Abstract
Recent reports have indicated that there is poor engraftment from hematopoietic stem cells (HSC) that have traversed cell cycle ex vivo. However, inducing cells to cycle in culture is critical to the fields of ex vivo stem cell expansion and retroviral-mediated gene therapy. Through the use of a xenograft model, the current data shows that human hematopoietic stem and progenitor cells can traverse M phase ex vivo, integrate retroviral vectors, engraft, and sustain long-term hematopoiesis only if they have had the opportunity to engage their integrin receptors to fibronectin during the culture period. If cultured in suspension under the same conditions, transduction is undetectable and the long-term multilineage regenerative capacity of the primitive cells is severely diminished.
A RESEARCH GOAL VITAL to the field of human gene therapy is to identify methods for stable transduction of pluripotent human hematopoietic stem cells (HSC). Retroviral vectors derived from the Moloney Murine Leukemia Virus are currently the most effective vehicles to stably integrate new genes into the host cell chromosomes. The major limitation of retroviral-mediated gene transfer is the requirement for DNA replication in the target cell.1 2 Hematopoietic stem cells are largely quiescent, and exogenous cytokines are commonly used to prompt cell division. For gene-modified stem cells to contribute to hematopoiesis throughout the lifetime of the recipient, it is crucial to define methods for transduction of HSC that do not initiate pathways of terminal differentiation.
Cell-free supernatant addition to marrow on an irradiated stromal support layer has been reported to be an effective method for transfer of genes into human hematopoietic progenitors.3-6 In addition, our group previously showed that the presence of a human stromal support layer during transduction was essential to preserve the ability of human CD34+ cells to sustain durable, long-term hematopoiesis in immune deficient mice.7 While stromal support increases levels of gene transduction of hematopoietic cells and preserves the clonogenic capacity, obtaining sufficient quantities of autologous stromal cells for clinical trials can be problematic and requires obtaining a bone marrow aspirate from the patient 2 weeks to 1 month before the stem cell harvest. Therefore, alternate methods of transduction that are equally effective would be preferable.
The use of the carboxyl (COOH) terminal domain of fibronectin (FN) to efficiently colocalize retroviral particles and hematopoietic target cells has been described by Moritz et al8, Hanenberg et al9, and Moritz et al.10 Their studies showed efficient transduction of human long-term culture-initiating cells (LTCIC) and murine reconstituting cells. In the current studies, we examined transduction of human stem and progenitor cells on the carboxy-terminal domain of FN11 in the bnx/hu xenograft model. The bnx/hu model of human hematopoiesis allows development of both T lymphocytes and myeloid cells from individual human hematopoietic stem or progenitor cells.7 We used the bnx/hu system to address an issue perhaps more crucial than the transduction level; the survival and maintenance of clonogenic capacity of primitive human reconstituting cells through a period of ex vivo culture. This factor is critical for any consideration of gene therapy using ex vivo manipulated bone marrow cells in fully myeloablated patients to ensure sustained production of both myeloid and lymphoid compartments from the transplanted cells.
Our previous studies identified two populations of hematopoietic cells from human bone marrow or mobilized peripheral blood stem cells (PBSC) that are capable of sustaining multilineage hematopoiesis in immune-deficient mice. The first population survives ex vivo culture in suspension with fetal calf serum and the cytokines, interleukin-3 (IL-3), IL-6, and stem cell factor (SCF), but can only sustain hematopoiesis for 4 to 5 months in the mice.7 The second population of cells, which we believe to be more primitive, requires stromal support during ex vivo culture to maintain the ability to engraft the mice and give rise to hematopoiesis for up to 1 year.7 We ascertained that the late graft failure observed with cells cultured in suspension could be partially, but not completely, ameliorated by inclusion of the cytokine FLT3 ligand (FL),12 which has been shown to stimulate the survival and/or proliferation of primitive human hematopoietic cells.13-17 In the current studies, we sought to determine whether a more crucial factor for maintaining the primitive population through a 72-hour ex vivo culture was engagement of the integrins very late antigen-4 (VLA-4) and/or VLA-5 to the FN carboxy-terminal domain. The effects of transduction of human CD34+ cells on stromal support versus FN or in suspension culture (over bovine serum albumin [BSA]-coated plates) were examined. The transduced cell populations were assessed for the extent of gene transfer into 14-day colony-forming progenitors and for survival and transduction of more primitive cells able to sustain human hematopoiesis in bnx mice for 6 to 12 months.
As we had previously observed, human cells cultured for 72 hours in suspension culture had a long-term survival defect and were not recovered in significant levels from any mouse. Human cells cultured 72 hours on stromal support or FN before transplantation had the highest rates of gene marking and engraftment and gave rise to equivalent percentages and lineages of mature human hematopoietic cells and progenitors in the marrow of the mice. Our data indicate that engagement of the integrins on HSC that bind FN (VLA-4 and VLA-5) throughout a 72-hour ex vivo culture with serum and cytokines is necessary to sustain the ability of the cells to mediate long-term hematopoiesis after transplantation.
MATERIALS AND METHODS
Preparation of CD34+ cells and stromal monolayers.
Normal human bone marrow cells were obtained from screens used to filter marrow during harvest of allogeneic donors. Use of these samples was approved by the Committee on Clinical Investigations at Childrens Hospital Los Angeles. Cells were incubated in basal bone marrow medium (BBMM) for 2 hours at 37°C with 5% CO2 to allow depletion of adherent stromal cells before isolation of hematopoietic progenitors. BBMM is Iscove’s Modified Dulbecco’s Medium (Irvine Scientific, Santa Ana, CA) with 20% heat-inactivated fetal calf serum (FCS; BioWhittaker, Walkersville, MD), 1% deionized BSA, fraction V, Sigma, St Louis, MO), 100 U/mL penicillin, 10 μg/mL streptomycin, 2 mmol/L L-glutamine (all from GIBCO, Grand Island, NY), 10-4 mol/L 2-mercaptoethanol, and 10-6 mol/L hydrocortisone (Sigma). After the stromal depletion step, the nonadherent hematopoietic cells were collected by vigorous flushing, and the plastic-adherent fraction was used to generate stromal cell monolayers as described below. CD34+ progenitors were isolated by incubation with the monoclonal antibody HPCA-1 (Becton Dickinson, San Jose, CA), followed by goat antimouse conjugated immunomagnetic beads (Dynal, Oslo, Norway) as described.18
The stromal layer was refed BBMM with horse serum (Gemini Bioproducts, Calabasas, CA) substituted for 50% of the FCS. Subconfluent layers of primary stromal cells were split by trypsinization (trypsin-EDTA; Irvine Scientific). Stroma was used as a supporting layer for transduction between passages 3 to 5, after hematopoietic cells and macrophages had been eradicated. Stromal cells were washed, irradiated (20 Gy), and plated at 2 × 105 cells per 25 cm2 vent-cap flask (Costar, Cambridge, MA) in BBMM the day before use.
Transduction of human CD34+ progenitors.
CD34+ cells were transduced in 25-cm2 vent-cap flasks (Costar) that had been previously coated with BSA, FN, or stromal cells. FN coating was done by incubating 5 mL of a 50 μg/mL solution of recombinant CH-296 (Retronectin, TaKaRa, Otsu, Japan) in a 25-cm2 flask for 2 hours at room temperature. The FN solution was removed and replaced with a 2% BSA solution (Fraction V; Sigma) to block nonspecific sites for an additional 30 minutes. BSA coating was performed by incubation of a 2% solution in 25-cm2 flasks for 2 hours at room temperature. FN and BSA flasks were rinsed in phosphate-buffered saline (PBS) and used directly without drying. CD34+ cells were added to each flask in 5 mL transduction medium and returned to the incubator for 15 to 30 minutes to reach 37°C and the proper pH before introduction of virus. Transduction medium was BBMM with 50 U/mL rH IL-6 (R & D Systems, Minneapolis, MN), 10 ng/mL rH IL-3 (Immunex Corp, Seattle, WA), and 50 ng/mL SCF (R & D Systems). FLT3 Ligand (generously donated by DNAX Corp, Palo Alto, CA), was added to designated flasks at a concentration of 100 ng/mL. For transduction, 5 mL of freshly thawed retroviral supernatant warmed to 37°C was added to each flask (5 mL Dulbecco’s modified Eagle’s medium [DME] with 10% FCS for sham flasks). Supernatant from the PG13/LN Gibbon Ape Leukemia Virus (GALV) pseudotype packaging cell line19 was used for all transductions. The PG13/LN supernatant had a titer of 5 × 106 infectious virions/mL, assayed on the human colon carcinoma cell line HT29 (American Type Culture Collections [ATCC], Rockville, MD) and was determined to be free of ecotropic, amphotropic, and GALV recombinant helper virus by polymerase chain reaction (PCR) for env and marker rescue assay on 3T3 and HT29 cells (ATCC). After the 72-hour in vitro transduction period, samples were plated in methylcellulose-based colony-forming unit (CFU) assays with and without the selective agent G418 (0.9 mg/mL active, screened lots from GIBCO/BRL, Grand Island, NY) to assess the extent of gene transfer into 14-day colony-forming progenitors as described.20 The remainders of each sample were transplanted into immune-deficient mice.
Mice.
All studies used 6-week old beige/nude/xid homozygous mice (bg.bg/nu.nu/xid.xid, NIH-3; Taconic Farms, Germantown, NY) bred at CHLA. Cotransplantation of transduced human progenitors and stromal cells producing IL-3 was performed as previously published, with sublethal conditioning performed by injection of 150 μg/kg 5-fluorouracil (5-FU) 48 to 72 hours before injection of human marrow.18 Mice were killed 6 to 12 months after transplantation with human cells. Bone marrow was flushed from the tibiae and femurs of each mouse and used for the assays described below.
Fluorescence-activated cell sorting (FACS) analysis.
Single cell suspensions from the marrow and spleen of cotransplanted mice were preincubated for 15 minutes on ice with unconjugated mouse immunoglobulin (MsIgG; Coulter, Hialeah, FL). Directly conjugated antibodies used to identify human-specific cell surface antigens were: HLE-1 (anti-CD45; Becton Dickinson [BD]), My9-RD1 (anti-CD33, Coulter), Leu-12 (anti-CD19, BD), Leu-3a (anti-CD4, BD), and Leu-2a (anti-CD8, BD). The antimouse CD45-R-PE antibody (Pharmingen, San Diego, CA) was used to identify murine leukocytes. After a 15-minute antibody binding period on ice, cells were depleted of red blood cells by resuspension in Ortho Lysis Buffer (BD), washed, and fixed in 1% paraformaldehyde. Samples were acquired on a Becton Dickinson FACScan and analyzed using the Cellquest software package (BD). Ten thousand events were acquired for each sample. Parallel staining and FACS analyses were done on normal human and nontransplanted bnxmouse bone marrow controls to confirm that none of the human-specific antibodies cross-reacted with murine cells.
Human-specific CFU plating.
To determine the number of clonogenic human hematopoietic progenitors engrafted within the murine bone marrow, cells harvested from each mouse were plated in human-specific CFU assay as described.7,12 18 Before plating, the bnx/hu bone marrow cells were incubated in BBMM for several hours to remove (by adherence) murine stromal cells, which secrete murine cytokines and invalidate the specificity for growth of human hematopoietic colonies measured in the assay. A total of 5 × 104 and 1 × 105 plastic nonadherent cells from engrafted and control mice were plated in duplicate in 1 mL of the medium in gridded culture dishes (Nunc, Naperville, IL), with and without G418 (0.9 mg/mL active compound; Gemini Bioproducts).
Inverse PCR.
DNA preparation and PCR for the neo gene was performed as described from total bone marrow and individual T-cell and myeloid clones, containing 200 to 1,000 cells.18,21,22 DNA from the tissues of each bnx/hu mouse was isolated by large-scale DNA preparation using standard methods as described.20,23 For inverse pCR analysis of neo-positive samples, half of the DNA obtained from each colony or 50 ng DNA from the tissues was digested with Taq1 restriction enzyme (GIBCO/BRL). A portion of each sample was then self-ligated and subjected to nested inverse PCR reactions as described.21 22
Statistical analyses.
The significance of each set of values was assessed using the two-tailed T-test assuming equal variance with the Excel 5.0 software (Microsoft Corp, Seattle, WA). Average values are listed with standard deviations, and standard error of the mean was used if all values were listed in table format.
RESULTS
Transduction of human CD34+ progenitors.
Human bone marrow was enriched for CD34+ progenitors by immunomagnetic separation. Enriched populations were 90% to 99% CD34+, as demonstrated by FACS analysis.24 The CD34+ cells were transduced by addition of supernatant from the retroviral vector LN, packaged in a GALV pseudotype by the cell line PG13.19 The LN vector carries the bacterial neomycin resistance gene25 and imparts resistance to the selective agent G418. Three supernatant additions were performed at 24-hour intervals over the course of 72 hours. The cytokines IL-3, IL-6, and SCF were present in all cultures, with or without inclusion of FLT3 ligand. Transductions were performed in suspension culture (over BSA-coated plates), on stromal support, or on plates coated with the recombinant FN fragment CH-296 (Retronectin). A portion of each transduced sample was plated in 14-day colony-forming assay with or without the selective agent, G418, to assess the extent of gene transfer into committed progenitors. The remainder of each sample was transplanted into a set of bnx mice to allow the subsequent study of more primitive human hematopoietic cells.
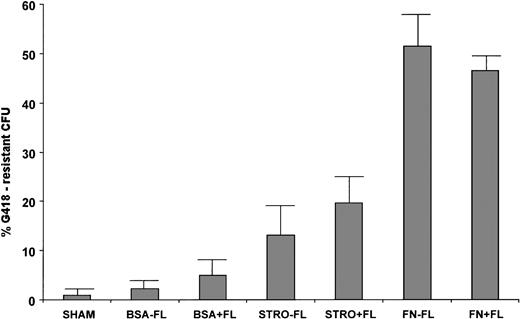
The levels of transduction in each condition before transplantation are shown in Table 1. Either stromal or fibronectin support was required to obtain efficient transduction of colony-forming progenitors with retroviral supernatant, and transduction in suspension resulted in low levels of transduction (average, 2.3% ± 1.7% G418R CFU). No significant increase in the extent of gene transduction into committed colony-forming progenitors in suspension was caused by inclusion of FL in the transduction media (average, 4.9% ± 2.4%, P > .05). The average levels of transduction in suspension culture, with or without FL, were not significantly higher than the SHAM background levels (average, 1.0% ± 0.6%, P > .05). Transduction on stromal support gave rise to slightly, but not significantly higher, percentages of G418R CFU in the current sets of experiments (likely due to the high level of variability [Table 1]). In the absence of FL, the average percent transduction on stroma was 13.1 ± 5.8 (P compared with BSA-FL > .05), and with addition of FL, the average percentage was 19.6 ± 6.6 (P compared with BSA+FL > .05 ). The average transduction levels obtained from the two sets transduced on stromal support did not differ significantly from one another (P > .05). Progenitors transduced on FN, either with or without FL, had significantly higher numbers of G418R CFU than those transduced on either BSA or stromal support. An average of 51.4 ± 7.4 was obtained on FN in the absence of FL (P = .006 compared with stro-FL), and an average of 46.5 ± 2.3 was obtained on FN with FL (P = .008 compared with stro + FL). Therefore, FN supported the best transduction of 14-day colony-forming progenitors (Fig 1). We next sought to examine transduction of more primitive cells on FN support, using the bnx/hu xenograft system.
Transduction of 14-Day Colony-Forming Progenitors
| . | G418R/ Total CFU . | % G418R . | bnx/hu Transplantation Set No. . |
|---|---|---|---|
| Experiment 1: Transduction condition | |||
| SHAM | 0/15 | 0 | — |
| BSA + 3/6/S | 0/24 | 0 | 1A |
| Stroma + 3/6/S | 0/40 | 0 | 1C |
| FN + 3/6/S | 28/39 | 71.8 | 1E |
| Experiment 2: | |||
| SHAM | 1/194 | 0.5 | — |
| BSA + 3/6/S | 3/212 | 1.4 | 2A |
| Stroma + 3/6/S | 69/251 | 27.5 | 2C |
| FN + 3/6/S | 122/235 | 51.9 | 2E |
| Experiment 3: | |||
| SHAM | 1/145 | 0.7 | — |
| BSA + 3/6/S/FL | 5/105 | 4.8 | 3B |
| Stroma + 3/6/S/FL | 57/199 | 28.6 | 3D |
| FN + 3/6/S/FL | 64/125 | 51.2 | 3F |
| Experiment 4: | |||
| SHAM | 0/215 | 0 | — |
| BSA + 3/6/S/FL | 6/197 | 3.0 | 4B |
| Stroma + 3/6/S/FL | 73/220 | 33.2 | 4D |
| FN + 3/6/S/FL | 109/243 | 44.9 | 4F |
| Experiment 5: | |||
| SHAM | 1/113 | 0.9 | — |
| BSA + 3/6/S | 1/177 | 0.5 | 5A |
| BSA + 3/6/S/FL | 5/109 | 4.6 | 5B |
| Stroma + 3/6/S | 10/111 | 9.0 | 5C |
| Stroma + 3/6/S/FL | 11/123 | 8.9 | 5D |
| FN + 3/6/S | 44/117 | 37.6 | 5E |
| FN + 3/6/S/FL | 64/156 | 41.0 | 5F |
| Experiment 6: | |||
| SHAM | 7/171 | 4.1 | — |
| BSA + 3/6/S | 16/221 | 7.2 | 6A |
| BSA + 3/6/S/FL | 18/258 | 7.0 | 6B |
| Stroma + 3/6/S | 37/234 | 15.8 | 6C |
| Stroma + 3/6/S/FL | 18/238 | 7.6 | 6D |
| FN + 3/6/S | 79/179 | 44.1 | 6E |
| FN + 3/6/S/FL | 95/194 | 49.0 | 6F |
| . | G418R/ Total CFU . | % G418R . | bnx/hu Transplantation Set No. . |
|---|---|---|---|
| Experiment 1: Transduction condition | |||
| SHAM | 0/15 | 0 | — |
| BSA + 3/6/S | 0/24 | 0 | 1A |
| Stroma + 3/6/S | 0/40 | 0 | 1C |
| FN + 3/6/S | 28/39 | 71.8 | 1E |
| Experiment 2: | |||
| SHAM | 1/194 | 0.5 | — |
| BSA + 3/6/S | 3/212 | 1.4 | 2A |
| Stroma + 3/6/S | 69/251 | 27.5 | 2C |
| FN + 3/6/S | 122/235 | 51.9 | 2E |
| Experiment 3: | |||
| SHAM | 1/145 | 0.7 | — |
| BSA + 3/6/S/FL | 5/105 | 4.8 | 3B |
| Stroma + 3/6/S/FL | 57/199 | 28.6 | 3D |
| FN + 3/6/S/FL | 64/125 | 51.2 | 3F |
| Experiment 4: | |||
| SHAM | 0/215 | 0 | — |
| BSA + 3/6/S/FL | 6/197 | 3.0 | 4B |
| Stroma + 3/6/S/FL | 73/220 | 33.2 | 4D |
| FN + 3/6/S/FL | 109/243 | 44.9 | 4F |
| Experiment 5: | |||
| SHAM | 1/113 | 0.9 | — |
| BSA + 3/6/S | 1/177 | 0.5 | 5A |
| BSA + 3/6/S/FL | 5/109 | 4.6 | 5B |
| Stroma + 3/6/S | 10/111 | 9.0 | 5C |
| Stroma + 3/6/S/FL | 11/123 | 8.9 | 5D |
| FN + 3/6/S | 44/117 | 37.6 | 5E |
| FN + 3/6/S/FL | 64/156 | 41.0 | 5F |
| Experiment 6: | |||
| SHAM | 7/171 | 4.1 | — |
| BSA + 3/6/S | 16/221 | 7.2 | 6A |
| BSA + 3/6/S/FL | 18/258 | 7.0 | 6B |
| Stroma + 3/6/S | 37/234 | 15.8 | 6C |
| Stroma + 3/6/S/FL | 18/238 | 7.6 | 6D |
| FN + 3/6/S | 79/179 | 44.1 | 6E |
| FN + 3/6/S/FL | 95/194 | 49.0 | 6F |
Methylcellulose-based colony-forming assays were plated 72 hours after initiation of transduction by the LN retroviral vector. Cells were transduced in suspension (BSA-coated plates), on stromal support, or on the COOH terminal domain of FN. In all cultures, IL-3, IL-6, and SCF (3/6/S) were used, with or without addition of FLT3 ligand (FL). Colonies were enumerated on day 14. The percentage of G418-resistant colony-forming progenitors was calculated as (no. of G418-resistant CFU/no. of total CFU) × 100. The values shown were obtained from 3,000 CD34+ cells plated with and without G418 (0.9 mg/mL).
Transduction of 14-day colony-forming progenitors. CD34+ progenitors from six normal human bone marrow samples were transduced in the presence of IL-3, IL-6, and SCF ± FL under the following conditions: BSA, in suspension culture over BSA-coated plates; STRO, on monolayers of irradiated allogeneic stromal cells; FN, on plates coated with the recombinant FN fragment CH-296 (Retronectin). After 72 hours and three additions of supernatant from the PG13/LN cell line, colony-forming assays were plated with and without the selective agent G418. Colonies were counted after 14 to 21 days growth. The percentages of transduction were calculated as (#G418-resistant CFU/# total CFU) × 100 = % G418-resistance.
Transduction of 14-day colony-forming progenitors. CD34+ progenitors from six normal human bone marrow samples were transduced in the presence of IL-3, IL-6, and SCF ± FL under the following conditions: BSA, in suspension culture over BSA-coated plates; STRO, on monolayers of irradiated allogeneic stromal cells; FN, on plates coated with the recombinant FN fragment CH-296 (Retronectin). After 72 hours and three additions of supernatant from the PG13/LN cell line, colony-forming assays were plated with and without the selective agent G418. Colonies were counted after 14 to 21 days growth. The percentages of transduction were calculated as (#G418-resistant CFU/# total CFU) × 100 = % G418-resistance.
Engraftment of human hematopoietic cells in bnx mice.
After in vitro transduction, each sample was cotransplanted with primary human stromal cells engineered to produce human IL-3 into 6-week old beige/nude/xid mice, as described.18 To transplant a series of 12 mice, in three groups, 4 × 106 purified CD34+ cells were initially inoculated into three separate transduction flasks to transplant four mice from each flask. The cell numbers transplanted after the in vitro period were not recounted or normalized, but reflected the input cell number.
Mice were killed 6 to 12 months after xenotransplantation. Bone marrow was recovered from the mice and analyzed for the percentage of human CD45+ cell engraftment by FACS analysis. No mouse transplanted in experiment #3 (Table 1) was engrafted by human cells due to inadequate marrow conditioning (frozen 5-FU is ineffective), so the experiment could not be included in subsequent evaluations. Immune-deficient mice that had received human cells transduced in suspension culture in the presence of IL-3, IL-6, and SCF (n = 11) lacked significant numbers of human cells in all organs 6 to 12 months after transplantation (BSA-FL, average, 0.05% ± 0.07% human CD45+ cells in the marrow, Table 2). In contrast, human cells transduced under the same conditions with addition of 100 U/mL FLT3 ligand (BSA+FL), were recovered from the marrow of four of seven mice. Human CD45+ cells, detected by FACS, averaged 0.9% ± 1.5% in this group (P = .06, not a significant difference). Nine of the 10 mice transplanted with human cells transduced on stromal support (stroma-FL) had significant levels of engrafted human hematopoietic cells in their marrow, averaging 3.1% ± 2.4% (Table2). Addition of FL to the transductions performed on stromal support did not significantly increase the average percentage of CD45+ cells in the marrow of the mice (average = 3.6% ± 2.6%, P > .05). The group of mice transplanted with cells transduced on FN without FL had levels of human cell engraftment averaging 3.2% ± 2.5%, similar to the levels obtained for the two stromal support sets. The group transplanted with human CD34+ cells transduced on FN+FL had slightly, but not significantly, higher levels of engraftment by human CD45+cells, averaging 5.2% ± 3.3% (P > .05). There was no statistically significant difference between the engraftment levels of mice transplanted with cells transduced on stromal support versus FN, with or without addition of FL (P > .05). All four sets did, however, have significantly higher engraftment levels than mice transplanted with cells maintained in suspension (BSA ± FL,P < .05).
Human Hematopoietic Lineages Recovered From the Bone Marrow of bnx Mice 6 to 12 Months Posttransplantation
| Mouse No. . | Transduction Conditions . | Months Engrafted . | % hu CD45+ . | % hu Cells of Each Lineage in bnx BM . | ||||
|---|---|---|---|---|---|---|---|---|
| BM . | spl . | CD4 . | CD8 . | CD33 . | CD19 . | |||
| 1A1 | BSA-FL | 8.5 | — | — | — | — | — | — |
| 1A2 | BSA-FL | 8.5 | 0.3 | — | — | — | — | — |
| 2A1 | BSA-FL | 10 | — | — | — | — | — | — |
| 2A2 | BSA-FL | 10 | — | — | — | — | — | — |
| 2A3 | BSA-FL | 10 | — | — | — | — | — | — |
| 5A1 | BSA-FL | 12 | — | — | — | — | — | — |
| 5A2 | BSA-FL | 12 | — | — | — | — | — | — |
| 5A3 | BSA-FL | 12 | — | — | — | — | — | — |
| 6A1 | BSA-FL | 6 | 0.2 | — | — | — | — | — |
| 6A2 | BSA-FL | 6 | — | — | — | — | — | — |
| 6A3 | BSA-FL | 6 | — | — | — | — | — | — |
| 4B1 | BSA + FL | 12 | 2.0 | — | 0.4 | 0.6 | 0.9 | — |
| 4B2 | BSA + FL | 12 | — | — | — | — | — | — |
| 4B3 | BSA + FL | 12 | 3.8 | — | 0.8 | 1.5 | 1.1 | — |
| 5B1 | BSA + FL | 12 | — | — | — | — | — | — |
| 6B1 | BSA + FL | 6 | — | 4.4 | — | — | — | — |
| 6B2 | BSA + FL | 6 | 0.2 | 0.2 | 0.1 | 0.1 | — | — |
| 6B3 | BSA + FL | 6 | 0.3 | 4.4 | 0.1 | 0.2 | — | — |
| 1C1 | Stroma-FL | 8.5 | 4.1 | — | 1.1 | 1.7 | 1.4 | — |
| 1C2 | Stroma-FL | 8.5 | 0.9 | — | 0.2 | 0.4 | 0.3 | — |
| 1C3 | Stroma-FL | 8.5 | 2.5 | — | 0.7 | 1.1 | 0.7 | — |
| 2C1 | Stroma-FL | 10 | 1.4 | — | 0.3 | 0.7 | 0.4 | — |
| 2C2 | Stroma-FL | 10 | 5.3 | — | 1.2 | 2.5 | 1.6 | — |
| 2C3 | Stroma-FL | 10 | — | — | — | — | — | — |
| 5C1 | Stroma-FL | 12 | 3.2 | — | 0.8 | 1.3 | 0.9 | — |
| 6C1 | Stroma-FL | 6 | 3.6 | — | 0.9 | 1.7 | 0.7 | — |
| 6C2 | Stroma-FL | 6 | 1.6 | 3.5 | 0.4 | 0.6 | 0.5 | — |
| 6C3 | Stroma-FL | 6 | 8.3 | 5.0 | 1.6 | 2.8 | 3.9 | — |
| 4D1 | Stroma + FL | 12 | 7.9 | — | 2.0 | 2.5 | 3.4 | — |
| 4D2 | Stroma + FL | 12 | 5.0 | — | 1.2 | 1.9 | 1.8 | — |
| 4D3 | Stroma + FL | 12 | 2.5 | — | 0.6 | 1.0 | 0.9 | — |
| 5D1 | Stroma + FL | 12 | 1.3 | — | 0.4 | 0.5 | 0.4 | — |
| 5D2 | Stroma + FL | 12 | 1.2 | — | 0.3 | 0.5 | 0.3 | — |
| 6D1 | Stroma + FL | 6 | 6.3 | 1.6 | 1.2 | 2.8 | 2.3 | — |
| 6D2 | Stroma + FL | 6 | 3.4 | 0.9 | 0.8 | 1.3 | 1.1 | — |
| 6D3 | Stroma + FL | 6 | 1.2 | — | 0.2 | 0.6 | 0.4 | — |
| 1E1 | FN-FL | 8.5 | 7.8 | 1.4 | 2.7 | 3.2 | 1.9 | — |
| 1E2 | FN-FL | 8.5 | 4.6 | — | 1.2 | 1.8 | 1.5 | — |
| 1E3 | FN-FL | 8.5 | — | — | — | — | — | — |
| 2E1 | FN-FL | 10 | 6.9 | — | 2.6 | 3.2 | 1.1 | — |
| 2E2 | FN-FL | 10 | 5.2 | — | 1.1 | 1.9 | 2.0 | — |
| 5E1 | FN-FL | 12 | 1.7 | — | 0.4 | 0.6 | 0.7 | — |
| 5E2 | FN-FL | 12 | 1.0 | — | 0.2 | 0.5 | 0.3 | — |
| 5E3 | FN-FL | 12 | 1.3 | — | 0.4 | 0.6 | 0.3 | — |
| 6E1 | FN-FL | 6 | 2.1 | — | 0.5 | 0.9 | 0.6 | — |
| 6E2 | FN-FL | 6 | 2.4 | — | 0.8 | 0.9 | 0.7 | — |
| 6E3 | FN-FL | 6 | 2.2 | — | 0.6 | 0.8 | 0.8 | — |
| 4F1 | FN + FL | 12 | 3.0 | 0.8 | 0.7 | 1.3 | 0.9 | — |
| 4F2 | FN + FL | 12 | 7.7 | — | 2.2 | 2.5 | 3.0 | — |
| 4F3 | FN + FL | 12 | 11.6 | 2.3 | 3.8 | 5.1 | 2.7 | — |
| 5F1 | FN + FL | 12 | 6.1 | — | 2.0 | 2.9 | 1.2 | — |
| 5F2 | FN + FL | 12 | 2.3 | — | 0.5 | 1.1 | 0.7 | — |
| 6F1 | FN + FL | 6 | 1.6 | — | 0.4 | 0.7 | 0.3 | — |
| 6F2 | FN + FL | 6 | 4.0 | 7.4 | 1.1 | 1.6 | 1.3 | — |
| 6F3 | FN + FL | 6 | 5.1 | 2.2 | 1.5 | 2.4 | 1.2 | — |
| Mouse No. . | Transduction Conditions . | Months Engrafted . | % hu CD45+ . | % hu Cells of Each Lineage in bnx BM . | ||||
|---|---|---|---|---|---|---|---|---|
| BM . | spl . | CD4 . | CD8 . | CD33 . | CD19 . | |||
| 1A1 | BSA-FL | 8.5 | — | — | — | — | — | — |
| 1A2 | BSA-FL | 8.5 | 0.3 | — | — | — | — | — |
| 2A1 | BSA-FL | 10 | — | — | — | — | — | — |
| 2A2 | BSA-FL | 10 | — | — | — | — | — | — |
| 2A3 | BSA-FL | 10 | — | — | — | — | — | — |
| 5A1 | BSA-FL | 12 | — | — | — | — | — | — |
| 5A2 | BSA-FL | 12 | — | — | — | — | — | — |
| 5A3 | BSA-FL | 12 | — | — | — | — | — | — |
| 6A1 | BSA-FL | 6 | 0.2 | — | — | — | — | — |
| 6A2 | BSA-FL | 6 | — | — | — | — | — | — |
| 6A3 | BSA-FL | 6 | — | — | — | — | — | — |
| 4B1 | BSA + FL | 12 | 2.0 | — | 0.4 | 0.6 | 0.9 | — |
| 4B2 | BSA + FL | 12 | — | — | — | — | — | — |
| 4B3 | BSA + FL | 12 | 3.8 | — | 0.8 | 1.5 | 1.1 | — |
| 5B1 | BSA + FL | 12 | — | — | — | — | — | — |
| 6B1 | BSA + FL | 6 | — | 4.4 | — | — | — | — |
| 6B2 | BSA + FL | 6 | 0.2 | 0.2 | 0.1 | 0.1 | — | — |
| 6B3 | BSA + FL | 6 | 0.3 | 4.4 | 0.1 | 0.2 | — | — |
| 1C1 | Stroma-FL | 8.5 | 4.1 | — | 1.1 | 1.7 | 1.4 | — |
| 1C2 | Stroma-FL | 8.5 | 0.9 | — | 0.2 | 0.4 | 0.3 | — |
| 1C3 | Stroma-FL | 8.5 | 2.5 | — | 0.7 | 1.1 | 0.7 | — |
| 2C1 | Stroma-FL | 10 | 1.4 | — | 0.3 | 0.7 | 0.4 | — |
| 2C2 | Stroma-FL | 10 | 5.3 | — | 1.2 | 2.5 | 1.6 | — |
| 2C3 | Stroma-FL | 10 | — | — | — | — | — | — |
| 5C1 | Stroma-FL | 12 | 3.2 | — | 0.8 | 1.3 | 0.9 | — |
| 6C1 | Stroma-FL | 6 | 3.6 | — | 0.9 | 1.7 | 0.7 | — |
| 6C2 | Stroma-FL | 6 | 1.6 | 3.5 | 0.4 | 0.6 | 0.5 | — |
| 6C3 | Stroma-FL | 6 | 8.3 | 5.0 | 1.6 | 2.8 | 3.9 | — |
| 4D1 | Stroma + FL | 12 | 7.9 | — | 2.0 | 2.5 | 3.4 | — |
| 4D2 | Stroma + FL | 12 | 5.0 | — | 1.2 | 1.9 | 1.8 | — |
| 4D3 | Stroma + FL | 12 | 2.5 | — | 0.6 | 1.0 | 0.9 | — |
| 5D1 | Stroma + FL | 12 | 1.3 | — | 0.4 | 0.5 | 0.4 | — |
| 5D2 | Stroma + FL | 12 | 1.2 | — | 0.3 | 0.5 | 0.3 | — |
| 6D1 | Stroma + FL | 6 | 6.3 | 1.6 | 1.2 | 2.8 | 2.3 | — |
| 6D2 | Stroma + FL | 6 | 3.4 | 0.9 | 0.8 | 1.3 | 1.1 | — |
| 6D3 | Stroma + FL | 6 | 1.2 | — | 0.2 | 0.6 | 0.4 | — |
| 1E1 | FN-FL | 8.5 | 7.8 | 1.4 | 2.7 | 3.2 | 1.9 | — |
| 1E2 | FN-FL | 8.5 | 4.6 | — | 1.2 | 1.8 | 1.5 | — |
| 1E3 | FN-FL | 8.5 | — | — | — | — | — | — |
| 2E1 | FN-FL | 10 | 6.9 | — | 2.6 | 3.2 | 1.1 | — |
| 2E2 | FN-FL | 10 | 5.2 | — | 1.1 | 1.9 | 2.0 | — |
| 5E1 | FN-FL | 12 | 1.7 | — | 0.4 | 0.6 | 0.7 | — |
| 5E2 | FN-FL | 12 | 1.0 | — | 0.2 | 0.5 | 0.3 | — |
| 5E3 | FN-FL | 12 | 1.3 | — | 0.4 | 0.6 | 0.3 | — |
| 6E1 | FN-FL | 6 | 2.1 | — | 0.5 | 0.9 | 0.6 | — |
| 6E2 | FN-FL | 6 | 2.4 | — | 0.8 | 0.9 | 0.7 | — |
| 6E3 | FN-FL | 6 | 2.2 | — | 0.6 | 0.8 | 0.8 | — |
| 4F1 | FN + FL | 12 | 3.0 | 0.8 | 0.7 | 1.3 | 0.9 | — |
| 4F2 | FN + FL | 12 | 7.7 | — | 2.2 | 2.5 | 3.0 | — |
| 4F3 | FN + FL | 12 | 11.6 | 2.3 | 3.8 | 5.1 | 2.7 | — |
| 5F1 | FN + FL | 12 | 6.1 | — | 2.0 | 2.9 | 1.2 | — |
| 5F2 | FN + FL | 12 | 2.3 | — | 0.5 | 1.1 | 0.7 | — |
| 6F1 | FN + FL | 6 | 1.6 | — | 0.4 | 0.7 | 0.3 | — |
| 6F2 | FN + FL | 6 | 4.0 | 7.4 | 1.1 | 1.6 | 1.3 | — |
| 6F3 | FN + FL | 6 | 5.1 | 2.2 | 1.5 | 2.4 | 1.2 | — |
Bone marrow and spleen samples were recovered from bnx mice 6 to 12 months after cotransplantation of transduced human hematopoietic cells and IL-3–producing stromal cells. The percentage of human CD45+ cells in the marrow (BM) and spleen (spl) were determined by FACS. In addition, marrow samples were tested for the percentages of human T, B, and myeloid cells that they contained. The percentages of human cells that were positive for each lineage marker by FACS analysis of the ungated bone marrow cells are shown. A dash indicates that no human hematopoietic cells were detected in that sample. In each experiment, normal human peripheral blood and age-matched, nontransplanted bnx mice were analyzed concurrently to verify the species-specificity of the monoclonal antibodies.
Next, the human hematopoietic lineages engrafted in each mouse were assessed by FACS and compared to determine whether the initial in vitro transduction conditions had influenced subsequent differentiation of the transplanted CD34+ cells. Concurrent normal human and nontransplanted age-matched bnx mice were used as controls to verify the species-specificity of each antibody at the concentration used. Lineages examined were CD19+ B lymphoid, CD4+ and CD8+ T lymphoid, and CD33+myeloid. The percentages of total bnx/hu bone marrow populations representing human cells of each lineage are shown in Table2. As we had previously observed in the bnx/hu cotransplantation system,7,12,18 21 there was an absolute lack of development of human CD19+ B lymphocytes from the human bone marrow-derived CD34+ cells in all mice tested. There was no significant difference in the relative levels of development of the T-cell or myeloid lineages in mice transplanted with human cells cultured with stromal support or FN, with or without FL (Table 2).
To quantitate the clonogenic human hematopoietic progenitors in the bnx/hu bone marrow, human-specific colony-forming assays were plated from all samples. Under the conditions used, murine colonies are unable to develop, as has been previously established.18 Very few human colonies were grown from the marrow of 11 bnx/hu mice that had received human bone marrow cultured for 72 hours in the absence of stromal support, FN, or FL (average, 0.4 ± 0.3 human CFU/3 × 105 bnx bone marrow cells, BSA-FL, Table 3). Cells cultured in suspension retained some degree of clonogenic capacity only when cultured with FL, but not with IL-3, IL-6, and SCF alone. Two of the seven mice transplanted with human cells transduced in suspension with FL had clonogenic progenitors in their marrow (average for the BSA + FL group, 4.4 ± 2.9; P > .05, not a significant difference). Variable numbers of human CFU of all lineages, burst-forming unit-erythroid (BFU-E), colony-forming unit–granulocyte-macrophage (CFU-GM), and colony-forming unit granulocyte, erythroid, monocyte, megakaryocyte (CFU-GEMM), were recovered from mice that had been transplanted with human CD34+ cells transduced in the presence of stromal support (average, 27 ± 7.6, Stro-FL and 27.6 ± 6.9, Stro + FL) and FN (average, 15.5 ± 4.2, FN-FL and 22.8 ± 6.8, FN + FL, Table 3). Although there was variability in the numbers of CFU obtained from individual mice within the four stroma and fibronectin groups, the average values did not differ significantly from group to group (P > .05). All groups of mice that had received human cells transduced on stromal support or FN did, however, have significantly higher levels of human clonogenic progenitor content than either group that had received cells transduced in suspension, with or without FL (P < .05).
Transduction of Total Bone Marrow and Clonogenic Progenitors Recovered From bnx/hu Mice 6 to 12 Months Posttransplantation
| bnx No. . | Transduction . | No. G418R/Total Hu CFU = %G418R . | PCR for neo Gene: Total BM . | Total No. BM Integrants . | No. T-Cell Integrants . | No. Myeloid Integrants . |
|---|---|---|---|---|---|---|
| 1A1 | BSA-FL | 0/03-150 | neg | NA | NA | NA |
| 1A2 | BSA-FL | 0/3 | neg | NA | NA | NA |
| 2A1 | BSA-FL | 0/0 | neg | NA | NA | NA |
| 2A2 | BSA-FL | 0/0 | neg | NA | NA | NA |
| 2A3 | BSA-FL | 0/0 | neg | NA | NA | NA |
| 5A1 | BSA-FL | 0/0 | neg | NA | NA | NA |
| 5A2 | BSA-FL | 0/0 | neg | NA | NA | NA |
| 5A3 | BSA-FL | 0/0 | neg | NA | NA | NA |
| 6A1 | BSA-FL | 0/1 | neg | NA | NA | NA |
| 6A2 | BSA-FL | 0/0 | neg | NA | NA | NA |
| 6A3 | BSA-FL | 0/0 | neg | NA | NA | NA |
| 4B1 | BSA + FL | 3/14 = 21.4% | + | 1 | 0 | 1 |
| 4B2 | BSA + FL | 0/0 | neg | NA | NA | NA |
| 4B3 | BSA + FL | 1/17 = 5.9% | + | 1 | 0 | 1 |
| 5B1 | BSA + FL | 0/0 | neg | NA | NA | NA |
| 6B1 | BSA + FL | 0/0 | neg | NA | NA | NA |
| 6B2 | BSA + FL | 0/0 | neg | NA | NA | NA |
| 6B3 | BSA + FL | 0/0 | neg | NA | NA | NA |
| 1C1 | Stroma-FL | 9/51 = 17.6% | + | 3 | −3-151 | − |
| 1C2 | Stroma-FL | 0/5 | + | 0 | − | − |
| 1C3 | Stroma-FL | 4/22 = 18.2% | + | 1 | − | − |
| 2C1 | Stroma-FL | 1/12 = 8.3% | + | 1 | − | − |
| 2C2 | Stroma-FL | 5/63 = 7.9% | + | 2 | − | − |
| 2C3 | Stroma-FL | 0/0 | neg | NA | NA | NA |
| 5C1 | Stroma-FL | 1/23 = 4.3% | + | 1 | − | − |
| 6C1 | Stroma-FL | 3/34 = 8.8% | + | 4 | 1 | 3 |
| 6C2 | Stroma-FL | 0/0 | neg | NA | NA | NA |
| 6C3 | Stroma-FL | 7/60 = 11.7% | + | 4 | 1 | 2 |
| 4D1 | Stroma + FL | 5/48 = 10.4% | + | 4 | − | − |
| 4D2 | Stroma + FL | 2/37 = 5.4% | + | 2 | − | − |
| 4D3 | Stroma + FL | 2/25 = 8.0% | + | 1 | − | − |
| 5D1 | Stroma + FL | 0/0 | + | 0 | − | − |
| 5D2 | Stroma + FL | 1/15 = 6.7% | + | 1 | − | − |
| 6D1 | Stroma + FL | 6/59 = 10.2% | + | 6 | 2 | 4 |
| 6D2 | Stroma + FL | 3/27 = 11.1% | + | 3 | 0 | 3 |
| 6D3 | Stroma + FL | 0/10 | + | 0 | 0 | 0 |
| 1E1 | FN-FL | 3/21 = 14.3% | + | 2 | − | − |
| 1E2 | FN-FL | 1/20 = 5.0% | + | 4 | − | − |
| 1E3 | FN-FL | 0/0 | neg | NA | NA | NA |
| 2E1 | FN-FL | 2/11 = 18.1% | + | 1 | − | − |
| 2E2 | FN-FL | 4/43 = 9.3% | + | 3 | − | − |
| 5E1 | FN-FL | 0/2 | + | 0 | − | − |
| 5E2 | FN-FL | 1/12 = 8.3% | + | 1 | − | − |
| 5E3 | FN-FL | 0/8 | + | 0 | − | − |
| 6E1 | FN-FL | 3/17 = 17.6% | + | 4 | 1 | 3 |
| 6E2 | FN-FL | 0/0 | neg | NA | NA | NA |
| 6E3 | FN-FL | 5/36 = 13.9% | + | 6 | 3 | 5 |
| 4F1 | FN + FL | 2/45 = 4.4% | + | 2 | − | − |
| 4F2 | FN + FL | 3/14 = 21.4% | + | 4 | − | − |
| 4F3 | FN + FL | 2/22 = 9.0% | + | 2 | − | − |
| 5F1 | FN + FL | 1/6 = 16.7% | + | 1 | − | − |
| 5F2 | FN + FL | 1/9 = 11.1% | + | 1 | − | − |
| 6F1 | FN + FL | 0/0 | + | 0 | 0 | 0 |
| 6F2 | FN + FL | 12/53 = 22.6% | + | 5 | 2 | 4 |
| 6F3 | FN + FL | 6/33 = 18.2% | + | 6 | 4 | 5 |
| bnx No. . | Transduction . | No. G418R/Total Hu CFU = %G418R . | PCR for neo Gene: Total BM . | Total No. BM Integrants . | No. T-Cell Integrants . | No. Myeloid Integrants . |
|---|---|---|---|---|---|---|
| 1A1 | BSA-FL | 0/03-150 | neg | NA | NA | NA |
| 1A2 | BSA-FL | 0/3 | neg | NA | NA | NA |
| 2A1 | BSA-FL | 0/0 | neg | NA | NA | NA |
| 2A2 | BSA-FL | 0/0 | neg | NA | NA | NA |
| 2A3 | BSA-FL | 0/0 | neg | NA | NA | NA |
| 5A1 | BSA-FL | 0/0 | neg | NA | NA | NA |
| 5A2 | BSA-FL | 0/0 | neg | NA | NA | NA |
| 5A3 | BSA-FL | 0/0 | neg | NA | NA | NA |
| 6A1 | BSA-FL | 0/1 | neg | NA | NA | NA |
| 6A2 | BSA-FL | 0/0 | neg | NA | NA | NA |
| 6A3 | BSA-FL | 0/0 | neg | NA | NA | NA |
| 4B1 | BSA + FL | 3/14 = 21.4% | + | 1 | 0 | 1 |
| 4B2 | BSA + FL | 0/0 | neg | NA | NA | NA |
| 4B3 | BSA + FL | 1/17 = 5.9% | + | 1 | 0 | 1 |
| 5B1 | BSA + FL | 0/0 | neg | NA | NA | NA |
| 6B1 | BSA + FL | 0/0 | neg | NA | NA | NA |
| 6B2 | BSA + FL | 0/0 | neg | NA | NA | NA |
| 6B3 | BSA + FL | 0/0 | neg | NA | NA | NA |
| 1C1 | Stroma-FL | 9/51 = 17.6% | + | 3 | −3-151 | − |
| 1C2 | Stroma-FL | 0/5 | + | 0 | − | − |
| 1C3 | Stroma-FL | 4/22 = 18.2% | + | 1 | − | − |
| 2C1 | Stroma-FL | 1/12 = 8.3% | + | 1 | − | − |
| 2C2 | Stroma-FL | 5/63 = 7.9% | + | 2 | − | − |
| 2C3 | Stroma-FL | 0/0 | neg | NA | NA | NA |
| 5C1 | Stroma-FL | 1/23 = 4.3% | + | 1 | − | − |
| 6C1 | Stroma-FL | 3/34 = 8.8% | + | 4 | 1 | 3 |
| 6C2 | Stroma-FL | 0/0 | neg | NA | NA | NA |
| 6C3 | Stroma-FL | 7/60 = 11.7% | + | 4 | 1 | 2 |
| 4D1 | Stroma + FL | 5/48 = 10.4% | + | 4 | − | − |
| 4D2 | Stroma + FL | 2/37 = 5.4% | + | 2 | − | − |
| 4D3 | Stroma + FL | 2/25 = 8.0% | + | 1 | − | − |
| 5D1 | Stroma + FL | 0/0 | + | 0 | − | − |
| 5D2 | Stroma + FL | 1/15 = 6.7% | + | 1 | − | − |
| 6D1 | Stroma + FL | 6/59 = 10.2% | + | 6 | 2 | 4 |
| 6D2 | Stroma + FL | 3/27 = 11.1% | + | 3 | 0 | 3 |
| 6D3 | Stroma + FL | 0/10 | + | 0 | 0 | 0 |
| 1E1 | FN-FL | 3/21 = 14.3% | + | 2 | − | − |
| 1E2 | FN-FL | 1/20 = 5.0% | + | 4 | − | − |
| 1E3 | FN-FL | 0/0 | neg | NA | NA | NA |
| 2E1 | FN-FL | 2/11 = 18.1% | + | 1 | − | − |
| 2E2 | FN-FL | 4/43 = 9.3% | + | 3 | − | − |
| 5E1 | FN-FL | 0/2 | + | 0 | − | − |
| 5E2 | FN-FL | 1/12 = 8.3% | + | 1 | − | − |
| 5E3 | FN-FL | 0/8 | + | 0 | − | − |
| 6E1 | FN-FL | 3/17 = 17.6% | + | 4 | 1 | 3 |
| 6E2 | FN-FL | 0/0 | neg | NA | NA | NA |
| 6E3 | FN-FL | 5/36 = 13.9% | + | 6 | 3 | 5 |
| 4F1 | FN + FL | 2/45 = 4.4% | + | 2 | − | − |
| 4F2 | FN + FL | 3/14 = 21.4% | + | 4 | − | − |
| 4F3 | FN + FL | 2/22 = 9.0% | + | 2 | − | − |
| 5F1 | FN + FL | 1/6 = 16.7% | + | 1 | − | − |
| 5F2 | FN + FL | 1/9 = 11.1% | + | 1 | − | − |
| 6F1 | FN + FL | 0/0 | + | 0 | 0 | 0 |
| 6F2 | FN + FL | 12/53 = 22.6% | + | 5 | 2 | 4 |
| 6F3 | FN + FL | 6/33 = 18.2% | + | 6 | 4 | 5 |
Bone marrow samples were recovered from bnx mice after engraftment periods of 6 to 12 months. Human-specific colony-forming assays with and without addition of the selective agent G418 were plated from the bone marrow of each mouse, and the results are shown as: no. of G418-resistant/total human CFU (% G418 − resistant CFU). PCR for the presence of the neogene in the LN proviral vector was performed on each marrow sample. Results of the PCR analyses are indicated as positive (+) or noneo gene detected (neg). Genomic DNA samples extracted from the marrow of each mouse that carried neo marked cells were further subjected to clonal analysis by inverse PCR. The number of proviral integrants detected in each sample (no. of PCR product bands/2 LTR bands per proviral integrant = no. of integrants) is listed. Individual human T-lymphoid cells (CD45+/CD3+) and myeloid progenitors (CD45+/CD33+ cells with small size and low side scatter) recovered from long-term engrafted mice from experiment no. 6 were deposited by ACDU into 96-well plates. Human granulocyte-macrophage clones were grown individually in methylcellulose medium with 0.9 mg/mL G418. Human T lymphocytes were expanded individually in medium containing rHuL-2 + PHA and irradiated feeders, then tested for the presence of the neo gene by PCR. Clonal integration analysis by inverse PCR was performed on the total marrow sample, and on each neo-positive human clone recovered from each mouse.
Abbreviation: NA, not applicable; noneo-marked cells were detected in the marrow of the mouse, so further analyses were not done.
A total of 3 × 105 bnx/hu bone marrow cells were plated ± G418 (0.9 mg/mL active) per sample.
A dash indicates that single-cell cloning was not performed.
Tissue distribution of vector-marked human cells in bnx mice.
It was possible that the differences in culture conditions during transduction, before transplantation, could cause variation in homing and/or sites of subsequent human hematopoiesis. The use of retroviral marking allows the tracking of a portion of the input cell population to each organ and can identify differences that might arise due to the method of ex vivo manipulation of the transplanted cells. Samples of the bone marrow, blood, and spleen from each long-term engrafted bnx mouse were therefore analyzed by FACS for the presence of human CD45+ cells, and organs (liver, lung, kidney, spleen, and bone marrow) were screened for the presence of LN provirus by PCR for the neo gene as described.18Vector-marked cells were most commonly detected in the bone marrow. No cells bearing neo provirus were recovered from the marrrow of mice transplanted with human cells transduced in suspension culture with IL-3, IL-6, and SCF (BSA-FL, Table 3). Addition of FL to the same transduction condition resulted in the presence of vector-marked cells in the marrow of two of seven mice (BSA + FL). Nine of the 10 mice that received human cells transduced in media containing IL-3, IL-6, and SCF with stromal support harbored cells marked with LN provirus in their bone marrow (stroma-FL, Table 3), and all eight mice transplanted with human cells transduced in 3/6/S/FL had marked marrow cells (Stroma + FL, Table 3). Nine of 11 mice transplanted with cells cultured on FN without FL had marked marrow cells, and eight of eight from the FN+FL group had neo-positive bone marrow. Only two mice from each group transplanted with cells cultured on stroma or FN had marked human cells in the spleen at the time of harvest, and no human cells bearing LN provirus were recovered from the liver, lung, or kidney of any animal (Table 3). Therefore, the major site for survival of marked human hematopoietic cells was the murine bone marrow, with few marked cells recovered from the other tissues after long-term engraftment.
Transduced human colony-forming progenitor recovery from the bone marrow of bnx mice.
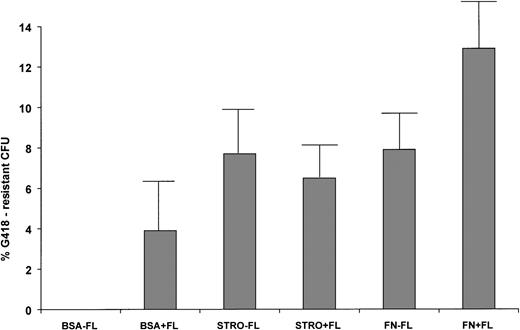
To further assess transduction levels of primitive human cells and survival of vector-marked human hematopoietic progenitors, human-specific colony-forming assays were plated with and without the selective agent G418 from the bone marrow of each mouse in the six groups. In two cases (mice 5D1 and 6F1, Table 3), the total marrow sample was positive for the neo gene, but no transduced progenitors were recovered. This data indicates that the neo gene in the total marrow sample was amplified from mature, marked cells that had lost clonogenic potential. Mice that had been transplanted with human cells transduced in the absence of stromal support, FN, or FL had no long-term engrafted human cells able to form colonies in the presence or absence of G418 (BSA-FL, Table 3). Only two of the seven mice transplanted with human CD34+ cells cultured on BSA-coated plates with FL had G418-resistant human CFU in their marrow (average, 3.9% ± 3.0%, P > .05). In contrast, the majority of the mice transplanted with cells cultured on stromal support or FN, ± FL, had reclonable, G418-resistant human hematopoietic progenitors in their marrow. The average percentages of G418R progenitors in each group were 7.7 ± 2.5 for STRO-FL, 6.5 ± 1.6 for STRO+FL, 7.9 ± 2.2 for FN-FL, and 12.9 ± 2.9 for FN+FL. Although all of the groups of mice transplanted with cells transduced on stromal or FN support had significantly higher levels of transduced, clonogenic progenitors than the BSA-FL group (P < .05), there was no statistical difference in the levels between the four stromal support or FN groups (P > .05, Fig 2). This data indicates that stromal and FN support were equivalent in their ability to promote survival and successful retroviral-mediated transduction of primitive, long-term engrafting human hematopoietic progenitors.
Transduction of clonogenic human progenitors recovered from long-term engrafted bnx/hu mice. Human-specific CFU were plated ± G418 from the marrow of each mouse. CFU were plucked from the plates and subjected to PCR for the neo gene to confirm the presence of integrated vector. The percentages of transduction were calculated as (#G418-resistant CFU/# total CFU) × 100 = % G418-resistance.
Transduction of clonogenic human progenitors recovered from long-term engrafted bnx/hu mice. Human-specific CFU were plated ± G418 from the marrow of each mouse. CFU were plucked from the plates and subjected to PCR for the neo gene to confirm the presence of integrated vector. The percentages of transduction were calculated as (#G418-resistant CFU/# total CFU) × 100 = % G418-resistance.
Clonal integration analysis.
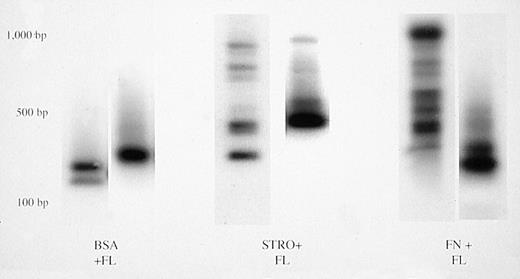
Each marrow sample that had been identified to contain cells marked by the LN vector (Table 3) was further subjected to inverse PCR to allow determination of the number of individual, marked human progenitors contributing to the neo-positive PCR signals. Retroviral vectors integrate into host cell chromosomes at random sites, generating unique restriction fragment length polymorphisms. Integrated vectors can therefore be used as clonal tags to identify all cells derived from a common progenitor.26-30 The results of the inverse PCR analyses are summarized in Table 3. Mice that had received human cells transduced in suspension with IL-3, IL-6, and SCF had no integrated LN provirus in their marrrow. Only mice #4B1 and 4B3, transplanted with human CD34+ cells transduced in suspension with FL, had single vector integrants detectable in their marrow (Fig 3). Mice that had received human cells transduced on stromal or fibronectin support ± FL had oligoclonal marking, with zero to six marked human progenitors contributing to hematopoiesis (Table 3, Fig 3). The average values in the latter four groups were not significantly different (P > .05). We conclude that transfer of the neo gene into a limited number of individual human progenitors capable of engrafting bnx mice for extended periods of time occurred to equivalent levels using either stromal or FN support during the transduction.
Clonal analysis by inverse PCR of bnx/hu bone marrow samples. Bone marrow was harvested and DNA was isolated after long-term engraftment by human CD34+ cells cultured in the conditions indicated. Clonal analysis was performed to determine the number of individual transduced progenitors that were contributing to hematopoiesis at the time of harvest.
Clonal analysis by inverse PCR of bnx/hu bone marrow samples. Bone marrow was harvested and DNA was isolated after long-term engraftment by human CD34+ cells cultured in the conditions indicated. Clonal analysis was performed to determine the number of individual transduced progenitors that were contributing to hematopoiesis at the time of harvest.
Human CD33+ myeloid and CD3+ lymphoid cells recovered from the bone marrow of the engrafted mice in experiment #6 were analyzed individually. Single cells were plated by automated cell deposition (ACDU) into 96-well plates and expanded to a size of 200 to 1,000 cells under lymphoid-specific or human myeloid-specific conditions as described.21,22 DNA was isolated from the individual clones and subjected to inverse PCR. No transduced T-cell or myeloid clones were recovered from mice transplanted with human CD34+ cells transduced in suspension with IL-3, IL-6, and SCF, with or without FL. Mice 6C1 and 6C3, transplanted with human cells transduced on stromal support without FL, had several separate proviral integration patterns among the myeloid clones, respectively, and each had a single integrant in the neo-positive human T-cell clones. Addition of FL to the transductions done on stromal support did not significantly increase the number of different clonogenic progenitors that were transduced. There was a small, but nonsignificant, increase in the number of clonongenic primitive cells that were transduced on FN, as compared with stromal support. Mice 6E1 and 6E3 had three and five myeloid cells transduced, respectively, and one and three T-cell clones. Mice 6F2 and 6F3, transplanted with human CD34+ cells transduced on FN with FL, had a slightly higher number of individual clones marked (four and five myeloid clones, and two and four T-lymphoid clones, respectively, Table 3). No matching patterns were obtained from human myeloid and T-cell clones recovered from the same mouse, which would have indicated that they were derived from the same stem cell. We have previously shown that this is an extremely rare event, using the current retroviral vector technology.21 We conclude that transduction for 72 hours on FN support in the presence of serum and cytokines gives rise to levels of gene transfer into primitive, reconstituting cells that are at least as effective as the levels achieved with stromal support. Furthermore, using the current clinically approved protocols, transduction on the FN matrix is essential to preserve the capacity of the human stem and progenitor cells to sustain long-term multilineage engraftment, if autologous stromal support is not used for practical or logistical reasons.
DISCUSSION
Our previous studies demonstrated that the presence of a stromal underlayer had dual benefits during ex vivo transduction of long-lived human CD34+ cells with cell-free retroviral supernatant. In addition to the enhancement of gene transfer into clonogenic progenitors, as had been previously shown by Moore et al,3we discovered that the presence of stroma during ex vivo transduction also maintained the ability of human progenitors to sustain long-term hematopoiesis in the bnx/hu system.7,12 We postulated that engagement of integrins was required to prevent induction of pathways of terminal differentiation in the cells. Wang et al31found that primitive hematopoietic cells were rescued from apoptosis by binding to stromal cells via the VLA-4 and vascular cell adhesion molecule-1 (VCAM-1) integrins. Chertkov et al32 had previously reported that murine stem cells cultured on stromal monolayers had better survival and levels of gene marking than cells kept on gelatin-coated plates with cytokines. We had shown that addition of FLT3 ligand to cells cultured in suspension could partially, but not completely, relace stromal support in maintaining the capacity of cultured cells to sustain long-term engraftment.12 Therefore, in the current studies, we compared culture in suspension with stromal and FN support, in the presence and absence of FLT3 ligand.
Immune-deficient mice transplanted with cells cultured on stromal support or FN fragments had comparable levels of human cell engraftment in their marrow, with no statistically significant difference. The cells in the BSA arm of the experiment were from the same donor and were maintained ex vivo under identical conditions, but in suspension culture, rather than adherent to the FN fragment. The cells maintained in suspension culture did not contribute to long-term hematopoiesis in the immune-deficient mice, suggesting that their long-term generative capacity had been severely diminished during the 72-hour ex vivo culture period. These data suggest that engagement of the integrins VLA-4 and VLA-5 to the FN COOH domain can maintain the ability of human CD34+ progenitors cultured 72 hours ex vivo in IL-3, IL-6, SCF, and serum to sustain long-term hematopoiesis, whereas that ability is lost by maintaining the cells under identical conditions, but in suspension culture.
Fragments of FN containing the RGDS, connecting segment-1 (CS-1), and heparin-binding domains have been shown to colocalize retroviral vector particles and hematopoietic cells, leading to efficient transduction of human hematopoietic progenitors.8-10 Verfaillie et al33 reported that primitive progenitors bind to the FN CS-1 domain via the VLA-4 integrin and lose adhesion as they differentiate. Hematopoietic progenitors and many differentiated cell types bind via the VLA-5 integrin to the FN RGDS domain.34,35 Because the CH-296 fragment includes both the CS-1 and the RGDS domains, it can be used for retroviral-mediated transduction of hematopoietic cells of both immature and mature phenotypes, including mature T lymphocytes.36
A number of current clinical gene therapy trials are using autologous stroma for ex vivo transduction of CD34+ cells due to the ability of stromal cells to enhance gene transfer into hematopoietic progenitors (reviewed in Nolta and Kohn22,37). However, stroma may produce negative regulators of hematopoiesis such as transforming growth factor (TGF)β and chemokines,38 is difficult to standardize for reproducibility, must be kept sterile for several weeks ex vivo before reinfusion of the gene therapy product into the patient, and may be difficult to expand from patients with disease or after chemotherapy.39 Obtaining autologous stromal cells requires a marrow aspirate to be drawn 1 month before the gene therapy procedure, which can pose risk to the patient, as with any invasive procedure. Additionally, expansion of stroma on a scale adequate for transduction of an entire inoculum of bone marrow or peripheral blood progenitors may not be possible within the period of time between the initial marrow aspirate and the harvest for the trial. Our studies have shown that FN-coated flasks replaced stromal support at the levels of gene transfer and stem cell survival. The present data suggests that the combination of FN, to enhance gene transfer, and FL, to support progenitor survival, is a simple, safe alternative for replacement of patient-derived stromal layers during clinical trials for human gene therapy.
ACKNOWLEDGMENT
We thank Donald Kohn, Ken Weinberg, Gay Crooks, Craig Jordan, and Robertson Parkman, as always, for useful discussion. This work would not have been possible without Sally Worttman, who heads our animal facility with care, and Renee Traub-Workman and Miriam Figueroa, who maintain the bnx mouse colonies with great dedication. Pat Snow provided excellent secretarial assistance.
Supported by Grants No. R01 DK53041 from the National Institutes of Health (NIH), NIDDK, SCOR #1-P50-HL54850 from the NIH, National Heart, Lung & Blood Institute, a James A. Shannon Director’s award from the NIH National Institutes of Diabetes and Digestive and Kidney Diseases, and a Career Development Award from the Childrens Hospital of Los Angeles Research Institute.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to J.A. Nolta, PhD, Division of Research Immunology/Bone Marrow Transplantation, Childrens Hospital Los Angeles, and the Department of Pediatrics, University of Southern California School of Medicine, 4650 Sunset Blvd, Mailstop #62, Los Angeles, CA 90027; e-mail: jnolta@chla.usc.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal