Abstract
Activated ABL oncogenes cause B-cell leukemias in mice and chronic myelogenous leukemia in humans. However, the mechanism of transformation is complex and not well understood. A method to rapidly and reversibly activate c-ABL was created by fusing the extra-cytoplasmic and transmembrane domain of the erythropoietin (EPO) receptor with c-ABL (EPO R/ABL). When this chimeric receptor was expressed in Ba/F3 cells, the addition of EPO resulted in a dose-dependent activation of c-ABL tyrosine kinase and was strongly antiapoptotic and weakly mitogenic. To evaluate the contributions of various ABL domains to biochemical signaling and biological effects, chimeric receptors were constructed in which the ABL SH3 domain was deleted (▵SH3), the SH2 domain was deleted (▵SH2), the C-terminal actin-binding domain was deleted (▵ABD), or kinase activity was eliminated by a point mutation, K290M (KD). The mutant receptors were stably expressed in Ba/F3 cells and analyzed for signaling defects, proliferation, viability, and EPO-induced leukemia in nude mice. When compared with the ability of the full-length EPO R/ABL receptor to induce proliferation and support viability in vitro, the ▵SH3 mutant was equivalent, the ▵SH2 mutant was moderately impaired, and the ▵ABD and KD mutants were profoundly impaired. None of these cell lines caused leukemia in mice in the absence of pharmacological doses of EPO. However, in mice treated with EPO (10 U/d), death from leukemia occurred rapidly with wild-type and ▵SH3. However, time to death was prolonged by at least twofold for ▵SH2 and greater than threefold for ▵ABD. This inducible model of ABL transformation provides a method to link specific signaling defects with specific biological defects and has shown an important role for the C-terminal actin-binding domain in proliferation and transformation in the context of this receptor/oncogene.
C-ABL IS A TYROSINE kinase proto-oncogene that is believed to be involved in growth control and DNA repair. Several oncogenes containing ABL have been discovered, including v-ABL, BCR/ABL, and TEL/ABL, each of which causes one or more types of leukemia. v-ABL, BCR/ABL, and TEL/ABL share the common feature that the ABL tyrosine kinase is activated and located at least in part in the cytoskeleton or at the cell membrane. In each case, activation of the ABL tyrosine kinase is believed to be a consequence of the new protein sequence outside the ABL domain. For example, the BCR/ABLoncogene is formed by a reciprocal translocation between chromosomes 9 and 22 that fuses the N-terminal portion of the BCR gene upstream of the c-ABL tyrosine kinase gene.1-7 There are multiple possible breakpoints in BCR, resulting in at least three different possible fusion proteins, p190BCR/ABL, p210BCR/ABL, and p230BCR/ABL. Each has increased tyrosine kinase activity and induces tyrosine phosphorylation of an overlapping set of cellular proteins.8-11 The activation of the ABL tyrosine kinase requires an N-terminal segment of BCR contained within amino acids 1-64 that is believed to induce the formation of oligomers.12
We have previously tested the hypothesis that oligomerization of ABL is sufficient to activate and deregulate ABL’s tyrosine kinase activity by constructing a synthetic oncogene in which oligomerization (dimerization) could be controlled by an exogenous ligand.13 Specifically, a chimeric receptor was constructed by fusing the extracellular ligand-binding domain of the erythropoietin receptor (EPO R)14 to c-ABL. When expressed in a nonleukemic, factor-dependent, murine hematopoietic cell line, Ba/F3, EPO induced a rapid, dose-dependent increase in tyrosine phosphorylation of the chimeric receptor itself and also induced phosphorylation of several other cellular proteins already known to be substrates for the BCR/ABL tyrosine kinase, such as Shc and CBL.15-17 EPO also caused a dose-dependent increase in viability and, at high doses, proliferation. In nude mice, Ba/F3 cells expressing EPO R/ABL caused a lethal leukemia if EPO was administered, but did not cause detectable disease in the absence of EPO administration.
In this study, we have examined the domains of ABL required for signaling and biological effects in the context of this chimeric receptor.
MATERIALS AND METHODS
EPO R/ABL chimeric receptor.
A cDNA encoding the chimeric EPO R/ABL receptor was previously described.13 A new series of chimeric receptor cDNAs was generated by replacing wild-type ABL with a set of ABL mutants previously described18 19 and ligating the new cDNAs into the expression vector pPL. The mutants will be described in detail below.
Cells and cell culture.
Ba/F3 cells20 were cultured at 37°C in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS) and 10% conditioned medium from WEHI-3B cells as a source of murine interleukin-3 (IL-3). The parental Ba/F3 cells used in these experiments do not express detectable endogenous EPO receptors and do not proliferate in response to EPO. As a control, Ba/F3 cells transfected with a wild-type murine EPO receptor were used,21 and these cells were shown to proliferate rapidly in response to exogenous EPO. Plasmids pPL EPO R/ABL and pGD, which contains a neomycin resistance gene, were cotransfected into Ba/F3 cells at a 20:1 molar ratio, respectively, using electroporation with a Bio-Rad Gene Pulsar (Bio-Rad, Richmond, CA), as described previously.22 Selection with G418 (1 mg/mL) in RPMI 1640 medium containing 10% WEHI conditioned medium was initiated 48 hours after electroporation. Multiple polyclonal cell lines were isolated from each transfection and further analyzed. Ba/F3 cells transformed by either p210BCR/ABL or p190BCR/ABL have been described previously.22
Flow cytometric analysis of EPO R expression and cell cycle.
Surface expression of EPO R was detected by fluorescence-activated cell sorting (FACS) using a polyclonal antiserum directed against the extracytoplasmic domain of human EPO R.23 Cell cycle analysis was performed by staining cells with propidium iodide. The cells were analyzed using a Coulter Epics V flow cytometer (Coulter Electronics, Miami, FL) and MultiCycle software (Phoenix Flow Systems, Phoenix, AZ).
Immunoblotting and immunoprecipitation.
Cells were deprived of growth factors by culturing in medium containing 10% FCS in RPMI 1640 overnight and then stimulated with either human recombinant EPO (Amgen Inc, Thousand Oaks, CA) or murine recombinant IL-3 (Upstate Biotechnology Inc, Lake Placid, NY) as indicated in each experiment. Aliquots containing equal numbers of cells were lysed in 1% Nonidet P40, 137 mmol/L NaCl, 1 mmol/L MgCl2, 10% Glycerol, 20 mmol/L Tris, pH 8.0, 1 mmol/L phenylmethylsulfonyl fluoride, 20 μgmL Aprotinin, 1 mmol/L Na orthovanadate, and 10 ng/mL leupeptin at 1 × 108 cells/mL. Immunoprecipitation was performed from lysates with 50 mL protein A Sepharose beads (Pharmacia, Uppsala, Sweden) after incubation with specific antibodies. Protein samples were separated under reducing conditions by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (6% to 12% polyacrylamide gradients) and electrophoretically transferred to PVDF membranes (Millipore, Bedford, MA). For immunoblotting, membranes were blocked in TBS (10 mmol/L Tris, pH 8.0, 150 mmol/L NaCl) containing 5% dry milk for 1 hour and then incubated with the appropriate primary antibodies in TBST (TBS with 0.05% Tween 20) overnight at 4°C. Primary antibodies were detected with a horseradish peroxidase-conjugated secondary antibody at a 1:5,000 dilution and later developed by a chemiluminescent reaction and exposed to radiographic film. Blots were stripped and reprobed with an antibody to CRKL to demonstrate equal loading.
Antibodies.
Antiphosphotyrosine monoclonal antibody (4G10) was provided by Dr Brian Druker (University of Oregon Health Sciences Center, Portland, OR).24 Anti-ABL, -SHP2, and -CBL antibodies were purchased from Santa-Cruz Biotechnology, Inc (Santa Cruz, CA). Anti-Shc antibody was purchased from Transduction Laboratory Inc (Lexington, KY), and anti-rasGAP antibody was purchased from Upstate Biotechnology Inc.
RNA extraction and Northern blotting.
Total cellular RNA was extracted from 2 × 107 cells using the guanidium thiocyanate method. Ten-microgram samples were subjected to 1% MOPS/formaldehyde agarose gel electrophoresis and blotted onto nitrocellulose membranes. The blot was hybridized with a 32P-labeled c-myc cDNA probe.25
Animal studies.
Seven- to 9-week-old NCr nu/nu mice (Taconic Laboratories, Germantown, NY) were maintained in bioclean conditions. All experiments included more than five animals in each group, and all experiments were repeated at least once. All experiments involving animals were reviewed and approved by the Dana-Farber Cancer Institute Animal Care and Use Committee. Ba/F3, Ba/F3 BCR/ABL, and Ba/F3 EPO R/ABL cells were injected intravenously in a small volume (<500 μL) in a tail vein. Mice then received human EPO by intraperitoneal injection 5 times/week.
RESULTS
EPO-dependent activation of tyrosine kinase activity in Ba/F3 cells.
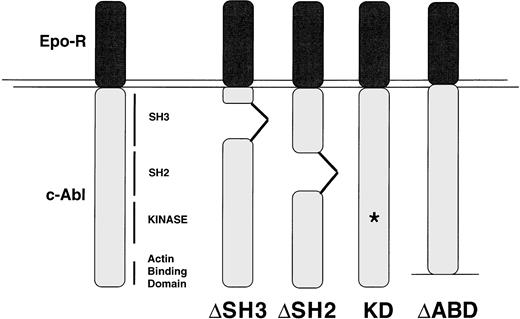
The IL-3–dependent Ba/F3 cell line was transfected with EPO R/ABL cDNAs as described in Materials and Methods. Multiple polyclonal, independently derived sublines from each transfection were generated. Four or more lines were then selected for further studies on the basis of having approximately equal surface expression of the appropriate chimeric receptor as measured by FACS. The predicted structures of the EPO R/ABL mutant chimeric receptors designed for the study are summarized in Fig 1. Equivalent expression was further confirmed in the selected lines by immunoblotting with an anti-ABL antibody (data not shown).
Structures of the mutant EPO R/ABL chimeric proteins. The EPO R/ABL constructs used in this study are summarized. ▵ABD, actin-binding domain (c-terminal 167 amino acids) deletion; ▵SH2, Src-homology domain 2 deletion; ▵SH3, Src-homology domain 3 deletion; KD, kinase dead has a point mutation at K290M. The location of the chimeric receptor proteins is indicated by an arrow.
Structures of the mutant EPO R/ABL chimeric proteins. The EPO R/ABL constructs used in this study are summarized. ▵ABD, actin-binding domain (c-terminal 167 amino acids) deletion; ▵SH2, Src-homology domain 2 deletion; ▵SH3, Src-homology domain 3 deletion; KD, kinase dead has a point mutation at K290M. The location of the chimeric receptor proteins is indicated by an arrow.
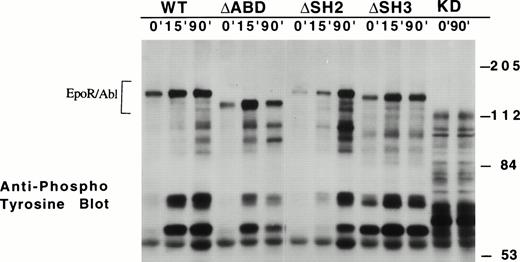
Using a dose of EPO of 2 U/mL, the activation of ABL tyrosine kinase activity over time was examined (Fig 2). After EPO treatment, each of the chimeric receptor proteins, with the exception of the kinase-inactive KD mutants, was phosphorylated on tyrosine within 5 minutes, and the phosphorylation persisted for more than 90 minutes. EPO also induced tyrosine phosphorylation of other cellular proteins (eg, pp140, pp120, pp68, and pp39 in Fig 2) in cell lines expressing wild-type, ΔABD, and ΔSH2 EPO R/ABL receptors. In some of the ΔSH3 sublines, there was detectable tyrosine phosphorylation of the chimeric receptor, even in the absence of EPO treatment, and these cell lines had a high tendency to evolve to factor-independence. EPO did not induce tyrosine phosphorylation of any cellular proteins in the KD sublines. These results indicate that the all chimeric receptors tested, except KD, are activated as tyrosine kinases after the addition of EPO.
Time course of ABL kinase activation in transfected Ba/F3 cell lines. The indicated cell lines were stimulated with EPO (2 U/mL) for 0, 15, and 90 minutes. Proteins phosphorylated on tyrosine were visualized by antiphosphotyrosine immunoblotting using 4G10. The location of the chimeric receptor proteins is indicated by a bracket.
Time course of ABL kinase activation in transfected Ba/F3 cell lines. The indicated cell lines were stimulated with EPO (2 U/mL) for 0, 15, and 90 minutes. Proteins phosphorylated on tyrosine were visualized by antiphosphotyrosine immunoblotting using 4G10. The location of the chimeric receptor proteins is indicated by a bracket.
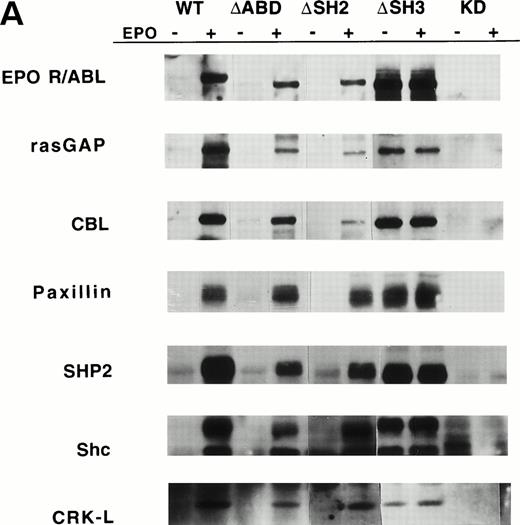
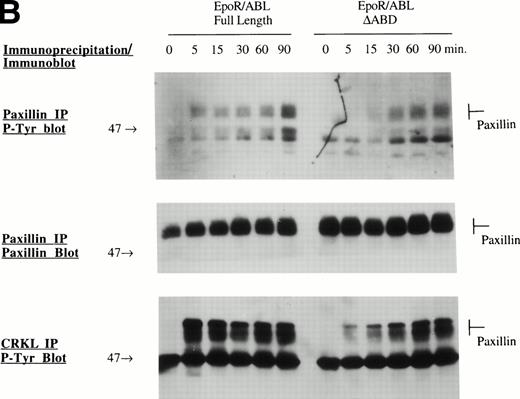
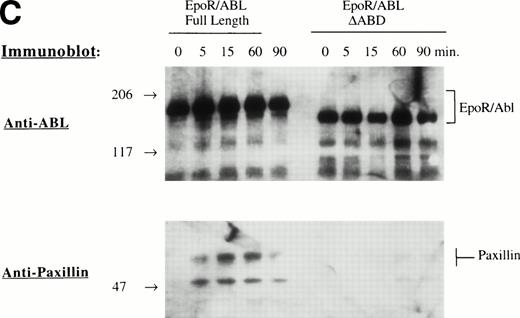
Several specific proteins are known to be tyrosine phosphorylated by either BCR/ABL or wild-type EPO R/ABL.13 The ability of mutant EPO R/ABL receptors to phosphorylate these proteins was therefore tested. Initially, cellular proteins were immunoprecipitated with antiphosphotyrosine antibody and blots were sequentially probed with antibodies to rasGAP, CBL, Paxillin, SHP2, Shc, and CRKL. In all cases, the results were confirmed by the reciprocal experiment, ie, immunoprecipitation with substrate-specific antibody followed by immunoblotting with antiphosphotyrosine antibody (Fig 3A). The full-length, ΔSH2, ΔSH3, and ΔABD chimeric receptors were able to phosphorylate rasGAP, Shc, and CRKL, although there were some qualitative differences, including decreased tyrosine phosphorylation of rasGAP and CBL by the ΔSH2 and ΔABD mutants (Fig 3A). Also, phosphorylation of several proteins was delayed in cells expressing the mutant ΔABD receptor (peak phosphorylation occurred at 30 to 60 minutes, rather than at 5 to 15 minutes in other cell lines). For example, the phosphorylation of paxillin, a cytoskeletal protein, was delayed by greater than 15 minutes in the ΔABD cell lines (Fig 3B), and a similar delay was observed for CBL (data not shown). The binding of CRKL to tyrosine phosphorylated paxillin was also delayed to a small degree (Fig 3B). Also, we found that the EPO R/ABL chimeric receptor transiently coprecipitated with paxillin after ligand activation, and this coprecipitation was reduced in cells expressing EPO R/ABL ΔABD chimeric receptors (Fig 3C).
Tyrosine phosphorylation of signaling proteins in Ba/F3 cells induced by EPO. (A) The indicated cell lines were IL-3 deprived for 6 hours and stimulated with medium alone or EPO (2 U/mL) for 30 minutes. Cell lysates were immunoprecipitated with antibodies as indicated, followed by antiphosphotyrosine immunoblotting with 4G10. (B) Ba/F3 cells expressing EPO R/ABL full length or EPO R/ABL ▵ABD chimeric receptors were IL-3 deprived and stimulated with EPO (2 U/mL) for 0 to 90 minutes. Lysates were immunoprecipitated with antipaxillin (upper two panels) or anti-CRKL (lower panel), followed by immunoblotting as shown. (C) In a similar experiment, lysates were subjected to immunoprecipitation with anti-EPO receptor antibody, followed by sequential immunoblotting with anti-ABL and anti-paxillin.
Tyrosine phosphorylation of signaling proteins in Ba/F3 cells induced by EPO. (A) The indicated cell lines were IL-3 deprived for 6 hours and stimulated with medium alone or EPO (2 U/mL) for 30 minutes. Cell lysates were immunoprecipitated with antibodies as indicated, followed by antiphosphotyrosine immunoblotting with 4G10. (B) Ba/F3 cells expressing EPO R/ABL full length or EPO R/ABL ▵ABD chimeric receptors were IL-3 deprived and stimulated with EPO (2 U/mL) for 0 to 90 minutes. Lysates were immunoprecipitated with antipaxillin (upper two panels) or anti-CRKL (lower panel), followed by immunoblotting as shown. (C) In a similar experiment, lysates were subjected to immunoprecipitation with anti-EPO receptor antibody, followed by sequential immunoblotting with anti-ABL and anti-paxillin.
These results indicate that each of the EPO R/ABL receptors studied here, except the KD mutant, activate tyrosine kinase activity in response to EPO, and that the SH2 domain is important for selecting some substrates, such as CBL, but not for many others. Furthermore, phosphorylation of some substrates, such as CBL and paxillin, is delayed in the absence of the actin-binding domain.
The C-terminal actin-binding domain of c-ABL is required for proliferation and viability signaling of the EPO R/ABL tyrosine kinase.
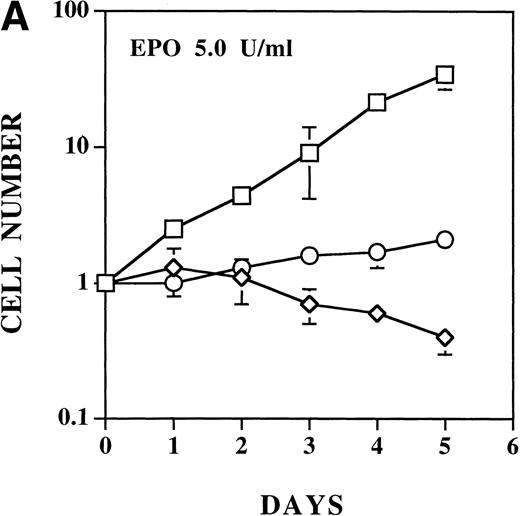
The ability of each EPO R/ABL receptor was tested for ability to support EPO-dependent proliferation and viability of Ba/F3 cells. EPO had no biological effect on Ba/F3 cells expressing the KD mutant, as expected (data not shown). In previous studies, we have shown that a concentration of EPO of 2 U/mL is sufficient to induce maximum proliferation of Ba/F3 cells expressing either wild-type EPO R/ABL or full-length EPO receptor.13 21 As noted above, the ΔSH3 mutant expressing cells tended to become factor independent in culture. When tested before this conversion, EPO induced equivalent or accelerated proliferation of ΔSH3 cells compared with cells expressing wild-type EPO R/ABL receptors. Compared with either wild-type or ΔSH3 receptor bearing cells, EPO-induced proliferation of either ΔSH2 or ΔABD receptor bearing cells was significantly impaired (Fig 4A).
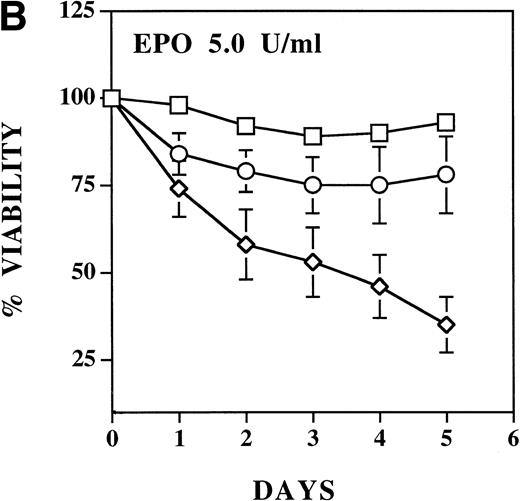
EPO induces a dose-dependent increase in viability and proliferation of Ba/F3 cells with wild-type and ▵SH2 but not with ▵ABD EPO R/ABL. Cells were cultured with 5 U/mL EPO, and the total number (A) and percentage (B) of viable cells was enumerated using 0.04% Trypan blue staining. (□) Wild-type; (◊) ▵ABD; (○) ▵SH2.
EPO induces a dose-dependent increase in viability and proliferation of Ba/F3 cells with wild-type and ▵SH2 but not with ▵ABD EPO R/ABL. Cells were cultured with 5 U/mL EPO, and the total number (A) and percentage (B) of viable cells was enumerated using 0.04% Trypan blue staining. (□) Wild-type; (◊) ▵ABD; (○) ▵SH2.
The ability of the mutant EPO R/ABL receptors to maintain viability in the absence of IL-3 was also examined (Fig 4B). Compared with the ability of the wild-type EPO R/ABL receptor to support viability, the ΔSH2 mutant was moderately impaired and the ΔABD mutant was profoundly impaired, even if the concentration of EPO was increased to 5 U/mL.
Delayed G1 progression in cells expressing EPO R/ABL receptors lacking the C-terminal actin-binding domain.
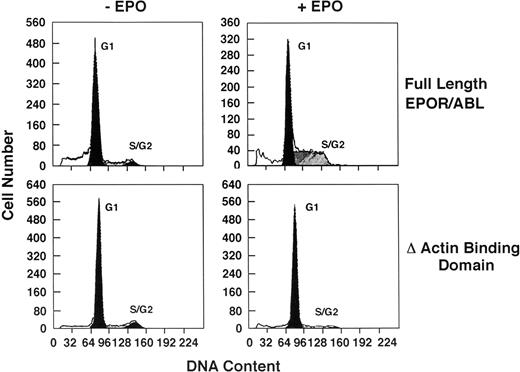
Sublines were IL-3 deprived for 16 hours, resulting in growth arrest in Go/G1. Cells were then cultured with EPO (5 U/mL) for 24 hours, and cell cycle status was analyzed by flow cytometry (Fig 5). Ba/F3 cells expressing either wild-type or ΔSH2 receptors readily entered S phase in response to EPO stimulation, whereas cells expressing ΔABD receptors remained in G0/G1. KD cells could not be studied because the cells die in less than 24 hours. Early response gene induction (c-myc) was examined by Northern blot. Myc was induced in cells expressing wild-type receptors, but not in ΔABD cells (Fig 6).
Cell cycle analysis of Ba/F3 cells expressing ▵ABD EPO R/ABL. Cells were first synchronized in G1 by IL-3 deprivation for 16 hours (−EPO) and then cultured with 5 U/mL EPO for another 24 hours (+EPO). Cell cycle analysis was performed with propidium iodide staining, analyzing 10,000 cells/point by flow cytometry. In the experiment shown, the proportion of cells in G1, S, and G2/M were, respectively, 83%, 12%, and 5% (full length −EPO); 55%, 45%, and 1% (full length +EPO); 90%, 7%, and 4% (▵ABD −EPO); and 89%, 8%, and 3% (▵ABD +EPO).
Cell cycle analysis of Ba/F3 cells expressing ▵ABD EPO R/ABL. Cells were first synchronized in G1 by IL-3 deprivation for 16 hours (−EPO) and then cultured with 5 U/mL EPO for another 24 hours (+EPO). Cell cycle analysis was performed with propidium iodide staining, analyzing 10,000 cells/point by flow cytometry. In the experiment shown, the proportion of cells in G1, S, and G2/M were, respectively, 83%, 12%, and 5% (full length −EPO); 55%, 45%, and 1% (full length +EPO); 90%, 7%, and 4% (▵ABD −EPO); and 89%, 8%, and 3% (▵ABD +EPO).
EPO fails to induce c-myc expression in ▵ABD cells. Full-length or ▵ABD EPO R/ABL transfected Ba/F3 cells were factor deprived overnight and then treated with EPO for 0 to 60 minutes. Fifteen micrograms of total cellular RNA was electrophoresed in each lane and analyzed by Northern blot hybridization with a c-myc cDNA as probe. Equal loading was confirmed by visualizing 18S and 28S RNA bands on the ethidium bromide-stained gel from which the blots were made.
EPO fails to induce c-myc expression in ▵ABD cells. Full-length or ▵ABD EPO R/ABL transfected Ba/F3 cells were factor deprived overnight and then treated with EPO for 0 to 60 minutes. Fifteen micrograms of total cellular RNA was electrophoresed in each lane and analyzed by Northern blot hybridization with a c-myc cDNA as probe. Equal loading was confirmed by visualizing 18S and 28S RNA bands on the ethidium bromide-stained gel from which the blots were made.
Generation of EPO-dependent leukemia in nude mice.
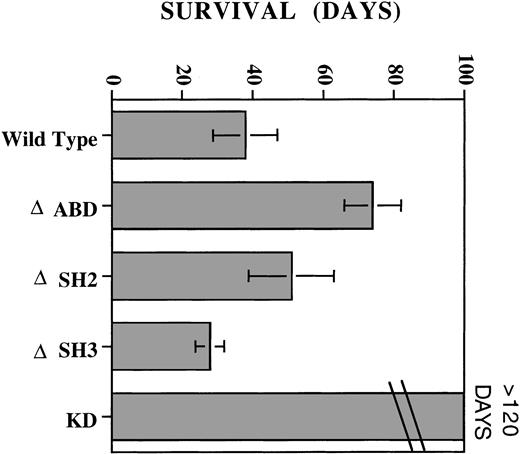
We compared the leukemic potential of cells expressing wild-type, ΔSH2, ΔSH3, ΔABD, and KD EPO R/ABL receptors after intravenous injection into nude mice. Groups of 5 to 6 mice were treated with EPO (10 U intraperitoneally daily for 5 days per week). In the absence of EPO administration, mice remained healthy for more than 90 days (data not shown). Nude mice receiving KD EPO R/ABL cells and EPO remained healthy and did not develop any signs of leukemia or tumor growth for greater than 120 days. The group receiving wild-type EPO R/ABL cells and EPO died between days 29 and 49. Those receiving ΔSH3 receptor cells and EPO died between days 23 and 33. Interestingly, cells expressing ΔSH2 and ΔABD receptors remained leukemic in mice with EPO, but time to death was prolonged compared with wild-type receptors (Fig 7).
Induction of EPO-dependent leukemia in nude mice. On day 0, mice received either 5 × 106 wild-type, ▵ABD, ▵SH2, ▵SH3, or KD EPO R/ABL cells via tail-vein injection, followed by daily injections of EPO intraperitoneally. Three to six mice were included in each group, and the experiment was performed two or more times. The figure shows the mean ± SD of days of survival after injection. EPO alone or EPO with parental Ba/F3 cells was not associated with mortality of the mice.
Induction of EPO-dependent leukemia in nude mice. On day 0, mice received either 5 × 106 wild-type, ▵ABD, ▵SH2, ▵SH3, or KD EPO R/ABL cells via tail-vein injection, followed by daily injections of EPO intraperitoneally. Three to six mice were included in each group, and the experiment was performed two or more times. The figure shows the mean ± SD of days of survival after injection. EPO alone or EPO with parental Ba/F3 cells was not associated with mortality of the mice.
DISCUSSION
Activation of the ABL proto-oncogene occurring as a result of chromosome translocations involving either the BCR or TEL genes is believed to cause both acute and chronic human leukemias. In both cases, the tyrosine kinase activity of ABL is increased, possibly as a result of oligomerization or clustering of ABL in the cytoplasm or cytoskeleton. The increased tyrosine kinase activity of BCR/ABL is essential for transformation10,26 and requires an N-terminal coiled-coil motif in BCR that may mediate self-association.12 There are three forms of BCR/ABL that are formed by different breakpoints within the BCR gene, each possibly associated with a different type of leukemia,27 suggesting that other domains in BCR may modulate the transforming activity in an unknown way. Each form contains the oligomerization motif and also contains tyrosine 177, a phosphorylation site that is important for transformation in vitro, possibly by forming a binding site for the SH2 domain of the adapter protein GRB2.28 The TEL-ABL fusion has been identified in several patients with acute leukemias.29 TEL contains a helix-loop-helix domain that may also mediate oligomerization, like the coiled-coil motif in BCR.30
We have previously tested the hypothesis that oligomerization was sufficient to activate c-ABL tyrosine kinase activity by constructing a chimeric receptor containing the ligand-binding domain of the EPO receptor and c-ABL.13 The resulting molecule, which functioned as a transmembrane receptor, proved to have EPO-dependent tyrosine kinase activity, phosphorylated several cytoplasmic proteins, and induced dose-dependent increases in viability, adhesion, and proliferation in the Ba/F3 cell line, thus mimicking the biological effects of BCR/ABL in this cell line. At low doses of EPO, the predominant effect was enhanced viability, whereas at higher doses (resulting in higher kinase activity), the receptor caused significant proliferation. This again was similar to the known biological effects of BCR/ABL in primary patient cells, where enhanced viability is more evident than autonomous proliferation.
A number of other techniques have been used to generate models in which the functions of ABL or BCR/ABL were inducible. For example, temperature-sensitive mutants of both v-abl and BCR/ABL have been studied,31,32 but have tended to be leaky and do not allow for in vivo use. Regulatable promoters have also been investigated, including the use of the estrogen receptor and metallothionine promoter.33 Also, recent preliminary studies with the FK1012 system and tetracycline inducible promoters have also been reported.34,35 The chimeric receptor reported here is of particular interest because of its in vivo applications, but does have the potential problem that ABL is tethered to the cell membrane. This is in contrast to BCR/ABL, which is located in the cytoplasm and cytoskeleton, but probably similar to v-abl, which is anchored to the membrane through myristylation.36
In this study, we have used the EPO R/ABL receptor to investigate the functions of several domains of ABL that have been previously implicated in transformation either in the context of BCR/ABL, TEL/ABL, or v-abl. Specifically, chimeric receptors were constructed that contained the external domain of the EPO receptor fused to c-ABL constructs in which the kinase was inactivated, or the SH2, SH3, or c-terminal 167 amino acids (actin-binding domain) were deleted. These receptors were then expressed in Ba/F3 cells and analyzed for their ability to activate signal transduction molecules and to support proliferation and viability. The cell line used here, Ba/F3, has been previously used extensively for evaluation of EPO signaling21 and is thus also of value in terms of comparing the signaling pathways activated by the chimeric EPO R/ABL with the wild-type EPO R.
Each of the mutants could induce EPO-dependent tyrosine phosphorylation of the receptor itself and cellular proteins, with the exception of the kinase-inactive mutant. Cells containing EPO R/ABL ΔSH3 displayed an increase in tyrosine phosphorylation of cellular proteins even in the absence of EPO. We looked for tyrosine phosphorylation of a number of proteins known to be substrates of BCR/ABL and/or v-abl, including rasGAP, paxillin, SHP2, Shc, CBL, and CRKL.15,16,24 37-39 When compared with EPO R/ABL, only a few differences were noted. The ΔSH2 receptor induced reduced tyrosine phosphorylation of CBL and rasGAP, suggesting that the SH2 domain of ABL could be important to select these substrates, and the ΔABD receptor induced reduced and delayed phosphorylation of the cytoskeletal protein paxillin and also of CBL. In contrast, deletion of the SH2, SH3, or actin-binding domains had no detectable effect on tyrosine phosphorylation of SHP2, Shc, or CRKL.
The role of the SH2 domain of BCR/ABL has been previously studied by a number of investigators, and the results have varied depending on the transformation target. In general, the SH2 domain has been found to be required for efficient transformation of fibroblasts, but not absolutely required for transformation of factor-dependent cell lines or primary hematopoietic cells.19,40,41 A number of proteins have been identified that can bind to the ABL SH2 domain, including CBL, Rin1, Shd, p62 (possibly p62 dok), and She.17 42-44 However, none of these has yet been proven to be required for BCR/ABL transformation. The results presented here show that the ΔSH2 receptor was impaired in its ability to promote EPO-dependent proliferation and viability in vitro, but caused an EPO-dependent, Ba/F3 cell leukemia in nude mice that was nearly as lethal as the wild-type chimeric receptor. The ΔSH2 receptor is defective in phosphorylating Cbl, but the other potential SH2-binding proteins have not yet been studied.
The role of the ABL SH3 domain in transformation is interesting and complex. Deletion of the SH3 domain from c-ABL causes oncogenic activation and is associated with an increase in ABL tyrosine kinase activity.45 It has been suggested that a cellular protein exists that downregulates ABL kinase activity when bound to its SH3 domain, thereby explaining the increased tyrosine kinase activity of SH3 deletion mutants. A number of potential SH3-binding proteins have been identified, including Abi1, Rin1, and Pag1.42,46 47 It is of interest that there is excellent in vitro evidence that Pag1 downregulates Abl tyrosine kinase activity. This 23-kD protein was originally identified by virtue of being induced by oxidative stress and may function as a thiol-specific antioxidant.
However, recent studies suggest that deletion of the SH3 domain from BCR/ABL rather than c-ABL may actually delay in vivo leukemogenesis, without significantly impairing growth factor-independence and other in vitro measures of transformation.48 Deletion of the SH3 domain did not affect any of the known cellular signaling pathways activated by BCR/ABL, including activation of MAPK, JNK, MYC, JUN, or STATs.48 However, there was a partial redistribution of BCR/ABL from the cytoskeleton/membrane compartment to the cytosol, and cellular localization may be important for BCR/ABL transformation, as will be discussed below.
In the studies presented here, deletion of the SH3 domain did not reduce transforming activities in vitro or in vivo of the chimeric receptor; in fact, the EPO R/ABL ΔSH3 was associated with in increase in spontaneous tyrosine phosphorylation of the chimeric receptor and cellular proteins (ie, phosphorylation in the absence of added EPO) and was associated with an increased tendency to convert to factor independence. We have not attempted to recover cell lines from mice receiving Ba/F3 cells expressing the ΔSH3 receptors to determine if they have converted to factor independence in vivo. However, we have tested Ba/F3 cells expressing the wild-type EPO R/ABL receptor for in vivo conversion to factor independence, and no such conversion has been observed in limited studies to date. Thus, overall, the EPO R/ABL ΔSH3 receptor described here behaves more like a regulatable version of v-abl, which has a deletion of SH3, rather than the SH3-deleted BCR/ABL described by Skorski et al.48 The differences could be explained by cellular localization. Both EPO R/ABL and v-abl are constitutively associated with the cell membrane, whereas the BCR/ABL ΔSH3 was found to lose part of its association with the cytoskeleton.48 If this line of reasoning is correct, tethering BCR/ABL ΔSH3 to the cell membrane would be predicted to reconstitute in vivo transforming functions.
The unexpectedly profound effects of deleting the actin-binding domain of EPO R/ABL are of interest and highlight the significance of subcellular localization for ABL transformation. The c-terminus of c-Abl has distinct binding sites for both G- and F-actin.8 The potential importance of the actin-binding domains in Abl biology has recently been highlighted by the finding that c-Abl is apparently involved in integrin and adhesion-mediated signaling.49 Interestingly, in untransformed cells, c-Abl is detected both in the nucleus and in the cytoplasm/cytoskeleton.50 However, BCR/ABL and TEL/ABL are both primarily, if not exclusively, cytoplasmic, with the majority found in the cytoskeleton distributed along actin filaments and in focal adhesion-like structures.51,52 There is now abundant evidence that BCR/ABL affects both the structure of the cytoskeleton, several cytoskeletal functions, and also adhesion of chronic myeloid leukemia cells to marrow stroma and to extracellular matrix proteins.51,53,54 However, previous studies have not consistently found a role for the actin-binding function of BCR/ABL in transformation, and the C-terminus of v-abl has not been shown to be required for transformation.55 In the current studies, a major requirement for the c-terminus of ABL was shown when studied in the context of the chimeric EPO R. The c-terminus was found to be necessary for both viability and proliferation in vitro, and in nude mice, absence of this domain significantly prolonged life span. The reasons are not yet clear, but the EPO R/ABL ΔABD receptor was highly active as a tyrosine kinase and phosphorylated most of the known kinase substrates of BCR/ABL. However, phosphorylation of at least one prominent substrate located in the cytoskeleton, paxillin, was both reduced and delayed compared with wild-type, and this suggests that this receptor may have lost access to a critical, but unknown, substrate in the cytoskeleton. There is a paradox in that ΔABD mutant cells have reduced viability in vitro, yet still cause leukemia in vivo, although delayed in onset. It is possible that in vivo there are sufficient growth factors (such as IL-3) in the marrow and spleen microenvironments to keep these cells alive, whereas the signal from the ΔABD mutant receptor alone is insufficient in vitro. There could also be a subset of cells that live long enough in vivo to gain new mutations that overcome the defect of the ΔABD mutant receptor. Additional studies to compare signaling of the wild-type and ΔABD receptors may be informative with regard to identifying important defects.
In conclusion, this chimeric EpoR/ABL receptor provides a new tool to examine the functions of specific signaling domains of ABL. Because ABL is tethered to the cell membrane, deletions or mutations that normally affect BCR/ABL function by changing cellular localization may have different effects in this chimeric molecule. Finally, unique effects, such as requirement for the c-terminal actin-binding domain, may be more prominent in this receptor than in other forms of activated ABL oncogenes.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to James D. Griffin, MD, Dana-Farber Cancer Institute, 44 Binney St, Boston, MA 02115; e-mail:james_griffin@dfci.harvard.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal