Abstract
Primary cutaneous B-cell lymphomas are B-cell non-Hodgkin’s lymphomas that arise in the skin. The major subtypes discerned are follicle center cell lymphomas, immunocytomas (marginal zone B-cell lymphomas), and large B-cell lymphomas of the leg. In this study, we analyzed the variable heavy chain (VH) genes of 7 of these lymphomas, ie, 4 follicle center cell lymphomas (diffuse large-cell lymphomas) and 3 immunocytomas. We show that all these lymphomas carry heavily mutated VH genes, with no obvious bias in VH gene usage. The low ratios of replacement versus silent mutations observed in the framework regions of 5 of the 7 lymphomas suggest that the structure of the B-cell antigen receptor was preserved, as in normal B cells that are selected for antibody expression. Moreover, evidence for ongoing mutation was obtained in 3 immunocytomas and in one lymphoma of large-cell type. In addition, in 1 immunocytoma, both IgG- and IgA-expressing clones were found, indicative of isotype switching. Our data provide insight into the biology of primary cutaneous B-cell lymphomas and may be of significance for their classification.
PRIMARY CUTANEOUS B-cell lymphomas (PCBCLs) are defined as B-cell non-Hodgkin’s lymphomas (B-NHLs) that arise in the skin, with no evidence of extracutaneous disease for a period of at least 6 months after diagnosis. PCBCLs generally have a much better prognosis than nodal B-NHLs of comparable histologic subtype.1-3 The European Organization for Research and Treatment of Cancer (EORTC) has proposed a new classification for PCBCLs, based on clinical, histological, and immunological criteria.3 The major subtypes are primary cutaneous follicle center cell lymphomas and immunocytomas (marginal zone B-cell lymphomas), both of indolent clinical behavior. Another group of PCBCLs, ie, large B-cell lymphomas of the leg, is considered to be of intermediate malignancy. Two provisionally designated entities are intravascular large B-cell lymphomas and plasmacytomas.
Although the nomenclature of PCBCLs suggests an established relationship with nodal B-NHL counterparts and/or with differentiation stages of normal B-cell ontogeny, virtually no data are as yet available on the composition of the B-cell antigen receptors (BCR) of PCBCLs. Normal B-cell maturation is characterized by stepwise alterations of the BCR. Naive B cells carry unmutated Ig variable heavy chain and light chain genes that are expressed at the cell surface as IgM and IgD isotypes.4,5 In germinal centers of secondary follicles, B cells proliferate and compete to bind antigens that are exposed at the surface of follicular dendritic cells (FDCs).6 Recognition of antigen elicits signals essential for proliferation and differentiation. During the subsequent cell divisions, somatic mutations are introduced in the variable Ig genes (reviewed by Kocks and Rajewsky7). Because of strict selection processes, the germinal center reaction finally yields B cells with nonrandom patterns of somatic mutations and augmented affinity for the recognized antigens.8 These post-germinal center cells, either memory B cells or plasma cells, often express heavy chain isotypes other than μ and δ,4 8 which has implications for the effector functions of the secreted Ig.
To obtain information on the maturational state of PCBCLs, we analyzed the variable heavy chain (VH) region genes of 7 of these lymphomas. We show that they all carry significantly mutated VH genes with mutation patterns reminiscent of antigen selection processes. This finding indicates that PCBCLs are derived from germinal center cells or their descendents. Moreover, evidence was obtained for ongoing somatic hypermutation and isotype switching, features that are shared with extracutaneous B-NHLs of mucosa-associated lymphoid tissue (MALT) and follicular lymphomas.
MATERIALS AND METHODS
Patient material.
Tissue material of 7 PCBCLs, ie, 4 follicle center cell lymphomas, 3 immunocytomas (marginal zone B-cell lymphomas), and 1 pseudolymphoma, was obtained from the Departments of Dermatology and Pathology of the Free University Hospital and the Department of Pathology of the Academic Medical Center (Amsterdam, The Netherlands). The diagnoses were based on the characteristic clinical and histologic criteria, described previously.3 The follicle center cell lymphomas showed a predominance of centroblasts and are further referred to as diffuse large B-cell lymphomas. Follow-up data confirmed the favorable prognosis of these lymphomas, with all patients alive and in complete remission 12 to 106 months after diagnosis. The clinical and histological data of the 8 patients are summarized in Table 1.
Description of Cases of PCBCL Analyzed in This Study
| Patient No. . | Morphology (type) . | Immunohistochemistry . | Patient Data . | ||||
|---|---|---|---|---|---|---|---|
| Ig Isotype . | CD21L* . | CD70 . | Sex . | Age (yr) . | Localization . | ||
| 1 | Large B-cell lymphoma | IgG | ++ | − | F | 40 | Head |
| 2 | Large B-cell lymphoma | Not clear | ++ | − | M | 68 | Thorax left |
| 3 | Large B-cell lymphoma | IgM, IgD | + | − | F | 66 | Left hip |
| 4 | Large B-cell lymphoma | IgM, IgD | − | − | M | 58 | Elbow, shoulder |
| 5 | Immunocytoma | IgM, faint IgD | +++ | + | M | 56 | Right leg |
| 6 | Immunocytoma | IgM, IgD | +++ | + | F | 74 | Chin |
| 7 | Immunocytoma | Not clear | − | − | M | 80 | Face |
| 8 | Pseudolymphoma | ND | ND | − | F | 46 | Nose |
| Patient No. . | Morphology (type) . | Immunohistochemistry . | Patient Data . | ||||
|---|---|---|---|---|---|---|---|
| Ig Isotype . | CD21L* . | CD70 . | Sex . | Age (yr) . | Localization . | ||
| 1 | Large B-cell lymphoma | IgG | ++ | − | F | 40 | Head |
| 2 | Large B-cell lymphoma | Not clear | ++ | − | M | 68 | Thorax left |
| 3 | Large B-cell lymphoma | IgM, IgD | + | − | F | 66 | Left hip |
| 4 | Large B-cell lymphoma | IgM, IgD | − | − | M | 58 | Elbow, shoulder |
| 5 | Immunocytoma | IgM, faint IgD | +++ | + | M | 56 | Right leg |
| 6 | Immunocytoma | IgM, IgD | +++ | + | F | 74 | Chin |
| 7 | Immunocytoma | Not clear | − | − | M | 80 | Face |
| 8 | Pseudolymphoma | ND | ND | − | F | 46 | Nose |
Abbreviation: ND, not determined.
+, minimal remnants; ++, nodular networks; +++, extensive, ill-defined FDC networks.
Immunohistochemistry.
The expression of surface Ig isotype, CD70, and the presence of FDCs was determined immunohistochemically on cryostat sections. Monoclonal antibodies specific for human Ig isotypes and CD21L (DRC-1) were purchased from DAKO (Glostrup, Denmark), except for anti-IgM, which was obtained from Becton Dickinson (Erembodegem-Aalst, Belgium). CLB-CD70/1, specific for CD70, was a kind gift from Dr R.A.W. van Lier (Central Laboratory of The Netherlands Red Cross Blood Transfusion Service, Amsterdam, The Netherlands). Acetone-fixed tissue sections were preincubated with 10% normal goat serum (Sera Lab, Sussex, UK) in phosphate-buffered saline (PBS) for 15 minutes. After 1 hour of incubation with the primary antibody, endogenous peroxidase was blocked for 10 minutes with 0.1% NaN3, 0.3% H2O2 in PBS. Subsequently, sections were incubated with biotin-conjugated rabbit antimouse (Dako) for 30 minutes. After incubation with a streptavidin-biotin-peroxidase complex (Dako) for 30 minutes, horseradish peroxidase activity was detected with 3-amino-9-ethylcarbazole (Sigma, St Louis, MO) and 0.03% H2O2. Sections were counterstained with haematoxylin (Merck, Darmstadt, Germany).
RNA isolation and cDNA synthesis.
Total cellular RNA was isolated from frozen tissue sections using the TRIZOL reagent (Life technologies, Breda, The Netherlands) according to the manufacturer’s instructions. For cDNA synthesis, 10 μg of RNA was incubated with 5 nmol of pd(N)6 primer (Pharmacia Biotech, Roosendaal, The Netherlands) for 10 minutes at 65°C. After cooling on ice, the reaction mixture was added to a final volume of 50 μL. It contained 400 U of Moloney murine leukemia virus (M-MLV) reverse transcriptase (Life Technologies, Breda, The Netherlands), 8 mmol/L dithiothreitol (DTT), 1 mmol/L of each dNTP, 1× first-strand buffer (50 mmol/L Tris-HCl, pH 8.3, 75 mmol/L KCl, 3 mmol/L MgCl2), and 60 U of RNAse inhibitor (Boehringer Mannheim, Almere, The Netherlands). The reaction was performed for 1 hour at 37°C. Subsequently, the enzyme was inactivated during 10 minutes at 95°C.
Polymerase chain reactions (PCRs).
The complementarity determining region 3 (CDR3) was amplified using a forward primer with specificity for framework region 3 (FR3) in combination with reverse primers specific for JH (JHseq), Cμ, Cγ (Cγ2), Cα, or Cδ (Table 2). Either, 1 μL of the cDNA reaction mixture was used or (for a nested PCR) 1 μL of PCR product from a VH family-specific PCR was used. The PCR mixture contained 1× Taq buffer (20 mmol/L Tris-HCl, 50 mmol/L KCl, pH 8.4), 0.2 mmol/L of each dNTP, 1.5 mmol/L MgCl2, 2 U of Taq polymerase (Life Technologies), and 0.5 μmol/L of each primer. First, 10 cycles of amplification were performed in the thermal cycler (PTC-100; MJ Research Inc, Watertown, MA), ie, successively 30 seconds at 95°C, 20 seconds at 57°C, and 20 seconds at 72°C. The next 40 cycles of amplification consisted of 30 seconds at 95°C, 20 seconds at 55°C, and 20 seconds at 72°C. The reaction was completed for 6 minutes at 72°C. PCR products were analyzed on a 3% Metaphor agarose gel (FMC Bioproducts, Rockland, ME). For the VH family-specific PCR, reactions were performed with one of the VH family-specific leader primers (Table 2), combined with the appropriate reverse primer, either JH, Cμ, Cγ, or Cα. The PCR reaction mixture was the same as for the CDR3-specific PCR, except that 1 U of Taq polymerase and 0.25 μmol/L of each primer was used. Thirty cycles of 30 seconds at 95°C, 30 seconds at 55°C, and 30 seconds at 72°C were performed. The reaction was terminated for 6 minutes at 72°C. The PCR products were analyzed on a 1% standard agarose gel (Sigma).
Primers Used for VH Gene Amplification
| Name . | Sequence (5′-3′) . |
|---|---|
| VH1 | AAATCGATACCACCATGGACTGGACCTGGAGG |
| VH1b | AAATCGATACCACCATGGACTGGACCTGGAG(C/A) |
| VH2a | AAATCGATACCACCATGGACACACTTTGCT(A/C)AC |
| VH2b | AAATCGATACCACCATGGACATACTTTGTTCCAC |
| VH3a | AAATCGATACCACCACCATGGAGTTTGGGCTGAGC |
| VH3b | AAATCGATACCACCACCATGGA(A/G)(C/T)T(G/T)(G/T)G(G/A)CT(G/C/T)(A/C/T)GC |
| VH4 | AAATCGATACCACCATGAAACACCTGTGGTTCTT |
| VH5 | AAATCGATACCACCATGGGGTCAACCGCCATC |
| VH6 | AAATCGATACCACCATGTCTGTCTCCTTCCTC |
| FR3 | GACACGGC(C/T)(C/G)T(G/A)TATTACTG |
| JH | GGACTAGTTTCTTACCTGAGGAGACGGTGACC |
| JHseq | ACCTGAGGAGACGGTGACC |
| Cμ | GGGTCGACGCAGGAGACGAGGGGGAAAAG |
| Cγ | GGGACTAGTAACTTTCTTGTCCACCTTGGTGTT |
| Cγ2 | AGACCGATGGGCCCTTGGTG |
| Cα | CGGGAAGACCTTGGGGCTG |
| Cδ | TGTCTGCACCCTGATATGATG |
| Name . | Sequence (5′-3′) . |
|---|---|
| VH1 | AAATCGATACCACCATGGACTGGACCTGGAGG |
| VH1b | AAATCGATACCACCATGGACTGGACCTGGAG(C/A) |
| VH2a | AAATCGATACCACCATGGACACACTTTGCT(A/C)AC |
| VH2b | AAATCGATACCACCATGGACATACTTTGTTCCAC |
| VH3a | AAATCGATACCACCACCATGGAGTTTGGGCTGAGC |
| VH3b | AAATCGATACCACCACCATGGA(A/G)(C/T)T(G/T)(G/T)G(G/A)CT(G/C/T)(A/C/T)GC |
| VH4 | AAATCGATACCACCATGAAACACCTGTGGTTCTT |
| VH5 | AAATCGATACCACCATGGGGTCAACCGCCATC |
| VH6 | AAATCGATACCACCATGTCTGTCTCCTTCCTC |
| FR3 | GACACGGC(C/T)(C/G)T(G/A)TATTACTG |
| JH | GGACTAGTTTCTTACCTGAGGAGACGGTGACC |
| JHseq | ACCTGAGGAGACGGTGACC |
| Cμ | GGGTCGACGCAGGAGACGAGGGGGAAAAG |
| Cγ | GGGACTAGTAACTTTCTTGTCCACCTTGGTGTT |
| Cγ2 | AGACCGATGGGCCCTTGGTG |
| Cα | CGGGAAGACCTTGGGGCTG |
| Cδ | TGTCTGCACCCTGATATGATG |
Cloning and sequencing of PCR products.
After excision of the PCR products from an agarose gel and isolation of DNA with the Qiaex kit (Qiagen, Hilden, Germany), the PCR products were ligated into pGEM-T vectors (Promega, Leiden, The Netherlands), according to the manufacturer’s instructions, and transformed into DH10b bacteria (Life Technologies). Subsequently, both strands of the inserts were sequenced from 4 or more colonies to obtain the sequence of the dominant clone, the consensus sequence. Sequencing was performed with an ABI sequencer (Perkin Elmer Corp, Norwalk, CT) using the dye-terminator cycle-sequencing kit (Perkin Elmer Corp), according to the manufacturer’s instructions. To determine the Taq error rate of our experimental design, 19 clones of CD79a and CD79b were sequenced. These clones were generated according to the same PCR and cloning procedures as used for the VH genes. The Taq error frequency thus established is 0.14%, which amounts to 0.4 mutation/VH clone.
Assignment of mutations.
The seqences found were compared with published germline sequences, using the Vbase database9 and DNAplot on the Internet (http://www.genetik.uni-koeln.de/dnaplot; programmed by H.H. Althaus) to identify mutations. Mutations at the last nucleotide position of the V gene were excluded from the mutational analysis, because they might result from nucleotide deletions at the joining sites. To calculate whether the excess or scarcity of replacement mutations in the FRs had occurred by chance, we used the binomial distribution model as proposed by Chang and Casali.10 In B cells selected for antibody expression, there is a counterselection for replacement (R) mutations in the FR to maintain the structure of the antibody. The ratios of replacement versus silent (R/S) mutations in the CDRs are often higher than expected. However, the R/S values found in the CDRs cannot be used as arguments for or against antigenic selection. Dörner et al11 showed that the R/S values of both FR and CDR were higher in nonproductive and therefore unselected rearrangements than in productive, antigen-selected rearrangements. Also, it can be imagined that, in an already selected Ig with optimal affinity, additional replacement mutations in the CDRs are unfavorable.
RESULTS
Morphology of PCBCLs.
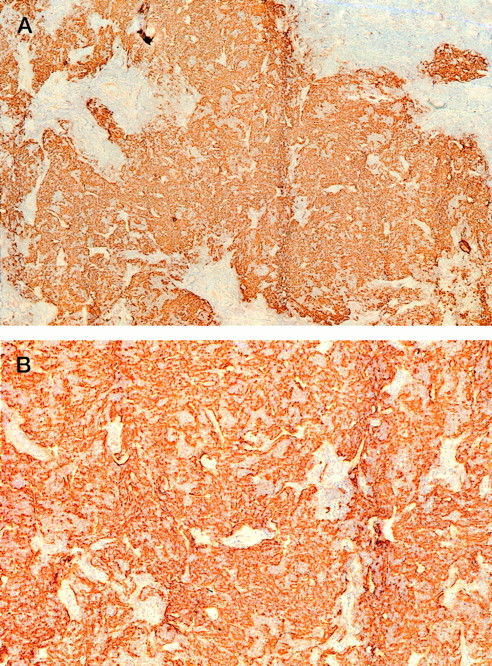
The clinical and histological data of the 7 PCBCLs analyzed are summarized in Table 1. The group comprised 4 follicle center cell lymphomas (all diffuse large B-cell lymphomas) and 3 immunocytomas (marginal zone B-cell lymphomas). In addition, a lesion showing the clinical and histologic features of a pseudolymphoma was included in this study (no. 8). Despite the fact that all lymphomas displayed a diffuse growth pattern, areas of FDCs were detected in 5 of the 7 lymphomas (nos. 1, 2, 3, 5, and 6). In PCBCLs no. 5 and 6, extensive ill-defined networks of FDCs were found (Fig 1), which suggests that the FDCs form an integral part of this neoplasm. Interestingly, CD70 expression was also found only in lymphomas no. 5 and 6 (not shown). In the other cases (no. 1, 2, and 3), the FDC clusters were more or less nodular and well circumscribed. Here, it is unclear whether they belong to the tumors or represent the remains of pre-existent reactive follicles that were infiltrated by the neoplastic B cells. These different patterns of FDCs in PCBCL have also been reported by Mori et al.12
Tissue section of lymphoma no. 5 stained for FDCs with antibodies against CD21L (DRC-1). Magnification (A) × 50 and (B) × 125.
Tissue section of lymphoma no. 5 stained for FDCs with antibodies against CD21L (DRC-1). Magnification (A) × 50 and (B) × 125.
Ig isotype expression.
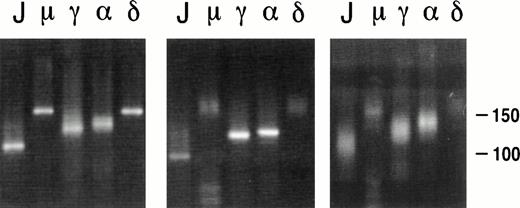
The Ig heavy chain isotype of the 7 PCBCLs studied was determined immunohistochemically (Table 1) and by PCR, ie, by amplifying the CDR3 region with an FR3 primer in combination with primers specific for the 5′ regions of Cμ, Cγ (Cγ2), Cα, or Cδ heavy chains (Table 2). Because of differences in length of the diversity (D) genes13 and random nucleotide additions by terminal deoxytransferase14 at junctions of the V-D and D-J gene segments, the size of the CDR3 regions varies considerably between B-cell clones.15 Therefore, a CDR3-specific PCR on a polyclonal B-cell population yields products of variable size, visible as multiple bands or a smear pattern after electrophoresis, whereas this PCR on a clonal population yields a single band (Fig 2). In our hands, this CDR3-specific PCR has proven very useful as a screening assay and as a sensitive method to identify the lymphoma-derived Ig when, due to the copresence of nonneoplastic B cells, multiple VH genes are amplified from a tissue specimen. With respect to the assessment of the Ig isotype expression, either immunohistochemically or by PCR, we encountered no discrepancies (compare Tables 1 and 3). For example, in lymphoma no. 6, clear membrane expression of both IgM and IgD was detected by immunohistochemistry (Table 1). As expected, products of single length were amplified by PCR using the FR3 primer in combination with JHseq, Cμ, and Cδ primers, whereas smears were obtained with Cγ2 and Cα as downstream primers (Fig 2, left panel). PCR of a pseudolymphoma yielded products of variable lengths in all lanes (Fig2, right panel). In 4 PCBCLs (no. 2, 4, 5, and 7), the two methods to assign the Ig isotype were complementary (Tables 1 and 3); immunostaining of lymphoma no. 5 showed clear membrane expression of IgM and, at most, very weak expression of IgD. However, using PCR, both IgM and IgD were clearly amplified from this lymphoma. Also, in cases no. 2 and 7, the Ig isotypes used could only be established by PCR. Alternatively, lymphoma no. 4 was found to express both IgM and IgD immunohistochemically, whereas by PCR only IgM expression was found. This was possibly due to the low amount of cDNA available from this lymphoma. In summary, 4 lymphomas (no. 3, 4, 5, and 6) were found to coexpress IgM and IgD, whereas 3 lymphomas (no. 1, 2, and 7) expressed IgG. Interestingly, the CDR3-specific PCR of lymphoma no. 7 yielded single bands in the lanes corresponding to the JHseq, Cγ2, and Cα primers, which is suggestive of the presence of clonal populations of both IgG and IgA isotypes (Fig 2, middle panel). To analyze whether the IgG and IgA products originated from the same tumor clone, the VH genes were amplified with VH family-specific primers in combination with the constant Cγ or Cα primers. Subsequent sequencing of the VH-Cγ and VH-Cα PCR products confirmed that they harbored the same VDJ rearrangement (not shown).
PCR analysis on the CDR3 region of lymphomas no. 6 (left panel) and 7 (middle panel) and the pseudolymphoma (right panel). The FR3 primer was used as upstream primer, and JHseq, Cμ, Cγ2, C, and Cδ primers were used as downstream primers, as indicated above the lanes.
PCR analysis on the CDR3 region of lymphomas no. 6 (left panel) and 7 (middle panel) and the pseudolymphoma (right panel). The FR3 primer was used as upstream primer, and JHseq, Cμ, Cγ2, C, and Cδ primers were used as downstream primers, as indicated above the lanes.
Ig Heavy Chain Gene Analysis of PCBCL
| Patient No. . | Ig Isotype3-150 . | VH Family . | Closest Germline Gene . | No. of Mutations . | % of Homology . | D Gene . | JH Gene . | Intraclonal Variation3-151 (no. of clones sequenced) . |
|---|---|---|---|---|---|---|---|---|
| 1 | γ | 5 | V5-51 (COS24) | 15 | 95 | NA | JH4b | 0 (4) |
| 2 | γ | 3 | V3-7 | 29 | 90 | NA | JH6c | 0 (4) |
| 3 | μ, δ | 3 | V3-23 | 52 | 82 | NA | JH4b | 0 (5) |
| 4 | μ | 3 | V3-7 | 15 | 95 | D21-9 | JH3b | 0.6 (8) |
| 5 | μ, δ | 3 | V3-30 (COS3) | 17 | 94 | NA | JH4b | 1.4 (5) |
| 6 | μ, δ | 4 | V4-61 (3d279d) | 35 | 88 | DXP4 | JH5b | 2.6 (5) |
| 7 | γ | 1 | V1-2 (DP8) | 37 | 87 | NA | JH4b | 3.4 (5) |
| 8 | α | 1 | V1-2 (DP8) | 37 | 87 | NA | JH4b | 0.2 (5) |
| Patient No. . | Ig Isotype3-150 . | VH Family . | Closest Germline Gene . | No. of Mutations . | % of Homology . | D Gene . | JH Gene . | Intraclonal Variation3-151 (no. of clones sequenced) . |
|---|---|---|---|---|---|---|---|---|
| 1 | γ | 5 | V5-51 (COS24) | 15 | 95 | NA | JH4b | 0 (4) |
| 2 | γ | 3 | V3-7 | 29 | 90 | NA | JH6c | 0 (4) |
| 3 | μ, δ | 3 | V3-23 | 52 | 82 | NA | JH4b | 0 (5) |
| 4 | μ | 3 | V3-7 | 15 | 95 | D21-9 | JH3b | 0.6 (8) |
| 5 | μ, δ | 3 | V3-30 (COS3) | 17 | 94 | NA | JH4b | 1.4 (5) |
| 6 | μ, δ | 4 | V4-61 (3d279d) | 35 | 88 | DXP4 | JH5b | 2.6 (5) |
| 7 | γ | 1 | V1-2 (DP8) | 37 | 87 | NA | JH4b | 3.4 (5) |
| 8 | α | 1 | V1-2 (DP8) | 37 | 87 | NA | JH4b | 0.2 (5) |
Abbreviation: NA, the D gene could not definitely be assigned to a germline D gene.
Heavy chain isotype expression as determined by PCR.
The intraclonal variation is indicated as the number of mutations observed per clone, compared with the consensus sequence.
VH, D, and JH gene usage.
VH genes were amplified with family-specific VH leader primers (Table2). On the PCR products thus obtained, we performed a nested CDR3-specific PCR to confirm that a particular VH product originated from the clonal population (data not shown). Subsequently, the VH product was cloned and sequenced. The nucleotide sequences have been deposited at the GenBank database (accession nos. AF052379 throughAF052386). The VH sequences were compared with the germline genes with the highest homology and, accordingly, the number of somatic mutations was determined (Table 3). Four PCBCLs used genes of the VH3 family, whereas VH1, VH4, and VH5 family genes were each found once. Comparison with germline JH gene segments showed that the JH4b gene was present in 4 rearrangements (lymphomas no. 1, 3, 5, and 7). The lymphomas no. 2, 4, and 6 used the JH6c, JH3b, and the JH5b genes, respectively. Corbett et al13 proposed stringent criteria for the assignment of D genes: at least 10 consecutive nucleotides of identity are required to confidently assign a D gene segment. According to these criteria, we could only assign the D21-9 and DXP4 gene segments of lymphomas no. 4 and 6, respectively.
Mutation patterns.
All lymphomas expressed extensively mutated VH genes, ranging from 15 to 52, with an average of 28.6 mutations per VH sequence (Table 3). Within our limited set of 7 PCBCLs, there was no obvious difference in the number of mutations between IgM+IgD+ or IgG+ lymphomas. Analysis of the distribution of replacement (R) versus silent (S) mutations10 demonstrated that 5 of the 7 PCBCLs (no. 2, 3, 4, 5, and 6) contained a significantly lower number of R mutations in the FRs (Table 4) than would be expected if mutations had occurred by chance alone, ie, in the absence of selective forces. Except for lymphoma no. 6, the R/S values within the CDRs were always higher than those within the FRs of the corresponding VH genes.
Analysis of VH Gene Mutations in PCBCLs
| Patient No. . | FR/CDR . | Observed . | Expected R/S4-150 . | P Value . | ||
|---|---|---|---|---|---|---|
| R . | S . | R/S . | ||||
| 1 | FR | 6 | 2 | 3 | 3.3 | .066 |
| CDR | 6 | 1 | 6 | 3.5 | ||
| 2 | FR | 10 | 8 | 1.3 | 2.8 | <.01 |
| CDR | 7 | 4 | 1.8 | 4.9 | ||
| 3 | FR | 18 | 19 | 0.95 | 2.9 | <.001 |
| CDR | 8 | 7 | 1.1 | 3.5 | ||
| 4 | FR | 2 | 8 | 0.25 | 2.8 | <.001 |
| CDR | 4 | 1 | 4 | 4.9 | ||
| 5 | FR | 1 | 8 | 0.13 | 2.9 | <.0001 |
| CDR | 5 | 3 | 1.7 | 3.9 | ||
| 6 | FR | 12 | 15 | 0.8 | 2.6 | <.01 |
| CDR | 2 | 6 | 0.33 | 4.5 | ||
| 7 | FR | 20 | 9 | 2.2 | 3.0 | .12 |
| CDR | 7 | 1 | 7 | 4.3 | ||
| Patient No. . | FR/CDR . | Observed . | Expected R/S4-150 . | P Value . | ||
|---|---|---|---|---|---|---|
| R . | S . | R/S . | ||||
| 1 | FR | 6 | 2 | 3 | 3.3 | .066 |
| CDR | 6 | 1 | 6 | 3.5 | ||
| 2 | FR | 10 | 8 | 1.3 | 2.8 | <.01 |
| CDR | 7 | 4 | 1.8 | 4.9 | ||
| 3 | FR | 18 | 19 | 0.95 | 2.9 | <.001 |
| CDR | 8 | 7 | 1.1 | 3.5 | ||
| 4 | FR | 2 | 8 | 0.25 | 2.8 | <.001 |
| CDR | 4 | 1 | 4 | 4.9 | ||
| 5 | FR | 1 | 8 | 0.13 | 2.9 | <.0001 |
| CDR | 5 | 3 | 1.7 | 3.9 | ||
| 6 | FR | 12 | 15 | 0.8 | 2.6 | <.01 |
| CDR | 2 | 6 | 0.33 | 4.5 | ||
| 7 | FR | 20 | 9 | 2.2 | 3.0 | .12 |
| CDR | 7 | 1 | 7 | 4.3 | ||
P values are the probability that the number of R mutations observed in the FRs was obtained by chance. Pvalues in boldface type indicate significance (P < .01).
See Chang and Casali.10
Intraclonal variation.
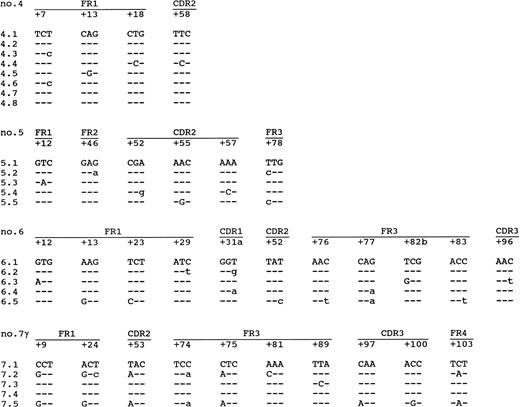
Information on the intraclonal variation was obtained by sequencing of each lymphoma at least four amplified VH molecules (Table 3 and Fig 3). No differences were found in the sequences of the individual clones of lymphomas no. 1 or 2, which are both IgG-expressing PCBCLs. Also, in lymphoma no. 3, which coexpresses IgM and IgD, no intraclonal variation was observed. However, lymphomas no. 4, 5, and 6 and the IgG-clone of no. 7 showed intraclonal variations of 0.6, 1.4, 2.6, and 3.4 mutations/clone, respectively. Moreover, in each of these lymphomas, mutations were found that were shared by more than one clone (Fig 3), which is strong evidence for ongoing somatic hypermutation rather than Taq error. Lymphomas no. 4, 5, and 6 coexpressed IgM and IgD (Tables 1 and 3), as determined by immunohistochemistry and PCR. The highest level of intraclonal variation was observed in the IgG clone of lymphoma no. 7, demonstrating that ongoing mutation is not necessarily confined to IgM+ PCBCLs. However, in contrast to the high mutation frequency of the IgG+ tumor cells, the intraclonal variation found in the IgA+ tumor cells amounted 0.2 mutations/clone, which does not exceed the Taq error frequency of 0.14% (∼0.4 mutation/clone). Yet, the consensus sequences of the IgG- and the IgA-expressing subclones were identical.
Intraclonal variation found in lymphomas no. 4, 5, and 6 and the IgG-clone of no. 7. Indicated are only the codons in which mutations were found. The first clone of each lymphoma is the consensus sequence. The mutations compared with this consensus sequence are shown. Replacement mutations are shown in capitals, and silent mutations are shown in small letters.
Intraclonal variation found in lymphomas no. 4, 5, and 6 and the IgG-clone of no. 7. Indicated are only the codons in which mutations were found. The first clone of each lymphoma is the consensus sequence. The mutations compared with this consensus sequence are shown. Replacement mutations are shown in capitals, and silent mutations are shown in small letters.
DISCUSSION
In this study, the VH genes of 7 PCBCLs were analyzed to learn what maturation steps were traversed by the tumor cells and to gain insight into the biological relation with other B-NHL. The PCR method used here has proven to be a fast and reliable approach to establish clonality at molecular level, despite the facts that only small amounts of frozen tissue were available and that the lymphomas harbored heavily mutated VH genes. To assay clonality with this method, 1 to 2 μg of RNA is sufficient, which is obtainable from 1 to 2 mg of lymphoma tissue. For comparison, for Southern blotting at least 30 μg genomic DNA is required, which equals 6 or 7 mg of tissue.
Within this limited set of PCBCLs, no bias in the use of VH genes was observed. Four of the lymphomas expressed VH3 genes (no. 2, 3, 4, and 5), whereas VH1, VH4, and VH5 genes were each found once (no. 7, 6, and 1, respectively). In normal peripheral B cells, VH3 genes are used in 56%, the VH4 and VH1 genes in 20% and 13%, respectively, whereas VH2, VH5, and VH6 are each found in less than 10%.16-18 In our study, 3 of the 4 large-cell PCBCLs use different members of the VH3 family. For nodal diffuse large B-cell lymphomas, one group reported that the VH4 gene was expressed in 15 of 17 cases studied.19 However, a more recent study observed no bias in VH gene usage within a set of 18 extracutaneous diffuse large B-cell lymphomas.20
The mutation frequency in the VH genes of the 7 PCBCLs studied here ranged from 15 to 52, with an average of 28.6 mutations (10%). This is significantly higher than the number of mutations found in normal B cells: for normal germinal center and memory B cells, Pascual et al4 reported average numbers of mutations of 5.7 (2%) and 9.5 (3%) in IgM- and IgG-derived VH sequences, respectively. Similar numbers of mutations were found by Tomlinson et al,21 ie, an average of 3.7 (1%) mutations per IgM-derived sequence and 10.2 (3%) mutations per IgG-derived sequence. We found high numbers of somatic mutations in both IgM-positive and IgG/IgA-positive PCBCLs, either of small- or large-cell types, without gross quantitative differences. Interestingly, all 4 IgM+ PCBCLs also coexpressed IgD (no. 3, 4, 5, and 6; Tables 1 and 3) and carried significantly mutated VH genes with low R/S ratios in the FRs. This contrasts with normal naive IgM+IgD+ B cells4 as well as with mantle cell lymphomas22and a subset of IgM+IgD+ B-cell chronic leukemias23 that are unmutated. It is possible that, in lymphomas no. 3, 4, 5, and 6, transformation from normal B cells to the IgM+IgD+ PCBCLs took place after their transition to a germinal center environment, but before the cells lost IgD expression. Interestingly, PCBCLs no. 5 and 6 were found to express CD70. These lymphomas may therefore bear a relationship with a recently described subset of germinal center B cells characterized by expression of IgD and CD70.24 It was hypothesized that these cells represent recent immigrants that are in the process of forming a germinal center. In accordance with this finding, a subset of IgM+IgD+ germinal center B cells has also been noticed by others, carrying, on average, 5.8 somatic mutations per VH gene.5 A minority of these CD38+IgM+IgD+ B cells harbored more than 10 mutations in the expressed VH genes. Because the patterns of somatic mutations in these cells were suggestive of antigen-driven selection processes, the investigators hypothesized that they might represent either germinal center founder cells derived from recirculating IgM+IgD+ memory cells or centrocytes differentiating into IgM+IgD+memory B cells.5
The presence of somatic mutations in the VH genes indicates that PCBCLs may be derived from germinal center cells or their descendants. Accordingly, in 5 of the 7 PCBCLs studied (no. 2, 3, 4, 5, and 6), the FRs displayed R/S ratios that are significantly lower than would be expected if random mutation would have occurred in the absence of selective forces. The apparant counterselection against R mutations in the FRs, which are essential for the integrity of the antibody, implies that expression of proper antigen receptors has been important for cell survival, at least at some stage(s) of development. However, the fact that the mutation patterns in these PCBCLs are reminiscent of antigen selection does not necessarily signify that lymphomagenesis itself is antigen-driven. In the diffuse large-cell lymphomas no. 1, 2, and 3, no intraclonal variation was observed. In these lymphomas, the somatic mutations were most likely introduced before or at the moment of complete transformation; therefore, it cannot be concluded that these lymphomas need Ig expression for their survival, let alone antigen recognition. The fact that the mutation frequency in these cases is abnormally high may be the result of a prolonged stay in the germinal center environment, possibly due to early, pretransforming genetic alterations. The findings within these PCBCLs are in accordance with a recent report by Gellrich et al,25 who analyzed, using single-cell PCR, the VH gene of a primary cutaneous immunoblastic B-cell lymphoma of the leg. It was demonstrated that the VH gene expressed by this lymphoma harbored 39 nucleotide differences compared with the most homologous VH gene (DP-54/V3-7). No evidence was obtained for ongoing somatic hypermutation. This subset of large-cell PCBCL, with a high load of somatic mutations without ongoing mutation, resembles noncutaneous diffuse large B-cell lymphomas.19 20However, our data do not provide an explanation for the difference in clinical behavior between cutaneous and extracutaneous diffuse large B-cell lymphomas.
Interestingly, in the PCBCLs no. 4, 5, 6, and 7, intraclonal variation was found, indicative of ongoing somatic hypermutation. The degree of intraclonal variation ranged between 0.6 and 3.4 mutations per clone. In lymphoma no. 7, clonal IgG and IgA gene products were found that contained the same VDJ rearrangement, indicating that they were isotype switch variants of the same tumor. It is remarkable that, whereas a high level of intraclonal variation was observed in the IgG clone (3.4 mutations/clone; Table 4), the IgA clone displayed no significant intraclonal variation. Still, the consensus sequences of the IgG-expressing and IgA-expressing clones proved to be exactly the same. The latter finding may indicate that the Ig heavy chain isotype switching occurred relatively late after the moment of transformation. The absence of somatic hypermutation in the IgA subset demonstrates that the ability to mutate may be abolished in the course of disease. This finding may have several explanations. It can be reasoned that the IgG clones were already functionally heterogeneous and that the isotype switch to IgA occurred in a nonmutating subclone. Alternatively, the shut-off of the mutation machinery may somehow have coincided with the process of isotype switching. It has been demonstrated that, in normal B cells, heavy chain class switch does not per se terminate somatic mutation.26 It is possible that additional genetic damage caused the termination of somatic hypermutation.
The VH mutation patterns of PCBCLs no. 4, 5, and 6 are also suggestive of clonal selection processes that favor BCR expression. In this subset of PCBCLs, the somatic mutations found may have been introduced both before and, contrary to their nonmutating counterparts, after the moment of complete transformation. It can be assumed that, among the mutations introduced in the lymphoma cells, there will be those that give rise to stopcodons or nonfunctional frameshifts. In fact, because the Ig genes are single-copy genes, they are particulary prone to inactivation by such mutations. Thus, the finding that actively mutating lymphomas express Ig is not trivial and implies that the tumorigenesis of these neoplasms may be BCR-guided. In this respect, the finding that two of four mutating PCBCLs (no. 5 and 6) contain elaborate networks of FDCs is noteworthy. Because normal skin does not harbor FDCs, this suggests that the FDCs may be an essential part of these neoplasms and possibly have a role in tumorigenesis. This idea is supported by the strong CD70 expression found on these particular PCBCLs: CD70 has been described to be a marker for mature B cells that have recently been primed by antigen in vivo.27 On the other hand, 2 PCBCLs that actively mutate their VH genes are devoid of FDCs (no. 4 and 7). Assuming that this is not due to not sampling error, this would signify that somatic hypermutation in PCBCLs does not necessarily depend on the presence of FDCs.
In line with the presumed analogy of the tertiary lymphoid tissue of skin (skin-associated lymphoid tissue [SALT]) and mucosa (MALT), some investigators propagate the idea that PCBCLs may be biologically related to malignant lymphomas of MALT.12,28 On morphological grounds, this has particulary been suggested for the immunocytomas of the skin. Although preliminary, our data provide support for this concept. Of the noncutaneous B-NHLs, ongoing somatic hypermutation is a characteristic feature of MALT lymphomas29-31 and follicular lymphomas.32-34MALT lymphomas arise at sites of chronic organ-specific inflammation caused by autoimmunity or specific infection, eg, Helicobacter pylori-associated gastritis35 (reviewed in Wright36). The suggestion of antigen-driven lymphomagenesis was strongly supported by the fact that small-cell gastric MALT lymphomas can be cured with antibiotics that eradicate Helicobacter pylori.37 In this respect, the evolvement of primary cutaneous immunocytoma in the context of local infection withBorrelia Burgdorferi38 is strikingly similar. Also, for these lymphomas, curative antibiotic treatment has been documented.39 In the 3 immunocytomas studied here, as well as in 1 of the diffuse large B-cell lymphomas, we obtained evidence of ongoing somatic hypermutation in extensively mutated VH genes. In addition, heavy chain isotype switching, as observed in immunocytoma no 7, has been observed in MALT lymphomas (unpublished results). The latter phenomenon is also a characteristic capacity of nodal follicular lymphomas.40 41 A potential relationship between PCBCLs and follicular lymphomas is supported by the presence of FDCs in the majority of the PCBCLs. Although our data suggest a relationship between PCBCLs and various forms of noncutaneous B-NHLs, at this moment no definite conclusions are allowed. Additional molecular data on the genetic defects have to be awaited to clarify this relationship.
ACKNOWLEDGMENT
The authors thank N.J. Ponne for technical assistance with sequencing and J.B.G. Mulder and P.J. van Beek for assistance with immunohistochemistry.
Supported by a grant from the Dutch Cancer Society (Grant No. AMC 95-957).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to C.J.M. van Noesel, MD, PhD, Department of Pathology, Academic Medical Center, Meibergdreef 9, 1105 AZ, Amsterdam, The Netherlands.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal