assigned to standard or intermediate risk protocols (78%) than MyAg− cases (65%; P = .001). They were also frequently prednisone responders (93% v 88%; P = .03), although this did not result in different rate of achievement of complete remission (Table 1). When hybrid cases26 were considered separately, these patterns of results did not change.
Response to treatment.
Six-year EFS of the cohort of studied patients was 66.4% (standard deviation [SD], 1.6) with median follow-up of 48 months; this was not significantly different from EFS of the 704 nonstudied patients (71.2%; SD, 1.9) (P = .23). The only pretreatment factors found to correlate favorably with EFS, also by multivariate analysis, were: age (1 to 9 years) (P = .0001), good prednisone response (P = .0002), and lower WBC count (P = .0033).
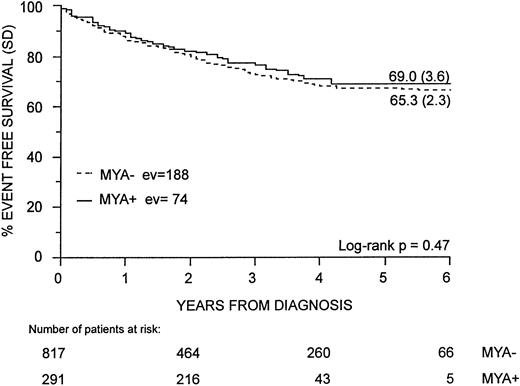
Both univariate and multivariate analyses suggest that the EFS of childhood ALL is not affected by MyAg coexpression. The 6-year EFS for MyAg+ALL was 69.0% (SD, 3.6). This was not significantly different from that of the 617 patients with MyAg−ALL who had a 6-year EFS of 65.3% (SD, 2.3) (P = .47) (Fig 2). Cases of hybrid ALL by BFM criteria did not have a poorer outcome either: their EFS (78.6%, SD, 4.4) was not different from that of nonhybrid cases (61.8%; SD, 3.1) (P = .15). The only exception was represented by the infant group: in fact, the 23 children younger than 12 months of age had a significantly worse outcome if they were MyAg+ (EFS, 0%) than if they were MyAg− (EFS, 43.7%; SD, 12.4) (P = .01). In the other age groups, MyAg coexpression was not associated with any difference in EFS: 69.5% (SD, 2.5) for MyAg− ALL versus 73.9% (SD, 3.7) for MyAg+ALL in children aged 1 to 9 years; 50.6% (SD, 5.3) for MyAg−ALL versus 54.9% (SD, 9.8) for MyAg+ALL in children older than 9 years. This pattern was not modified when hybrid cases were considered separately. Moreover, none of the six analyzed myeloid markers was independently associated with a different outcome.
Comparison of EFS in MyAg+ALL and MyAg−ALL. EFS in MyAg+ALL is not significantly different than in MyAg−ALL. Follow-up time is 72 months and median follow-up is 48 months. ev, events; MYA, My Ag.
Comparison of EFS in MyAg+ALL and MyAg−ALL. EFS in MyAg+ALL is not significantly different than in MyAg−ALL. Follow-up time is 72 months and median follow-up is 48 months. ev, events; MYA, My Ag.
MyAg coexpression was not associated with a different outcome within the different immunophenotypic subgroups. Among the 122 patients with T-lineage ALL, the 35 MyAg+ cases had a 6-year EFS of 46.7% (SD, 8.7), which was comparable to that of the 87 patients with MyAg− ALL (EFS 47.1%; SD, 5.5). The 256 MyAg+ cases of B-lineage ALL had a EFS of 72.1% (SD, 3.9), which was comparable to that of the 530 patients with MyAg−ALL (EFS, 68.1%; SD, 2.5).
All other evaluated parameters, in particular, WBC count, organomegaly, FAB morphology, risk-directed therapy, response to steroids, CD10 expression in B-lineage ALL, B-lineage subgroups, could not demonstrate any statistically significant difference for EFS in MyAg+versus MyAg−ALLs.
DISCUSSION
MyAg coexpression is not a rare event in childhood ALL. In our experience, it occurs in about one third of all cases. This frequency is higher than in previous studies, which reported a range between 4% and 22%3,16-24 (Table 3). However, it is comparable to the 10% to 54% range reported for adult patients.10-15 The sensitivity of the most recent methods used in our study could possibly explain the different percentages reported by most investigators. In a recent report, Uckun et al25 found a 16.6% incidence of MyAg coexpression among 1,557 patients enrolled in the Childrens Cancer Group (CCG) protocols. Such apparently lower incidence may be explained by their choice to include only two MyAg, CD13 and CD33, in the panel of the MoAbs used for central investigation of immmunophenotype. The investigators suggest that the reported incidence of CD13 and CD33 coexpression in childhood ALL should be representative of overall MyAg expression. Although we may confirm that CD13 and CD33, alone or in combination, are the most frequently observed MyAg, in our series they might account for about one half of all cases coexpressing at least one MyAg. Actually, in our study, four more antigens were evaluated to analyze a wider panel of myeloid antigenic molecules.19,39 Using the “minor” ones (CD11b, CD14, and CD15), 47 additional cases (16%) were identified. False positive results may be easily ruled out in our setting, based on our study design: immunonophenotyping was performed centrally by flow cytometry, using highly specific MoAbs for all cases; thus even weak antigen expression might be detected. It is to be noted that limit for positivity in single fluorescence analysis was 20%, while it was 30% in the study by Uckun et al.25 Double fluorescence analysis was always performed to confirm all cases, including those that showed low percentages of positive cells as having coexpressing blasts. This should avoid the main difficulties in interpreting data on MyAg+ALL. In fact, results could be altered by using the overlapping of percentages to identify coexpression, because of the presence of residual normal myeloid cells in the samples.19,25 In our series, cases coexpressing CD13 and/or CD33 on more than 30% of blasts were 152 (16% of all cases), the same percentage found by Uckun et al.25
A Comparative Review of Studies on Clinical Significance of MyAg Coexpression in ALL
| Author, Year . | No. of Cases . | MyAg+ALL No. (%) . | MyAg Used . | % IFI . | Confirmation of Coexpression . | Correlation With EFS . |
|---|---|---|---|---|---|---|
| Pui et al, 1990 | 372 | 61 (6.4) | CD11b, 13, 14 CD15, 33, 36 | 25 | DF | No (FU, 36 mo) |
| Wiersma et al, 1991 | 236 | 52 (22) | CD13, 33, 14 | 30 | OVL DF | Yes (FU, 40 mo) |
| Borowitz et al, 1991 | 1141 | (7) (15) | CD13, 33 CD13, 33 | 30 20 | 30% OVL ND | No No |
| Pui et al, 1991 | 410 | 25 (6.1) | CD11b, 13, 14 CD15, 33, 36 | 20 2 MyAg+ | ND | |
| Fink et al, 1993 | 206 | 24 (11) | CD13, 33, 65, 15 14, 11b, GA, 41 | OVL | Yes (median FU, 44 mo) | |
| Ludwig et al, 1994 | 736 | 50 (7) | CD13, 33, 65 | 20 | DF | ND |
| Reiter et al, 1994 | 975 | 51 (5) | CD13, 33, 65 | 20 | 20% OVL | No |
| Uckun et al, 1997 | 1557 | 260 (16.6) | CD13, 33 | 30 | No (FU, 73 mo) |
| Author, Year . | No. of Cases . | MyAg+ALL No. (%) . | MyAg Used . | % IFI . | Confirmation of Coexpression . | Correlation With EFS . |
|---|---|---|---|---|---|---|
| Pui et al, 1990 | 372 | 61 (6.4) | CD11b, 13, 14 CD15, 33, 36 | 25 | DF | No (FU, 36 mo) |
| Wiersma et al, 1991 | 236 | 52 (22) | CD13, 33, 14 | 30 | OVL DF | Yes (FU, 40 mo) |
| Borowitz et al, 1991 | 1141 | (7) (15) | CD13, 33 CD13, 33 | 30 20 | 30% OVL ND | No No |
| Pui et al, 1991 | 410 | 25 (6.1) | CD11b, 13, 14 CD15, 33, 36 | 20 2 MyAg+ | ND | |
| Fink et al, 1993 | 206 | 24 (11) | CD13, 33, 65, 15 14, 11b, GA, 41 | OVL | Yes (median FU, 44 mo) | |
| Ludwig et al, 1994 | 736 | 50 (7) | CD13, 33, 65 | 20 | DF | ND |
| Reiter et al, 1994 | 975 | 51 (5) | CD13, 33, 65 | 20 | 20% OVL | No |
| Uckun et al, 1997 | 1557 | 260 (16.6) | CD13, 33 | 30 | No (FU, 73 mo) |
Only series analyzed by flow cytometry have been shown.
Abbreviations: % IFI, percentage of cells positive by single fluorescence analysis; DF, double fluorescence staining; OVL, overlapping of percentages of positive cells; FU, follow-up; mo, months; ND, not done; GA, glycophorin A.
The frequency of myeloid coexpression was comparable in T- and in B-ALL, as well as in all subgroups of B-lineage ALL. In particular, CD10−, B-lineage ALL (pre-pre–B ALL) had the same incidence of MyAg coexpression than other more mature types, while Ludwig et al16 suggested a particularly high frequency (50%). Moreover, CD13 and CD33 were not typical of this group, while the most frequent MyAg was CD15, in agreement with previous reports.22,40 The presence of CD15 in infant ALL has already been found in a larger group of our patients,32even if it could not be confirmed in this limited subset of infants. The correlation of MyAg coexpression, the peculiar chromosomal translocation t(4;11), and poor prognosis in infant and pre-pre–B ALL has often been suggested.41 T-ALL of intermediate maturational stage showed a particularly high number of MyAg+ cases. This difference with previous observations19 22 may be due to the small numbers of T-ALL MyAg+ cases analyzed therein.
Coexpression of MyAg+ALL was not associated with any of the presenting features, in particular previous reports34prompted us to explore any association with those presenting features known to bear an adverse prognostic value, such as infant or older age and higher leukocyte count. Interestingly, poor steroid response on day 7, indeed a powerful independent adverse prognostic factor in the BFM-chemotherapy setting,3,42 was not associated with MyAg coexpression. On the contrary, MyAg+ cases were more frequently good steroid responders. Accordingly, they were more frequently eligible for the non-high (standard and intermediate) risk groups. This also agrees with a recent report from our group, describing a higher incidence of MyAg coexpression in the ALL subset identified by the prospective molecular screening of the t(12; 21) translocation,43 which is associated with favorable prognosis.44 45 Thus, our data confirm that MyAg coexpression is usually associated with a constellation of favorable presenting features. For this reason, the MyAg+ cases have a nonrandom distribution toward the lower risk groups. Finally, myeloid antigen coexpression is not associated with a different outcome in childhood ALL. Because only infant patients with MyAg+ALL have shown a worse prognosis, their presence could affect statistical analysis in small series. This conclusion on our large series of unselected, newly diagnosed childhood ALL cases is in agreement with the recent report from the CCG25 for patients who are treated with a BFM-based chemotherapy.
In conclusion, our large, prospective study of coexpression of six different MyAg in childhood ALL confirms it is not associated either with immunophenotype, response to therapy, or long-term outcome and thus has no prognostic value, as far as aggressive treatment is used.46,47 Therefore, it should not be used for any therapeutic choice, including patient stratification or indication for bone marrow transplantation, with the exception of infant leukemia. Nevertheless, continuous use of a wide panel, including many different MyAg (up to six in our experience), is justified by the possibility of identifying cases of undifferentiated leukemia and FAB type Mo AML48 and also to define in the majority of cases a leukemia-associated immunophenotype for individual patients.48-51 This information, jointly with molecular studies, might be highly valuable when drawing prospective studies of minimal residual disease aimed to tailor treatment.
We also suggest that the term “hybrid” leukemia for MyAg+ALL is not supported by any clinical evidence of heterogeneity. On the contrary, it could induce incorrect impressions of “myeloid” or “atypical” characteristics and might be misleading.
ACKNOWLEDGMENT
The authors thank all investigators from AIEOP Institutions: L. Felici (Cl. Pediatrica, Ancona); N. Santoro (Cl. Pediatrica I, Bari); T. Santostasi (Cl. Pediatrica II, Bari); P. Cornelli (O. Riuniti, Bergamo); A. Pession (Cl. Pediatrica III, Bologna); A. Arrighini (Cl. Pediatrica, Brescia); G. M. Fiori (Div. Oncoematologia Pediatrica, Cagliari); R. Galanello (Cl. Pediatrica II; Cagliari); A. Sciotto (Div. Oncoematologia Pediatrica, Catania); S. Magro (Div. Ematologia, Catanzaro); A. Lippi (O. Meyer, Firenze); M. Cominetti (O. Galliera, Genova); C. Rosanda (O. Gaslini, Genova); F. Massolo, (Cl. Pediatrica II, Modena); A. Murano (Cl. Pediatrica I, Napoli); M.F. Pinta (O. Pausillipon, Napoli); S. Auricchio, (Cl. Pediatrica II, Napoli); A. Correra (O. SS. Annunziata, Napoli); L. Zanesco (Cl. Pediatrica II, Padova), G. Fugardi (Cl. Pediatrica I, Palermo); G. Izzi (Div. Ematologia Pediatrica, Parma); M. Aricò (Cl. Pediatrica, Pavia); A. Amici (Cl. Pediatrica, Perugia); R. Di Lorenzo (Div. Ematologia, Pescara); C. Favre (Cl. Pediatrica III, Pisa); F. Nobile (Div. Ematologia, Reggio Calabria); A.M. Testi (Cattedra di Ematologia, La Sapienza, Roma); G. De Rossi (O. Bambino Gesù, Roma); B. Werner (Cl. Pediatrica, La Sapienza, Roma); D. Gallisai (Cl. Pediatrica, Sassari); C. D'Ambrosio (Cl. Pediatrica, Siena); E. Barisone (Cl. Pediatrica, Torino); P. Tamaro (Cl. Pediatrica, Trieste); L. Nespoli (Cl. Pediatrica, Varese); G. Marradi (Cl. Pediatrica, Verona). We also wish to thank Dr Lucia Masiero for statistical assistance and Dr M. Spinelli, G. Giacometti and B. Michielotto for technical work.
Supported by AIRC (Associazione Italiana per la Ricerca sul Cancro); by AIL (Associazione Italiana Leucemie), Progetto “30 Ore per la Vita”.
Presented in part at the XXIX Congress of SIOP, held in Instanbul, September 23-27, 1997.
Address reprint requests to Maria Caterina Putti, MD, Dipartimento di Pediatria, Via Giustiniani, 3, 35128 Padova, Italy.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal