Abstract
Phenotypic characterization of Diamond Blackfan Anemia (DBA) patients and their relatives was performed in 54 families. Complete blood count, fetal hemoglobin level, erythrocyte i antigen expression, and erythrocyte adenosine deaminase (eADA) activities were quantitated in patients and relatives. eADA was elevated in 28 of 34 transfusion-independent DBA patients, whereas persistence of erythrocyte i antigen was noticed in only 10 of 20 DBA patients. High eADA activities were also found in 14 of 149 healthy family members, allowing us to identify an isolated high eADA phenotype in these families. In contrast, increase in erythrocyte i antigen expression, elevated fetal hemoglobin levels, and macrocytosis were much less frequently noted in nonaffected members of the DBA families studied. Importantly, isolated high eADA phenotype was found to be significantly associated with genetic markers on chromosome 19 that segregate with the DBA phenotype. Isolated high eADA phenotype thus seems to reflect a silent phenotype of DBA in affected families. These findings suggest that elevated eADA activity in unaffected individuals needs to be taken into account during genetic assessment of DBA families and could be used for accurate assessment of mode of inheritance.
LINKAGE ANALYSIS of Diamond Blackfan anemia (DBA) multiplex families has recently assigned this disorder to a locus on 19q13.2.1 Interestingly, most families shared the same chromosomal linkage, although there were marked differences in the phenotypic expression of the disease, including associated malformations, therapeutic outcomes, and mode of inheritance. Previous studies have identified several biological features in DBA patients, as well as in some of their relatives. These include increases in fetal hemoglobin levels, macrocytosis, persistence of erythrocyte i antigen expression, and elevated erythrocyte adenosine deaminase activity (eADA).2 3
In the present study, we have systematically investigated each of these abnormalities in all members of 54 DBA families. In addition, we performed DNA analysis with markers linked to the DBA locus on chromosome 19q13.2 in most family members. eADA was elevated in 28 of 34 transfusion-independent DBA patients, whereas persistence of erythrocyte i antigen was noticed in only 10 of 20 DBA patients. High eADA activities were also found in 14 of 149 healthy family members, allowing us to identify an isolated high eADA phenotype in these families. Importantly, isolated high eADA phenotype was found to be significantly associated with genetic markers on chromosome 19 that segregate with the DBA phenotype. These data strongly support the previous suggestion that isolated high eADA level could represent a silent phenotype of this disease. This parameter is thus highly relevant for familial studies.
MATERIALS AND METHODS
Study design.
The French National Register of DBA, initiated in 1985, includes 158 patients whose diagnoses fit all the criteria defined by the DBA study group of the European Society for Pediatric Haematology and Immunology. The criteria for the diagnosis of DBA include occurrence of anemia before 2 years of age, exclusion of recent parvovirus B19 infection by both serology and polymerase chain reaction (PCR) analysis of bone marrow cells, and exclusion of Fanconi anemia by a negative chromosome fragility test. The French DBA register was approved by the Commission Nationale Informatique et Libertés, and the study protocol was approved by the human experimentation committee of Hôpital Bicêtre (France). Informed consent was obtained from each individual and from the parents of children included in the genetic study.
Clinical and biological evaluation were performed on all affected individuals and on all of the available family members from 54 different families (63 patients, 93 parents or relatives, 56 siblings). Detailed physical examination was performed on all individuals. Biochemical and hematologic evaluation included complete blood cell count with red blood cell (RBC) indices, hemoglobin electrophoresis, fetal hemoglobin level determination by resistance to alkaline denaturation,4 erythrocyte i antigen expression, and quantification of eADA and erythrocyte purine nucleoside phosphorylase (PNP) activities. Biochemical and hematologic evaluations were not performed on blood samples from individuals receiving regular blood transfusions. Macrocytosis or elevation of fetal hemoglobin were not considered to be relevant indicators of DBA phenotype in one individual receiving chemotherapy and in another individual with chronic alcoholism.
Flow cytometry analysis of blood group i antigen expression.
RBCs from patients, siblings, and relatives were collected in EDTA and were rapidly cryopreserved in liquid nitrogen until used. Blood samples from common donors and from a rare individual of the OI-negative phenotype (Guillard) were collected and treated similarly. Analysis of blood group i antigen expression was performed on a FACScan cytometer (Becton Dickinson, San Jose, CA) using the murine monoclonal antibody anti-i (NaM61-1A2, IgM) donated by Dr D. Blanchard (Nantes, France). In 0.15 mol/L phosphate-buffered saline (PBS), 3 × 105 RBCs were incubated for 30 minutes at 22°C with three preparations of the hybridoma culture supernatants diluted 1:20, 1:40, and 1:80. After several washings in PBS supplemented with 0.5% bovine serum albumin, the cells were incubated for 30 minutes at 22°C with a fluorescein-conjugated anti-mouse IgM (Immunotech, Marseille, France). After several additional washes, the microagglutinates were gently dissociated by micropipetting and recovered as single-cell suspensions (assessed by light scatter) and subjected to fluorescence analysis. OI-negative cells (Guillard) which exhibited high levels of blood group i antigen were used as positive controls. Blood group i expression on RBCs from a population of 31 healthy adults and from the OI-negative adult controls was estimated using serial dilutions (1:20, 1:40, 1:80) of the murine monoclonal anti-i antibody (supernatant) by direct immunofluorescence analysis on a flow cytometer. Under saturing conditions (1:20 dilution), the mean fluorescence intensity was 64.7 ± 31.7 for normal adult RBCs and 893 ± 244 for OI-negative cells, indicating a low level of expression of i antigen on adult RBCs and a significant overexpression of i antigen on OI-negative cells.
Assessment of eADA and PNP.
eADA and PNP activities were assessed in hemolysates by radioisotopic methods with paper chromatography separation of substrates and products on Whatman P81 paper (Whatman, Maidstone, UK).5 Results were expressed both as an absolute value and as the ratio of ADA to PNP activities. Mean values from control population are 1.35 ± 0.36 nmol/mn/mg hemoglobin for eADA, and 144 ± 19 nmol/mn/mg hemoglobin for erythrocyte PNP.
Haplotype assessment.
Genetic haplotype was studied as a part of the linkage study using microsatellites flanking the region identified in familial cases.1 Genomic DNA was extracted from peripheral blood leukocytes according to standard procedures. The polymorphic dinucleotide repeats D19S200, D19S197, and D19S408, assigned to the 19q13 region,6 were chosen for the determination of haplotypes. The markers D19S200 and D19S408 flank the DBA gene locus and their relative order, with the approximate genetic distances, is centromere-D19S200-1.5 cm-D19S197-1.9 cm-D19S408-telomere.1 PCR of polymorphic markers was performed at optimized conditions as described previously.1 The PCR reactions were performed in a reaction volume of 10 μL with 20 ng of genomic DNA and a γ-32P-ATP end-labeled primer in 96-well microtitre plates. The PCR products were separated by polyacrylamide gel electrophoresis and visualized by autoradiography. The genetic haplotypes were assigned manually.
Statistical analysis.
Analysis was focused on a subset of eight families presenting with both classical DBA patient(s) and seemingly healthy individuals with isolated high eADA activity. Because DNA samples were not available for two of these eight families, correlation studies between phenotype and genetic haplotypes could be performed in only six of the families. Diagnosis was evoked from the occurrence of erythroblastopenia, between birth and 14 months of age. Three patients were supposed to be sporadic cases, whereas the three other families included between two and three DBA cases. Genetic haplotype of each family member was compared with those of the index DBA case, and assessed to be either the same or different. Statistical analysis of relationship between genetic haplotype and clinical phenotype was then performed using Yates corrected Chi-square test, and computing of exact 95% limits of confidence interval of odds ratio using Epi-Info 6.04b7 or Statistica 4.0 B (Statsoft, Inc, Tulsa, OK) software.
RESULTS
Phenotypic characterization of patients.
Of the 63 DBA patients studied, 16 came from 7 multiplex families whereas the other 47 were considered sporadic cases. Among these 63 individuals, 34 patients were transfusion independent; eADA, PNP, mean corpuscular volume (MCV), and fetal hemoglobin level were quantitated in these 34 individuals. As shown in Fig 1, eADA activity was found to be significantly above normal levels in 28 of the 34 patients. In contrast, PNP activity was always within the normal range (data not shown). Persistent increased erythrocyte i antigen expression (3 to 10 times higher than normal) was noted in 10 of 20 patients studied. In our study we did not find any correlation between eADA activity and persistence of erythrocyte i antigen expression. Fetal hemoglobin levels were elevated in 21 DBA patients whereas macrocytosis was noted in 16 DBA patients. Neither fetal hemoglobin levels nor MCV correlated with any of the other biochemical and hematologic parameters studied.
Distribution of eADA in DBA patients, their siblings, their parents, and normal controls. eADA and PNP activities were assessed in hemolysates by radioisotopic methods with paper chromatography separation of substrates and products, as described in Materials and Methods and by Pérignon et al.5
Distribution of eADA in DBA patients, their siblings, their parents, and normal controls. eADA and PNP activities were assessed in hemolysates by radioisotopic methods with paper chromatography separation of substrates and products, as described in Materials and Methods and by Pérignon et al.5
Phenotypic characterization of families.
We also studied 149 apparently healthy members from 54 DBA families. Fourteen of the 149 healthy individuals showed elevated eADA (observed values ranging from 2.29 to 8.6 nmol/mn/mg hemoglobin; normal values, 1.35 ± 0.36 nmol/mn/mg hemoglobin). This group of individuals with high eADA activity consisted of 8 siblings, 1 father, 3 mothers, and 2 relatives (grandmother and great aunt) of the affected members. These data are not sufficient to support a hypothesis of parental imprinting. Interestingly, the erythrocyte i antigen expression was found to be significantly elevated (1.5 to 3 times as compared with healthy adults) in 5 of the 79 nonaffected family members studied, whereas fetal hemoglobin and MCV were found significantly elevated in only 2 of 149.
Correlation studies between eADA activity and gene haplotype.
Haplotype analysis performed on 6 families in which DBA was associated with isolated high eADA activity showed a cosegregation of the 19q markers of the DBA patients with isolated high eADA phenotype in 14 of 15 individuals (Fig 2A through F and Table 1). On the other hand, among 17 nonaffected individuals who showed an eADA activity in the normal range, 3 shared the 19q markers haplotype of the affected individual of the family. Statistical analysis of data showed a significant association between the genetic haplotype and increased eADA activity (P < .0001). In contrast, no significant association was noted in this study between genetic haplotypes of DBA patients and other hematologic markers (persistent erythrocyte i antigen expression, fetal hemoglobin, MCV).
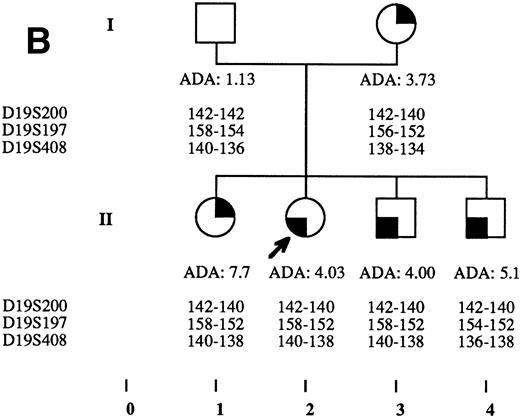
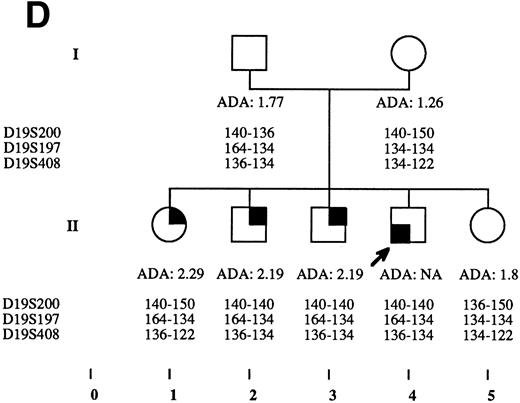
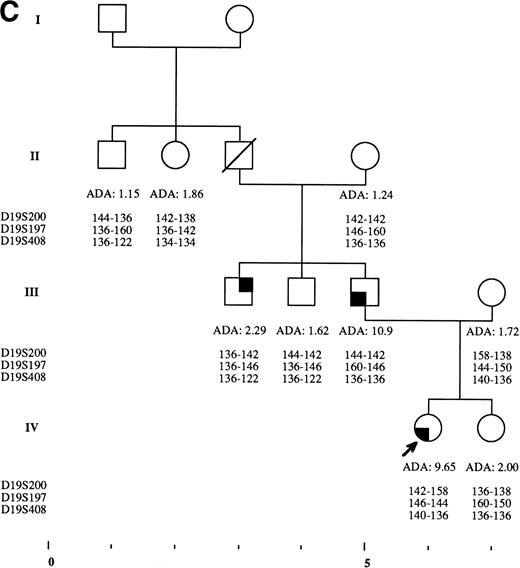
Pedigrees of families simultaneously showing DBA and isolated high eADA phenotype. (A) Recombination events occurred on meiosis from individuals III-3 and II-5. Allele sizes for microsatellite D19S200 could not be determined for individuals II-13 and III-13, and they were therefore not included in the haplotype analysis. (B) Unlike the three DBA cases of this family, individual II-1 was previously diagnosed as transient erythroblastopenia, and her mother (I-3) was healthy. (C) DBA index case of this family (patient IV-6) responded to interleukin-3 and is still independent of transfusions and steroids on the long-term follow-up. (D) All three siblings showed high eADA levels (II-1, -2, -3) and have normal blood count and hemoglobin electrophoresis, without known history of anemia. (E) Individuals I-2 and II-3 show classical DBA phenotype. eADA of individual II-2 was found increased at birth and remains over normal limits without any episode of anemia, and no other biological abnormality. eADA measurements of individuals I-1 and I-3 were performed in another laboratory, and results are within normal values for the technique considered. (F) Individual II-3 does not share the same haplotype as the DBA index case of the family. NA, not available.
Pedigrees of families simultaneously showing DBA and isolated high eADA phenotype. (A) Recombination events occurred on meiosis from individuals III-3 and II-5. Allele sizes for microsatellite D19S200 could not be determined for individuals II-13 and III-13, and they were therefore not included in the haplotype analysis. (B) Unlike the three DBA cases of this family, individual II-1 was previously diagnosed as transient erythroblastopenia, and her mother (I-3) was healthy. (C) DBA index case of this family (patient IV-6) responded to interleukin-3 and is still independent of transfusions and steroids on the long-term follow-up. (D) All three siblings showed high eADA levels (II-1, -2, -3) and have normal blood count and hemoglobin electrophoresis, without known history of anemia. (E) Individuals I-2 and II-3 show classical DBA phenotype. eADA of individual II-2 was found increased at birth and remains over normal limits without any episode of anemia, and no other biological abnormality. eADA measurements of individuals I-1 and I-3 were performed in another laboratory, and results are within normal values for the technique considered. (F) Individual II-3 does not share the same haplotype as the DBA index case of the family. NA, not available.
Correlation Between Genetic Haplotype and ADA Level in Families With Occurrence of DBA and Isolated High ADA Level
| . | Genetic Haplotype Within the Family . | ||
|---|---|---|---|
| Same as DBA Case . | Different From DBA Case . | Total . | |
| eADA | |||
| Increased* | 14 | 1 | 15 |
| Normal | 3 | 16 | 19 |
| Total | 17 | 17 | 34 |
| . | Genetic Haplotype Within the Family . | ||
|---|---|---|---|
| Same as DBA Case . | Different From DBA Case . | Total . | |
| eADA | |||
| Increased* | 14 | 1 | 15 |
| Normal | 3 | 16 | 19 |
| Total | 17 | 17 | 34 |
Index DBA case of each family is indicated with an arrow on pedigrees in Fig 2A through F. Statistical analysis performed through Yates corrected Chi-square test: P < .0001; odds ratio = 74.67; exact confidence limits of odds ratio, (5.87 − 3,342.87).7
Both isolated high eADA individuals and other DBA cases within the family with high eADA activity are considered increased eADA.
Because these data suggest that elevated high eADA could be of use in assessing the mode of inheritance, we reexamined families on the basis of the measured eADA activity. Dominant mode of inheritance was confirmed through an elevation of eADA in 2 families. In 3 other families in which the mode of inheritance could not be established, dominant mode of inheritance could be deduced from the finding of apparently healthy individuals with high eADA activity. In 1 multiplex family, reassessment of previously healthy individuals suggested reappraisal of mode of inheritance from recessive to dominant. Sporadic occurrence of DBA was confirmed in 46 families, with normal or low levels of eADA in parents or siblings.
DISCUSSION
In the present study, we have been able to show a significant association between DBA phenotype and isolated high eADA activity phenotype on the basis of haplotype analysis of six DBA families. This finding of high eADA activity in apparently normal family members could be an important tool for a more precise characterization of nuclear families. Isolated high eADA phenotype can represent a silent phenotype, linked to the 19q13.2 DBA locus in affected families. We have also confirmed the previous report of high prevalence of increase in eADA activity in transfusion-independent DBA patients.
Previous studies have already described such a biochemical abnormality in DBA families but could not prove any association between these phenotypes. Glader and Backer8 had reported an elevation of eADA activity in 26 of 29 transfusion-independent patients, as did Filanovskaya et al9 in 10 of 13 DBA patients. The reduced frequency of this feature in DBA patients in a third study (elevation of eADA in 9 of 19 DBA patients only) could be explained by the inclusion of transfusion-dependent patients.10,11 Elevation of eADA was confirmed to be a persistent feature in DBA patients over years, as shown through serial measurements.8 High eADA activity in healthy relatives from DBA-affected individuals has also been previously reported by different groups. Among 8 families where both parents were assessed, 2 of 16 parents were found to exhibit isolated high eADA activity.3,10 Our data confirm overall prevalence of asymptomatic high eADA phenotype in one of the parents of sporadic DBA cases, with 14 of 149 individuals originating from 8 of 54 French families in our series (9.4% of individuals, 14.8% of families). Previous studies reported this finding in 10 of 44 cases (22%).8-11 Other nonspecific parameters have also been described in DBA (persistence of increased erythrocyte i antigen expression, increase in fetal hemoglobin level, macrocytosis). According to the present study, they seem to be less frequently abnormal than eADA, and no significant association could be found between any of these parameters and genetic markers on the 19q DBA locus.
The pathophysiological link between DBA and elevation of eADA remains unclear. Besides primary ADA deficiencies associated with immune deficiency12 and hemolytic anemia with major increase in eADA activity,13 several hematologic disorders are characterized by a nonspecific increase in ADA activity. Indeed, increased eADA level has been reported in myeloproliferative syndromes, dyskeratosis, and megaloblastic anemia.8 In contrast, eADA was reported to be normal in patients with transient erythroblastopenia in childhood.14 As others, we have shown that the increase in erythrocyte enzyme activity does not correlate with biological signs of stress hematopoiesis, steroid treatment response, or lymphocyte ADA activity (data not shown).8 ADA is one of the enzymes of the nucleoside catabolism pathway, converting adenosine to inosine. Two major isoforms have been characterized in the erythrocytes.15 Analysis of these isoforms did not show any alteration in DBA,10,11 indicating that this observed ADA abnormality is not caused by an imbalance between isoforms of the enzyme, but rather by an overall increase in the concentration of the isoforms. Recent progress in molecular biology favors this hypothesis, because most familial cases of DBA are linked on chromosome 19 (19q13.2),1 whereas the gene coding for ADA is located on chromosome 20.16
Is the increase in eADA activity a secondary phenomenon linked to erythroblastopenia (or one of its consequences), or do both eADA increase and DBA phenotype result from a common genetic defect, like an alteration of a promoter or of an enhancer regulating the expression of two genes involved in different metabolic pathways? Mice models with mutations in the coding sequence of c-kit or of its ligand, resulting in strains of mice with both macrocytic anemia and elevated nucleoside deaminase activity, favor the first hypothesis.17
Application of the present findings show that some isolated DBA cases correspond to a dominant mode of inheritance with a variable phenotypic expression within the family. The present study also provides some new insights into the heterogeneity encountered in DBA. Previous studies have emphasized the wide range of clinical presentation of DBA, which includes long-term transfusion dependence, steroid responsiveness, and spontaneous remissions. Our data highlight three biochemical phenotypes, sometimes simultaneously present within a same family: DBA cases with increased eADA activity, DBA cases with normal eADA activity, and nonanemic individuals with high eADA activity. The existence of a completely silent phenotype is furthermore suggested by the occurrence of 3 of 17 healthy individuals sharing the same genetic haplotype as the corresponding DBA case. Thus, a normal eADA activity in a healthy individual from a DBA family is not sufficient to provide accurate genetic counseling.
Our clinical and biochemical findings, along with analysis of molecular basis of DBA, highlight the usefulness of complete phenotypic and genetic characterization of all family members in DBA cases to provide better genetic counseling to the affected families. Furthermore, findings of linkage between markers from 19q13 region and isolated high eADA phenotype can be used to identify a subclinical expression of DBA in the affected families. It is anticipated that the identification of the gene(s) responsible for the DBA phenotype will enable us to finally understand the relationship between the classical DBA phenotype and the intrafamilial asymptomatic isolated high eADA phenotype.
ACKNOWLEDGMENT
We acknowledge the DBA working groups of the European Society for Pediatric Hematology and Immunology (ESPHI), and of the Société d’Hématologie et d’Immunologie Pédiatrique (SHIP). We are grateful to V. Drouin and M. Cadoudal for their technical assistance in the determination of eADA. We are grateful to Drs Narla Mohandas, Philippe Gascard, and Joel Chasis for their careful help to improve this manuscript.
Supported in part by Association Française contre les Myopathies (A.F.M.), Généthon, Direction de la Recherche Clinique (DRC) (grant CRC 950183), and Assistance Publique-Hôpitaux de Paris. Haplotyping was performed thanks to grants from the Children’s Cancer Foundation of Sweden, the Swedish Medical Research Council, and the DBA Foundation Inc.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to T.N. Willig, MD, Bldg 74-157, Lawrence Berkeley Laboratory, One Cyclotron Rd, Berkeley, CA 94720; e-mail:tnwillig@lbl.gov.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal