Abstract
Interleukin-15 (IL-15) is produced by human bone marrow (BM) stromal cells and can induce CD34+ hematopoietic progenitor cells (HPCs) to differentiate into CD56+CD3−natural killer (NK) cells in the absence of stromal cells. IL-15 mediates its effects by signaling through the β and γcchains of the IL-2/15 receptor (R). The c-kit ligand (KL), also produced by stromal cells, enhances the expansion of NK cells from CD34+ HPCs in the presence of IL-15, but alone has no ability to differentiate NK cells. Mice deficient in KL do not appear to have a quantitative deficiency in NK cells, suggesting that other stromal cell factors may contribute to NK cell expansion. Flt3 ligand (FL) is also produced by BM stromal cells and has homology with KL. Furthermore, mice with a targeted disruption of the FL gene have reduced numbers of NK cells. We evaluated here the effects of FL on human NK cell development and expansion from CD34+ HPCs. Like KL, FL significantly enhanced the expansion of NK cells from CD34+ HPCs in the presence of IL-15, compared with IL-15 alone. However, FL alone had no effect on NK cell differentiation. We therefore explored the mechanism by which FL promotes IL-15–mediated NK cell development. FL was found to induce IL-2/15Rβ (CD122) expression on CD34bright HPCs. The CD34brightCD122+ cell coexpressed CD38, but lacked expression of CD7, CD56, NK cell receptors (NKRs), or cytotoxic activity in the absence of IL-15. Using limiting dilution analysis in the presence of IL-15 alone, we demonstrated that the FL-induced CD34brightCD122+ HPCs had an NK cell precursor frequency 20- to 60-fold higher than the CD34dim/negCD122− HPCs and 65- to 235-fold higher than fresh CD34+ HPCs. KL had similar effects as FL, but induced a significantly lower percentage of CD34brightCD122+ cells (P ≤ .01). Both FL and KL also increased IL-15R transcript in CD34+ HPCs. Culture of CD34+ HPCs in FL or KL, followed by culture in IL-15 alone, induced expression of both C-type lectin and Ig-superfamily NKRs on CD56+ cells. These data collectively support a role for FL in early human NK cell development. FL or KL generate a unique CD34brightCD122+CD38+ human NK cell intermediate from CD34+ HPCs that lacks NK features yet is IL-15–responsive. IL-15 is then required for the induction of CD56 and NKRs, LGL morphology, cytotoxic activity, and the ability to produce abundant cytokines and chemokines.
NATURAL KILLER (NK) cells are large granular lymphocytes (LGLs) that play an important role in the innate or antigen nonspecific immune response to infection.1-3Although it is known that NK precursor cells reside in the bone marrow (BM), their phenotype(s) and the factors that regulate their differentiation into mature NK cells are incompletely understood.4,5 Interleukin-2 (IL-2) has been used extensively to study NK cell development from CD34+hematopoietic progenitor cells (HPCs) in vitro6-9; however, IL-2 is produced exclusively by antigen-activated T cells and is not found within the BM stroma.10,11 Furthermore, NK cells develop normally in mice lacking T cells12 and in mice that bear a disrupted IL-2 gene,13,14 yet are absent in mice15,16 and humans17 which lack either the β or γc signaling components of the IL-2 receptor (IL-2R). Collectively, these data suggest that other factors that bind to the IL-2R are critical for NK cell development.11
IL-15 is produced by human BM stromal cells, binds to and signals through the IL-2Rβγ, and can induce the differentiation of NK cells from CD34+ HPCs in vitro.11,18 Mice that cannot express IL-15 in their BM stroma lack NK cells.19,20Another stromal cell factor, c-kit ligand (KL), can significantly enhance the expansion of NK cells from CD34+ HPCs in combination with IL-15, but alone has no effect on NK cell differentiation.11 However, mice that lack KL have not been reported as having NK cell deficiencies,21 suggesting that other stromal factors may contribute to the expansion of NK cells. c-Kit, the KL receptor, is a member of the class III receptor tyrosine kinase (RTK) family that also includes flt3.22-26 Flt3 is expressed almost exclusively on early hematopoietic CD34+stem cells,26-31 whereas c-kit appears to be expressed on primitive and mature hematopoietic cells as well as on blood NK cells.24,32,33 Flt3 ligand (FL) has strong homology with KL and is also produced by stromal cells.34-37 Several studies have shown that FL maintains and stimulates the proliferation of primitive murine and human HPCs and synergizes with a number of other growth factors, including IL-3, IL-6, IL-7, IL-11, IL-12, and granulocyte colony-stimulating factor (G-CSF).38-42Interestingly, mice with a genetically disrupted FL gene exhibit a deficiency in early lymphopoiesis, with low to absent mature NK cells, implicating FL as an important factor for murine NK cell development.43 Recently, Williams et al44showed that c-kit+Sca2+IL-2/15Rβ−Lin−murine BM cells incubated in a combination of IL-6, IL-7, KL, and FL subsequently developed into functional NK1.1+ NK cells when cultured in IL-15. In the present study, we have investigated the role of FL in the regulation of human NK cell development from BM-derived CD34+ HPCs in vitro and compared the effect of FL to that of KL. We identify a novel population of human CD34+ HPCs that express IL-2/15Rβ after culture in FL or KL. This cell type is IL-15–responsive and thus appears to represent a distinct intermediate in human NK cell development.
MATERIALS AND METHODS
Growth factors.
Purified recombinant human (rh) FL, rhKL (specific activity, >105 U/mg), and rhIL-15 (specific activity, 1.49 × 109 U/mg) were kindly provided by Immunex Research and Development Corp (Seattle, WA). rhIL-2 (specific activity, 1.53 × 107 U/mg) was obtained from Hoffman LaRoche (Nutley, NJ). rhIL-7, rhIL-9, transforming growth factor-β1 (TGF-β1), and tumor necrosis factor-α (TNF-α) were purchased from Peprotech Inc (Rocky Hill, NJ). rhIL-12 (specific activity, 4.5 × 106 U/mg) was a gift of Dr Stanley Wolf (Genetics Institute, Cambridge, MA).
Monoclonal antibodies (MoAbs).
The following MoAbs were purchased from Becton Dickinson (San Jose, CA): anti-CD34-phycoerythrin (PE), anti-CD34-fluorescein isothiocyanate (FITC), anti-CD2-FITC (Leu-5b), anti-CD3-FITC (Leu-4), nonreactive mouse Ig (MsIg)-FITC (control-FITC), and MsIg-PE (control-PE). The anti-CD16-FITC MoAb was obtained from CalTag Laboratories (San Francisco, CA), and anti-CD56- (NKH-1) PE was obtained from Coulter Immunotech (Hialeah, FL). The mouse-antihuman NK cell receptor (NKR) MoAbs used for this study were EB6 (anti-CD158a), GL 183 (anti-CD158b), Zin 276 (anti-p70/CDw159), FSTR 172 (anti-p50.3), and Zin 270 (anti-NKG2A), kindly provided by Drs Alessandro and Lorenzo Moretta (Università di Genova, Genova, Italy).45-47
Purification of human BM HPCs.
BM was aspirated from the posterior iliac crest of healthy adult donors after obtaining informed consent. BM mononuclear cells (MNC) were separated by density centrifugation over a Ficoll-Hypaque (Sigma, St Louis, MO) gradient at 400g for 30 minutes. CD34+cells were enriched from MNC by affinity chromatography using the Ceprate LC device (CellPro, Bothel, WA), following the manufacturer’s instructions. The enriched cells were then stained with anti-CD34-PE (CD34+Lin−) population on a FACStar Plus cell sorter (Becton Dickinson). Cells obtained in this manner were routinely ≥98% pure.11
Long-term suspension culture of CD34+ HPCs.
Sorted CD34+Lin− HPCs were cultured in 96-well microplates at a concentration of 2 × 104 in 200 μL of complete RPMI-1640 medium (RPMI-1640 [GIBCO, Grand Island, NY] supplemented with 10% heat-inactivated human AB serum [HAB; C-six; Diagnostics, Mequon, WI] and antibiotics [Sigma]) and the indicated growth factors: rhFL, IL-15, and KL at 100 ng/mL; rhIL-2, IL-7, and IL-9 at 10 ng/mL; rhIL-12 at 10 U/mL; and TNF-α and TGF-β1 at 20 ng/mL. At day 7, day 14, and every 3 days thereafter, half of the culture medium was replaced with fresh medium containing 10% HAB and the same concentration of growth factors. After 21 days, the cells were enumerated and analyzed for morphology, cell surface phenotype, cytotoxic activity, and cytokine production. Cell enumeration was performed in triplicate by vital dye exclusion using a hemacytometer. In some experiments, after culture for 21 days, cells were cultured for an additional 14 days in the presence or absence of additional cytokines as indicated.
Immunophenotype analyses.
Cultured cells were harvested, incubated on ice with an excess of nonreactive mouse IgG for 10 minutes, and then stained on ice for 15 to 30 minutes with various combinations of the following directly conjugated MoAb: anti-CD56-PE and FITC-conjugated MoAb reactive against CD2, CD3, and CD16. For analysis of NKR expression, cultured cells were stained with unconjugated mouse-antihuman NKR MoAb, washed, stained with a secondary FITC-conjugated goat antimouse IgG (Sigma), washed twice, and then stained with directly conjugated anti-CD56-PE. Background fluorescence was determined by analysis of cells identically stained with nonreactive isotype control MoAbs. Cells were analyzed using an MNC gate on a FACScan (Becton Dickinson) with the Lysis II software program.
Cytotoxicity and proliferation assays.
Cytotoxic activity of cultured cells was determined in a standard51Cr-release cytotoxicity assay against the NK-sensitive K562 cell line, as previously described,11 at an E:T ratio of 8:1. Results of cytotoxicity assays represent the mean ± SEM of triplicate wells. Cell proliferation was measured by [3H]-thymidine incorporation during the final 24 hours of a 96-hour incubation at 37°C.11 Results of proliferation assays represent the mean ± SEM of triplicate wells and are expressed as cpm of [3H]-thymidine incorporation.
Measurement of NK cell cytokine production.
Three-week cultures of CD34+ HPCs in FL plus IL-15 or IL-15 alone were harvested and separated by fluorescence-activated cell sorting (FACS) into CD56+CD3− and CD56−CD3− subsets. Sorted (≥98% purity) CD56+CD3− and CD56−CD3− populations were plated at 50,000 cells/well in complete RPMI-1640 medium. After resting overnight in the absence of any cytokines, cells were costimulated with 100 ng/mL rhIL-15 and 10 U/mL of rhIL-12. After 48 hours of incubation, cell-free culture supernatants were collected and frozen at −70°C. Commercial enzyme-linked immunosorbent assay (ELISA) kits were used for the determination of interferon-γ (IFN-γ; Endogen, Woburn, MA), TNF-α, TGF-β (Genzyme, Cambridge, MA), and macrophage inflammatory protein-1α (R & D Systems, Minneapolis, MN) levels, following the manufacturer’s instructions. All supernatants were assayed simultaneously.
Limiting dilution analysis (LDA).
As shown below, there is a striking positive correlation between the expression of CD56 in these cultured cells and the expression of NKRs, LGL morphology, cytotoxic activity, and the potential for cytokine production, all of which define NK cells. Furthermore, CD56− cells in culture lack these properties. Therefore, we were able to use CD56 expression as an accurate readout for NK cell development in our LDA of NK cell precursor frequency. Human CD34+ HPCs were isolated as described above, were plated by limiting dilution, and were either assayed for NK cell precursor frequency 2 weeks after being plated in 100 ng/mL IL-15 (day 0 LDA) or first cultured in 100 ng/mL KL or FL for 21 days, washed, and then cultured for 2 weeks in IL-15 (day-21 LDA). For the LDA, cells were plated in a limiting dilution fashion from a concentration of 5,000 cells/well to 21 cells/well in 96-well plates with 15 replicates per cell concentration. One half of the cell culture medium was removed every 5 days and replaced with fresh IL-15–supplemented medium. After 2 weeks of culture in IL-15, the cells from each well were stained with anti-CD56-PE, washed, and analyzed for CD56 expression by flow cytometry, and wells were scored positive or negative compared with identical wells stained with the nonreactive PE-conjugated isotype control MoAb. BM-derived NK cells have very high (ie, ≥102PE log fluorescence) surface density expression of CD56 (Fig 1A). Thus, CD56+ wells were clearly and easily demarcated from identical wells stained with isotype control MoAb. In some experiments, CD34brightCD122+ and CD34dim/negCD122− cells were sorted after CD34+ HPC culture in FL alone and plated in an LDA for NK cell progenitor frequencies as described above. The NK cell progenitor frequency was calculated as the reciprocal of the concentration of cells that resulted in 37% negative wells using Poisson statistics and the weighted mean method.48 49
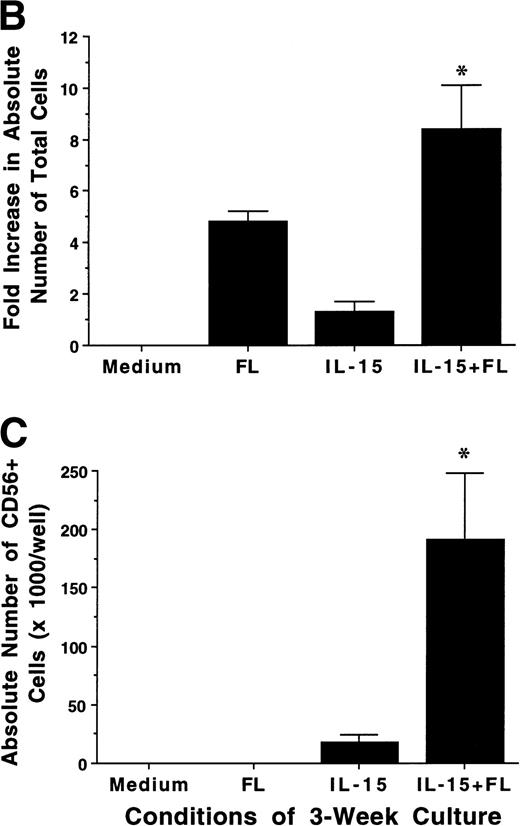
FL enhances IL-15–mediated development of CD56+ CD3− NK cells from CD34+ HPCs in vitro. (A) Flow cytometric analysis of CD56 and CD3 expression on MNCs generated in 21-day cultures of CD34+ HPCs under the indicated conditions (right column). Nonreactive MsIg isotype control MoAb were used to determine background fluorescence (left column). Results displayed are representative of 10 separate experiments. (B) Fold increase in the absolute number of MNCs after 21 days of culture of CD34+ HPCs in the indicated conditions. (C) The absolute number of CD56+CD3− cells after a 21-day culture of CD34+ HPCs in the indicated conditions. These values were calculated by multiplying the absolute number of viable MNCs by the percentage of cells that were CD56+CD3− by flow cytometric analysis. Results shown in (B) and (C) represent the mean ± SEM of five separate experiments. The asterisk indicates a value of P ≤ .025 compared with IL-15 alone.
FL enhances IL-15–mediated development of CD56+ CD3− NK cells from CD34+ HPCs in vitro. (A) Flow cytometric analysis of CD56 and CD3 expression on MNCs generated in 21-day cultures of CD34+ HPCs under the indicated conditions (right column). Nonreactive MsIg isotype control MoAb were used to determine background fluorescence (left column). Results displayed are representative of 10 separate experiments. (B) Fold increase in the absolute number of MNCs after 21 days of culture of CD34+ HPCs in the indicated conditions. (C) The absolute number of CD56+CD3− cells after a 21-day culture of CD34+ HPCs in the indicated conditions. These values were calculated by multiplying the absolute number of viable MNCs by the percentage of cells that were CD56+CD3− by flow cytometric analysis. Results shown in (B) and (C) represent the mean ± SEM of five separate experiments. The asterisk indicates a value of P ≤ .025 compared with IL-15 alone.
Cell cycle analysis.
Cell cycle status was examined by staining cells with either propidium iodide (PI), as described previously,50 or Hoechst 33342, as follows. One million viable cells were stained with 2 μg/mL Hoechst 33342 in 1 mL of complete RPMI-1640 medium and incubated at 37°C for 45 minutes. After centrifugation, supernatants were decanted and cells were stained with CD56-PE MoAb for 15 minutes on ice and then washed with phosphate-buffered saline (PBS) containing 2 μg/mL Hoechst 33342. After being fixed in PBS with 2% formaldehyde and 2 μg/mL Hoechst 33342, cells were simultaneously analyzed for cell cycle status and CD56 expression by flow cytometry.
Analysis of flt3, c-kit, and IL-15Rα expression by reverse transcription-polymerase chain reaction (RT-PCR).
Total RNA was prepared using RNAzol in accordance with the method of Chomczynski and Sacchi.51 cDNA synthesis for RT-PCR was performed at 42°C for 1 hour in a 30 μL reaction mixture containing 2 μg of total RNA, 2 μmol/L of random hexamer primers, 300 U Moloney’s murine leukemia virus (MMLV) reverse transcriptase (GIBCO), and dNTPs at 0.2 mmol/L each (GIBCO), followed by 5 minutes of incubation at 95°C and then incubated on ice for 5 minutes. Five microliters of the resultant cDNA product was amplified by PCR with primers for flt329 or c-kit,52 as described. Primers for IL-15Rα amplification were designed from the published IL-15Rα sequence.53 The forward primer (ACCTTCCACAGGAACCACAG) and reverse primer (AGGTAGCATGCCAGGAGAGA) yield a 213-bp product. The products were separated on 2% agarose gel containing 0.2 μg/mL ethidium bromide. The integrity of RNA samples was verified by amplification of the β-actin transcript.11
Statistical analysis.
Experimental groups were compared using the Student’s t-test, where indicated, with P ≤ .05 considered significant.
RESULTS
FL synergizes with IL-15 in the generation of human CD56+NK cells from CD34+ BM HPCs.
We examined the ability of FL, IL-15, or a combination of FL plus IL-15 to promote the development of human NK cells from BM HPCs in 3-week cultures. Purified CD34+Lin− HPCs (2 × 104/well) cultured in FL (100 ng/mL) alone exhibited a 4.6- ± 0.7-fold increase in total MNC number, but did not give rise to any CD56+ NK cells. Culture of CD34+ HPCs in IL-15 alone (100 ng/mL) for 3 weeks resulted in only a 1.3- ± 0.5-fold increase in total MNC number, but 74% ± 6.9% of cells expressed CD56 at high density (CD56bright), which produced a low absolute number of CD56+ NK cells. However, culture of CD34+ HPCs with FL plus IL-15 resulted in an 8.4- ± 1.7-fold increase in total MNC number, with 72% ± 8.6% of cells CD56bright, which produced a 14.4- ± 6-fold greater absolute number of CD56+CD3− NK cells compared with IL-15 alone (P ≤ .025; Fig 1A through C). The CD56+cells generated by culture in FL plus IL-15 or IL-15 alone exhibited LGL morphology, an absence of CD3, and minimal surface density expression of CD2 and CD16 (data not shown).
Analysis of NK cell cytotoxic activity, cytokine production, and NKR expression.
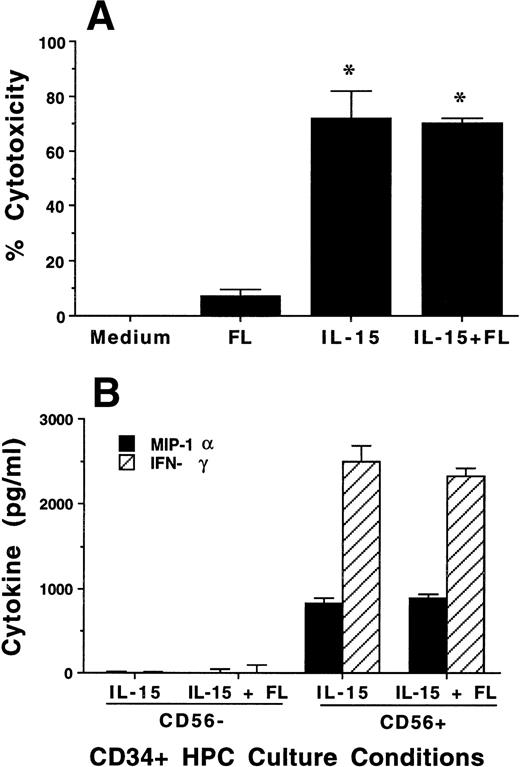
To assess the functional characteristics of the CD56+ cells derived from culture of CD34+ HPCs in FL plus IL-15, we tested cytotoxic activity and cytokine production. After 3 weeks of culture in the presence of FL, MNCs had no CD56 expression and no significant cytotoxic activity against K562 target cells, which lack MHC class I. In contrast, CD34+ HPCs cultured in FL plus IL-15 or IL-15 alone had high CD56 expression (Fig 1) and consistently exhibited greater than 70% cytotoxicity against K562 target cells (Fig 2A).
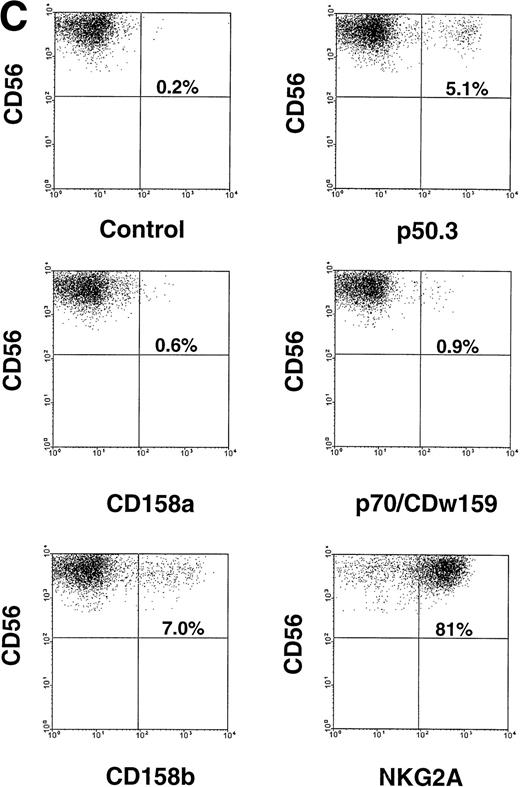
Characterization of NK cell cytotoxicity, cytokine production, and NKR expression by MNCs, CD56+CD3−, and CD56−CD3− cells generated from CD34+ HPCs in various conditions. (A) Cytotoxicity assay. MNCs were harvested after 21-day culture with indicated cytokines, washed, and tested for cytotoxic activity against the NK-sensitive K562 target cell line at an 8:1 E:T ratio. (B) Cytokine (IFN-γ) and chemokine (MIP-1) production. MNCs were harvested after 21-day culture with indicated cytokines, sorted into CD56+CD3− and CD56−CD3− fractions, and costimulated with IL-15 plus IL-12 for 48 hours. Cell-free supernatants were harvested and tested for IFN-γ and MIP-1 by ELISA. For both (A) and (B), results represent the mean ± SEM of four separate experiments. The asterisk in (A) denotes a value of P ≤ .01 compared with culture in FL alone. (C) NKR expression. NK cells generated in vitro by culture in FL for 3 weeks, and then in IL-15 for 2 weeks, were stained with anti-NKR and anti-CD56 MoAbs and analyzed by flow cytometry as described in Materials and Methods. Flow cytometry data are representative of 3 separate donors, which are summarized in Table 1.
Characterization of NK cell cytotoxicity, cytokine production, and NKR expression by MNCs, CD56+CD3−, and CD56−CD3− cells generated from CD34+ HPCs in various conditions. (A) Cytotoxicity assay. MNCs were harvested after 21-day culture with indicated cytokines, washed, and tested for cytotoxic activity against the NK-sensitive K562 target cell line at an 8:1 E:T ratio. (B) Cytokine (IFN-γ) and chemokine (MIP-1) production. MNCs were harvested after 21-day culture with indicated cytokines, sorted into CD56+CD3− and CD56−CD3− fractions, and costimulated with IL-15 plus IL-12 for 48 hours. Cell-free supernatants were harvested and tested for IFN-γ and MIP-1 by ELISA. For both (A) and (B), results represent the mean ± SEM of four separate experiments. The asterisk in (A) denotes a value of P ≤ .01 compared with culture in FL alone. (C) NKR expression. NK cells generated in vitro by culture in FL for 3 weeks, and then in IL-15 for 2 weeks, were stained with anti-NKR and anti-CD56 MoAbs and analyzed by flow cytometry as described in Materials and Methods. Flow cytometry data are representative of 3 separate donors, which are summarized in Table 1.
The monocyte-derived cytokines IL-12 and IL-15 are potent costimulators for IFN-γ and MIP-1α production by mature NK cells.54 55 Three-week cultures of CD34+ HPCs in FL plus IL-15 or in IL-15 alone were harvested and separated by FACS into CD56+CD3− and CD56−CD3− subsets. Equal numbers of each subset (5 × 104 per well) were then costimulated with IL-15 plus IL-12 for 48 hours, after which cell-free culture supernatants were harvested and assayed for IFN-γ or MIP-1α protein production by ELISA. CD56+ cells generated from CD34+ HPCs cultured either in FL plus IL-15 or in IL-15 alone produced abundant amounts of IFN-γ and MIP-1α after 48 hours of costimulation with IL-15 plus IL-12, whereas CD56−cells from the same cultures showed little or no production of above-noted cytokines (Fig 2B). There was no spontaneous production of immunomodulatory cytokines, ie, IFN-γ, MIP-1α, TGF-β, or TNF-α, detected from supernatants in the above-noted culture conditions, from cells cultured in FL or KL alone for 3 weeks, or after an additional 2 weeks of culture in IL-15 (data not shown).
We assessed CD56− and CD56+ BM-derived NK cell progenitors and BM-derived NK cells, respectively, for NKR expression. CD34+ HPCs cultured for 3 weeks in FL or KL alone did not express CD56 or NKR (not shown). However, after the washout of FL or KL and culture in IL-15 for an additional 2 weeks, NKR expression was observed within the CD56+ fraction of cells. This included modest but distinct expression of Ig-superfamily killer inhibitory receptor (KIR) antigens on CD56+ NK cells, as well as abundant expression of the C-type lectin NKR on CD56+ cells. CD56− cells did not express NKR. These results are summarized for three donors in Table 1 and a representative panel of two-parameter histograms is shown in Fig 2C. Thus, after culture in FL and IL-15, all NK cell phenotypic and functional characteristics were restricted to the CD56+ subset of cells.
NKR Surface Expression by CD56+ Cells Derived From CD34+ HPCs Cultured in FL for 21 Days, Followed By Culture in IL-15 for 14 Days
| NKR . | NKR Subfamily . | % of NKR Expression in CD56+ Cells . | |||
|---|---|---|---|---|---|
| Donor 1 . | Donor 2 . | Donor 3 . | Mean ± SEM . | ||
| CD158a | KIR2DL1/S1 | 0.59 | 1.14 | 0.56 | 0.76 ± 0.2 |
| CD158b | KIR2DL2/L3/S2 | 7.03 | 3.10 | 6.80 | 5.6 ± 1.0 |
| p70/CDw159 | KIR3DL1/S1 | 0.88 | 0.37 | 0.04 | 0.43 ± 0.2 |
| p50.3 | KIR2DS4 | 5.1 | 0.08 | 0.10 | 1.76 ± 1.7 |
| NKG2A | C-type lectin | 80.72 | 47.8 | 76.42 | 68.3 ± 8.4 |
| NKR . | NKR Subfamily . | % of NKR Expression in CD56+ Cells . | |||
|---|---|---|---|---|---|
| Donor 1 . | Donor 2 . | Donor 3 . | Mean ± SEM . | ||
| CD158a | KIR2DL1/S1 | 0.59 | 1.14 | 0.56 | 0.76 ± 0.2 |
| CD158b | KIR2DL2/L3/S2 | 7.03 | 3.10 | 6.80 | 5.6 ± 1.0 |
| p70/CDw159 | KIR3DL1/S1 | 0.88 | 0.37 | 0.04 | 0.43 ± 0.2 |
| p50.3 | KIR2DS4 | 5.1 | 0.08 | 0.10 | 1.76 ± 1.7 |
| NKG2A | C-type lectin | 80.72 | 47.8 | 76.42 | 68.3 ± 8.4 |
Purified CD34+ HPCs were cultured in FL (100 ng/mL) for 21 days, washed, and cultured in IL-15 (100 ng/mL) for an additional 14 days. Cells were harvested and stained with anti-NKR MoAbs and anti-CD56-PE as described in Materials and Methods. The flow cytometry dot-plot for donor 1 is shown in Fig 2C. No detectable NKR or CD56 expression was observed in the 3-week cultures before the addition of IL-15. Similar NKR profiles were obtained when KL (100 ng/mL) was substituted for FL in these experiments (not shown). Individual results from 3 donors, as well as the mean ± SEM, are shown.
Comparison of the effects of FL and KL on NK cell precursor frequency.
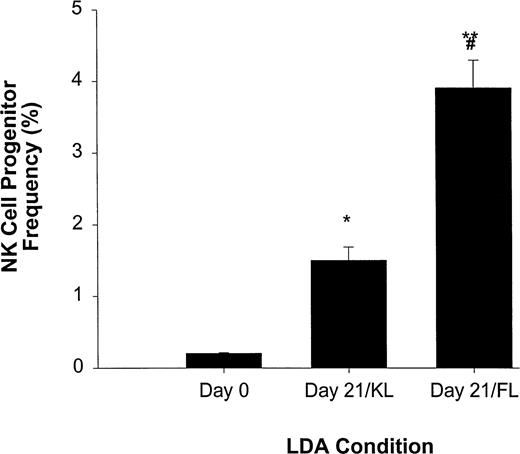
We have previously reported that KL, a BM stromal factor with strong homology to FL, can synergize with IL-15 to enhance the expansion of CD56+ NK cells from CD34+ HPCs.11We therefore directly compared FL and KL for their ability to increase NK cell precursor frequency among CD34+ HPCs in an LDA (Fig 3). NK cell precursor frequency in freshly isolated CD34+ HPCs cultured in IL-15 alone was 1 in 483 ± 16 cells, or approximately 0.21% ± 0.007%. However, when CD34+ HPCs were first cultured for 3 weeks in KL and then analyzed for NK cell precursor frequency in subsequent culture with IL-15 alone, there was a significant (P ≤ .005) increase in precursor frequency to 1 in 67 ± 8.3 cells (1.5% ± 0.19%). Likewise, initial 3-week culture in FL increased the subsequent NK cell precursor frequency in IL-15 alone to 1 in 25 ± 2.5 cells (3.9% ± 0.4%; P ≤ .002), which was significantly higher than that observed in KL (P ≤ .01; Fig 3).
LDA of NK cell precursor frequency within freshly isolated CD34+ HPCs (day 0) or CD34+ HPCs cultured for 21 days in KL (day 21/KL) or FL (day 21/FL). CD34+ HPCs cultured in KL (*P ≤ .005) or FL (**P ≤ .002) had significantly increased NK cell precursor frequency compared with freshly isolated CD34+ HPCs. In addition, the 21-day/FL cultures had significantly greater NK cell precursor frequencies when compared with 21-day/KL culture (#P≤ .01). The results represent the mean ± SEM of three independent experiments.
LDA of NK cell precursor frequency within freshly isolated CD34+ HPCs (day 0) or CD34+ HPCs cultured for 21 days in KL (day 21/KL) or FL (day 21/FL). CD34+ HPCs cultured in KL (*P ≤ .005) or FL (**P ≤ .002) had significantly increased NK cell precursor frequency compared with freshly isolated CD34+ HPCs. In addition, the 21-day/FL cultures had significantly greater NK cell precursor frequencies when compared with 21-day/KL culture (#P≤ .01). The results represent the mean ± SEM of three independent experiments.
Identification of an NK cell precursor after culture in FL or KL.
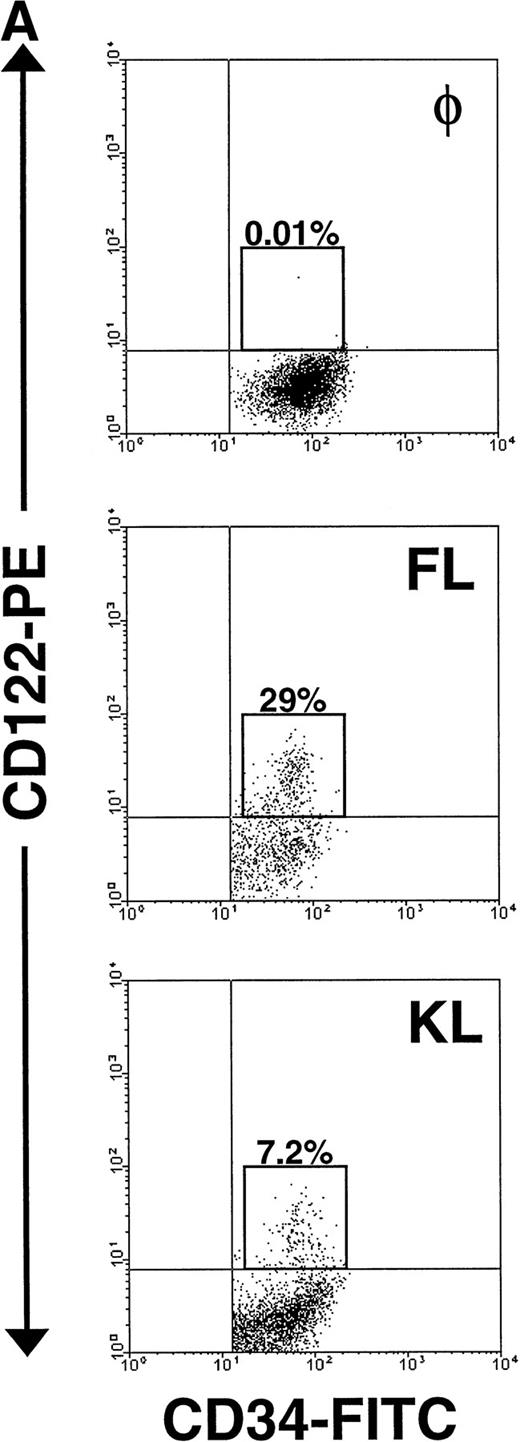
The significant increase in NK cell precursor frequency after culture of CD34+ HPCs in FL or KL suggested that these factors may be responsible for the generation of an intermediate, IL-15–responsive NK cell precursor. The IL-2/15Rβ chain (CD122) is required for IL-15 signaling.18 We therefore examined CD122 expression by flow cytometric analysis on both freshly isolated human CD34+HPCs and CD34+ HPCs cultured in FL for 10 days. Fresh CD34+ BM HPCs expressed no detectable CD122 on their surface, consistent with earlier reports.44 56 However, after 10 days of culture in FL alone, 20% ± 3% of CD34+ HPCs became CD122+. Interestingly, this fraction was among the cells expressing CD34 with relatively high surface density, ie, the CD34bright subset (Fig 4A).
Expression of CD122 (IL-2/15Rβ) protein and IL-15R mRNA on CD34+ HPCs after culture in FL or KL. (A) Flow cytometric analysis of CD122 on freshly isolated CD34+HPCs (day 0) or CD34+ HPCs cultured in FL or KL for 10 days. The percentage of CD34+CD122+ cells (gated population) is indicated for each condition. (B) RT-PCR for IL-15R mRNA transcript expression. CD34+ HPCs from 2 donors were examined for IL-15R on day 0 (lanes 1 and 4), day-10 culture in KL (lanes 2 and 5), day-10 culture in FL (lanes 3 and 6), and 10-day culture in FL followed by culture in IL-15 for 14 days (lane 7). Lane 8 was H2O control. RT-PCR analysis was performed as described in Materials and Methods.
Expression of CD122 (IL-2/15Rβ) protein and IL-15R mRNA on CD34+ HPCs after culture in FL or KL. (A) Flow cytometric analysis of CD122 on freshly isolated CD34+HPCs (day 0) or CD34+ HPCs cultured in FL or KL for 10 days. The percentage of CD34+CD122+ cells (gated population) is indicated for each condition. (B) RT-PCR for IL-15R mRNA transcript expression. CD34+ HPCs from 2 donors were examined for IL-15R on day 0 (lanes 1 and 4), day-10 culture in KL (lanes 2 and 5), day-10 culture in FL (lanes 3 and 6), and 10-day culture in FL followed by culture in IL-15 for 14 days (lane 7). Lane 8 was H2O control. RT-PCR analysis was performed as described in Materials and Methods.
To determine the NK cell precursor frequency in these CD34+HPC subsets after the 10-day culture in FL, FACS-sorted CD34brightCD122+ and CD34dim/negCD122− cells were compared using LDA. The NK cell precursor frequency in CD34brightCD122+ cells was between 20- and 60-fold higher than the NK cell precursor frequency among the CD34dim/neg CD122− subset and between 65- and 235-fold higher than the frequency in fresh CD34+ HPCs (Table 2). Thus, it appears that culture of human CD34+ HPCs in FL induces IL-2/15Rβ (CD122) expression in CD34bright HPCs, creating a cell that, by our assays, represents a phenotypic and functional intermediate within human NK cell development. CD34brightCD122+ is the phenotype that identifies this subset after culture in FL but before culture in IL-15.
Human NK Cell Precursor Frequency of Fresh CD34+ HPCs or 10-Day Culture of CD34+ HPCs in FL
| Donor . | Fresh CD34+ HPCs . | 10-Day Culture of CD34+ HPCs in FL . | |
|---|---|---|---|
| CD34brightCD122+ . | CD34dim/negCD122− . | ||
| 1 | 1:458 | 1:7 | 1:147 |
| 2 | 1:471 | 1:2 | 1:127 |
| 3 | 1:527 | 1:5 | 1:161 |
| Donor . | Fresh CD34+ HPCs . | 10-Day Culture of CD34+ HPCs in FL . | |
|---|---|---|---|
| CD34brightCD122+ . | CD34dim/negCD122− . | ||
| 1 | 1:458 | 1:7 | 1:147 |
| 2 | 1:471 | 1:2 | 1:127 |
| 3 | 1:527 | 1:5 | 1:161 |
Purified CD34+ HPCs were either assayed immediately for NK cell precursor frequency by LDA or further cultured in FL (100 ng/mL) for 10 days, sorted by FACS into CD34brightCD122+ or CD34dim/negCD122− subsets (>98% pure), and then plated for LDA. In each LDA, cell populations were cultured in IL-15 (100 ng/mL) for an additional 14 days, stained with anti-CD56-PE MoAb, and analyzed for CD56 expression and NK cell precursor frequency, as described in Materials and Methods.
The CD34brightCD122+ subset is CD38+CD7−CD56− by flow cytometric analysis. Before culture in IL-15, the CD34brightCD122+ subset does not express any of the NKR shown in Table 1 and Fig 2C, and it does not have any cytotoxic activity against NK targets (data not shown). Although we were unable to detect IL-15Rα protein expression by flow cytometric analysis of this subset, we were able to easily detect the expression of IL-15Rα transcript after culture of CD34+ HPCs in FL (Fig 4B).
Importantly, KL has similar, albeit less dramatic, effects as FL in the generation of this NK cell intermediate. After a 10-day culture of CD34+ HPCs in KL, 7.5% ± 0.7% of the CD34+ HPCs coexpressed CD122, again within the CD34bright fraction (Fig 4A). This was significantly lower than the percentage of CD34brightCD122+ cells found after culture with FL (P ≤ .01). This is consistent with the significantly lower NK cell precursor frequency seen when CD34+ HPCs were cultured for 21 days in KL compared with FL, as shown in Fig 3. The IL-15Rα transcript was also detected after incubation of CD34+ HPCs in KL (Fig 4B).
The effect of IL-15 on NK cell development.
As described above, there is a striking correlation between the expression of CD56 and the appearance of NK cell function. The mechanism by which CD56+ NK cells develop from their CD34+CD56− precursor populations after the addition of IL-15, ie, differentiation or proliferation, is unknown. We therefore examined CD34+ HPCs after 21 days of culture in FL or KL and quantitated the MNC number, viability, percentage of apoptosis, and cell cycle status at 3-day intervals after the addition of IL-15. Absolute cell number did not change significantly over 14 days after the addition of IL-15, yet CD56+ cells went from less than 1% to greater than 80%. Cell cycle analysis demonstrated that a constant fraction (14% ± 2.1%) of cells were in G2/S phase over the subsequent 14-day culture with IL-15, which was approximately equal to the fraction undergoing apoptosis (15% ± 1.4%). When analyzed on days 10 through 12 after addition of IL-15 (ie, days 31 to 33), 13% ± 1.5% of the CD56− fraction was cycling (G2/S), whereas only 2.2% ± 0.6% of the CD56+ fraction was cycling. The absolute increase in CD56+ cells from day 10 (36,450) to day 12 (81,420) could not be accounted for by proliferation, as virtually every CD56+ cell on day 31 would have to double once in 48 hours, data not supported by the cell cycle analysis. These data are summarized in Table 3 and suggest that the increase in CD56+ NK cells in the presence of IL-15 is more likely the result of NK precursor cell differentiation to mature NK cells rather than the selective outgrowth of a small fraction of CD56+ cells. However, it does not suggest that all CD34+ HPCs cultured in FL or KL were committed to NK cell development before the addition of IL-15. Indeed, when CD34+ HPCs after 21 days of culture in FL or KL, but before culture in IL-15, were exposed to other hematopoietic factors, such as G-CSF, granulocyte-macrophage colony-stimulating factor (GM-CSF), erythropoietin, or thrombopoietin, we observed morphologic evidence of differentiation to other lineages (not shown).
Time Course of Cell Number, CD56 Expression, Viability, Percentage of Apoptosis, and Cell Cycle Status of CD34+HPCs Cultured in FL or KL for 21 Days, Followed by the Addition of IL-15 for 14 Days
| Day of Culture . | FL → FL + IL-15 . | KL → KL + IL-15 . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of Cells/Well (×1,000) . | % CD56+ . | % Nonviable . | % Apoptosis . | % Cycling . | No. of Cells/Well (×1,000) . | % CD56+ . | % Nonviable . | % Apoptosis . | % Cycling . | |
| Day 21 | 154 ± 22 | 0.8 ± .05 | 2.5 ± 0.4 | 21 ± 8 | 14 ± 2 | 170 ± 28 | 0.7 ± .03 | 2.2 ± 0.5 | 13 ± 1 | 13 ± 2 |
| Day 24 | 171 ± 15 | 9.5 ± 2.2 | 2.6 ± 0.5 | 14 ± 3 | 12 ± 3 | 186 ± 20 | 3.7 ± 0.9 | 1.8 ± 0.4 | 14 ± 3 | 12 ± 1 |
| Day 27 | 163 ± 21 | 13 ± 2.8 | 5.7 ± 1.2 | 15 ± 2 | 10 ± 2 | 170 ± 22 | 11 ± 3.1 | 5.8 ± 1.0 | 10 ± 2 | 14 ± 3 |
| Day 30 | 135 ± 18 | 27 ± 2.5 | 6.3 ± 0.9 | 12 ± 2 | 12 ± 4 | 170 ± 18 | 25 ± 4.3 | 5.1 ± 0.6 | 11 ± 2 | 11 ± 3 |
| Day 33 | 138 ± 10 | 59 ± 6.4 | 9.8 ± 1.5 | 11 ± 2 | 14 ± 3 | 165 ± 17 | 54 ± 9.6 | 8.3 ± 0.7 | 12 ± 1 | 15 ± 1 |
| Day 36 | 138 ± 11 | 94 ± 3.1 | 8.6 ± 1.2 | 14 ± 1 | 11 ± 1 | 168 ± 15 | 83 ± 2.6 | 16 ± 2.1 | 13 ± 1 | 12 ± 2 |
| Day of Culture . | FL → FL + IL-15 . | KL → KL + IL-15 . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of Cells/Well (×1,000) . | % CD56+ . | % Nonviable . | % Apoptosis . | % Cycling . | No. of Cells/Well (×1,000) . | % CD56+ . | % Nonviable . | % Apoptosis . | % Cycling . | |
| Day 21 | 154 ± 22 | 0.8 ± .05 | 2.5 ± 0.4 | 21 ± 8 | 14 ± 2 | 170 ± 28 | 0.7 ± .03 | 2.2 ± 0.5 | 13 ± 1 | 13 ± 2 |
| Day 24 | 171 ± 15 | 9.5 ± 2.2 | 2.6 ± 0.5 | 14 ± 3 | 12 ± 3 | 186 ± 20 | 3.7 ± 0.9 | 1.8 ± 0.4 | 14 ± 3 | 12 ± 1 |
| Day 27 | 163 ± 21 | 13 ± 2.8 | 5.7 ± 1.2 | 15 ± 2 | 10 ± 2 | 170 ± 22 | 11 ± 3.1 | 5.8 ± 1.0 | 10 ± 2 | 14 ± 3 |
| Day 30 | 135 ± 18 | 27 ± 2.5 | 6.3 ± 0.9 | 12 ± 2 | 12 ± 4 | 170 ± 18 | 25 ± 4.3 | 5.1 ± 0.6 | 11 ± 2 | 11 ± 3 |
| Day 33 | 138 ± 10 | 59 ± 6.4 | 9.8 ± 1.5 | 11 ± 2 | 14 ± 3 | 165 ± 17 | 54 ± 9.6 | 8.3 ± 0.7 | 12 ± 1 | 15 ± 1 |
| Day 36 | 138 ± 11 | 94 ± 3.1 | 8.6 ± 1.2 | 14 ± 1 | 11 ± 1 | 168 ± 15 | 83 ± 2.6 | 16 ± 2.1 | 13 ± 1 | 12 ± 2 |
Purified CD34+ HPCs were plated at a density of 2 × 104 cells per well and first cultured in medium containing FL or KL (100 ng/mL) for 21 days. IL-15 was then added to culture in combination with either FL or KL, respectively, for an additional 14 days. Cells were examined at 3-day intervals after the addition of IL-15, as described in Materials and Methods.
Synergistic effect of FL with other cytokines upon CD56+NK cell generation from CD34+ HPCs.
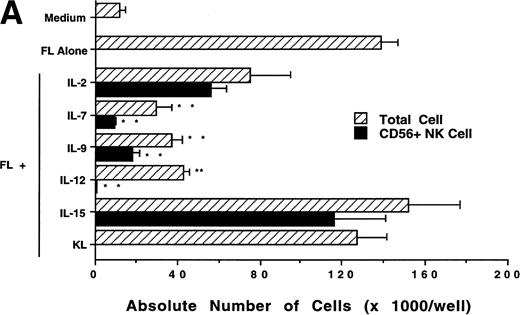
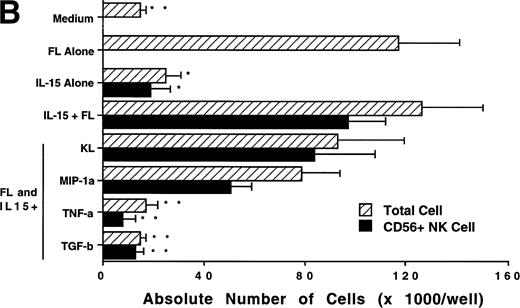
We next investigated whether FL could combine with other cytokines that have been shown to act in early hematopoiesis38-42 for the production of CD56+ NK cells from CD34+ HPCs. CD34+ HPCs were cultured for 3 weeks with rhIL-2, IL-7, IL-9, IL-12, IL-15, and KL in the presence of FL. The absolute number of CD56+CD3− cells generated among total cells in each culture condition is shown in Fig 5A. A direct comparison of IL-2 and IL-15 in combination with FL showed that these cytokines were not significantly different in their ability to generate CD56+CD3− NK cells in this culture system. Both IL-7 and IL-9 demonstrated weak synergy with FL in the production of CD56+CD3− NK cells. However, these factors alone yielded no CD56+CD3− NK cells (data not shown). IL-12, alone or in combination with FL, did not stimulate production of CD56+CD3− NK cells in this culture system. The combination of KL and FL did not generate any CD56+CD3− NK cells, and FL, KL, and IL-15 together were no more effective in generating CD56+CD3− NK cells from CD34+HPCs than were FL plus IL-15 or KL plus IL-15 (Fig 5B). When CD34+ HPCs were cultured with FL and IL-15 and TNF-α, the generation of CD56+CD3− NK cells was inhibited by 90% ± 7% (FL plus IL-15 v FL plus IL-15 plus TNF-α; P ≤ .005). Similarly, the addition of TGF-β to cells being cultured with FL plus IL-15 resulted in a 72% ± 8% reduction in the generation of CD56+CD3−NK cells (FL plus IL-15 v FL plus IL-15 plus TGF-β; P≤ .005). MIP-1α did not significantly inhibit the generation of NK cells from CD34+ HPCs (Fig 5B). Thus, the most potent combinations for the generation of CD56+CD3− NK cells from CD34+BM HPCs were FL plus IL-15 or KL plus IL-15, and TNF-α, TGF-β can function as potent negative regulators of NK cell development.
Effect of FL in combination with various cytokines on the generation of CD56+CD3− NK cells from CD34+ HPCs. Purified CD34+ HPCs were plated in complete RPMI-1640 medium in the presence of the indicated cytokines at the concentrations described in Materials and Methods. Results represent the mean ± SEM of total MNC number and absolute CD56+ NK cell number of five separate experiments. *P ≤ .01 and **P ≤ .005 compared with cultures with FL plus IL-15.
Effect of FL in combination with various cytokines on the generation of CD56+CD3− NK cells from CD34+ HPCs. Purified CD34+ HPCs were plated in complete RPMI-1640 medium in the presence of the indicated cytokines at the concentrations described in Materials and Methods. Results represent the mean ± SEM of total MNC number and absolute CD56+ NK cell number of five separate experiments. *P ≤ .01 and **P ≤ .005 compared with cultures with FL plus IL-15.
Expression and functional assessment of flt3 and c-kit transcript during NK cell differentiation.
CD34+ BM HPCs, BM-derived CD56+CD3− NK cells, CD56bright and CD56dim blood NK cells, CD3+ T cells, and unfractionated peripheral blood lymphocytes (PBLs) were examined by RT-PCR for their expression of flt3 and c-kit transcripts. Amplification of the flt3 transcript (210 bp) was observed in CD34+ BM cells from five consecutive normal donors. However, 5 samples of BM-derived CD56+CD3− NK cells obtained from a 21-day culture of CD34+ HPCs with FL plus IL-15, as well as 5 samples of CD56bright, CD56dim blood NK cells, and CD3+ T cells were negative for the flt3 mRNA transcript (Fig 6A). One of 5 unfractionated PBL samples was positive for flt3 mRNA, which may be explained by expression of flt3 in rare circulating CD34+ HPCs. The c-kit transcript (1,090 bp) was observed in all 5 samples of CD34+ BM cells, BM-derived CD56+CD3− NK cells obtained from a 21-day culture of CD34+ HPCs with FL plus IL-15, as well as CD56bright blood NK cells, CD3+ T cells, and unfractionated PBLs. The CD56dim blood NK cell subset was negative for c-kit mRNA, consistent with our previous functional observations.33,57 To confirm the absence or presence of functional gene products in blood NK cell subsets, sorted CD56bright cells were cultured in IL-2, FL plus IL-2, or KL plus IL-2 for 96 hours and assayed for a proliferative response (Fig6B). CD56bright NK cells cultured with FL plus IL-2 demonstrated no enhancement of [3H]-thymidine incorporation over the same cells cultured with IL-2 alone. In contrast, a parallel culture of CD56bright NK cells in KL plus IL-2 showed a proliferative synergy compared with those cells cultured in IL-2 alone. This is consistent with our earlier observations of a functional c-kit receptor on this NK cell subset.33 Thus, flt3 and c-kit appear to have distinct patterns of expression during NK cell differentiation.
(A) RT-PCR analysis of flt3 and c-kit mRNA expression in HPCs and various lymphocyte populations. Lanes 1 through 3, purified CD34+ HPCs from 3 normal BM donors; lanes 4 and 5, CD56+CD3− NK cells generated from CD34+ HPCs after culture in FL plus IL-15 for 3 weeks; lanes 6 and 7, CD56bright blood NK cells; lanes 8 and 9, CD56dim blood NK cells from two normal blood donors; lane 10, unfractionated PBL; lane 11, blood CD3+ T cells. (Below) Human β-actin control of the identical samples. (B) Proliferation of CD56bright NK cells in response to various concentrations of IL-2, IL-2 plus FL (100 ng/mL), and IL-2 plus KL (100 ng/mL). The results are representative of three separate experiments and indicate the mean cpm ± SEM of triplicate measures.
(A) RT-PCR analysis of flt3 and c-kit mRNA expression in HPCs and various lymphocyte populations. Lanes 1 through 3, purified CD34+ HPCs from 3 normal BM donors; lanes 4 and 5, CD56+CD3− NK cells generated from CD34+ HPCs after culture in FL plus IL-15 for 3 weeks; lanes 6 and 7, CD56bright blood NK cells; lanes 8 and 9, CD56dim blood NK cells from two normal blood donors; lane 10, unfractionated PBL; lane 11, blood CD3+ T cells. (Below) Human β-actin control of the identical samples. (B) Proliferation of CD56bright NK cells in response to various concentrations of IL-2, IL-2 plus FL (100 ng/mL), and IL-2 plus KL (100 ng/mL). The results are representative of three separate experiments and indicate the mean cpm ± SEM of triplicate measures.
DISCUSSION
In the present study, we investigated the role of FL in the regulation of human NK cell development from BM-derived CD34+ HPCs and compared the effect of FL with that of KL. Neither FL nor KL had any ability to induce NK cell differentiation from CD34+ HPCs in the absence of IL-15. However, the ability of both FL and KL to significantly increase NK cell precursor frequency among CD34+ HPC before the addition of IL-15 strongly suggested that these factors contribute to the survival and/or expansion of an IL-15–responsive NK precursor cell. Indeed, we demonstrated that incubation of CD34+ HPCs in FL or KL resulted in the generation of a novel human NK cell intermediate characterized by CD34bright and CD122 (IL-2/15Rβ) expression. It is unclear if activation of either c-kit or flt3 by their respective ligands induces the IL-15R components on a fraction of CD34+ cells or whether there is a selective expansion of a small CD34brightCD122+ subset. Our failure to detect CD122 expression by flow cytometric analysis of 10,000 CD34+ cells before culture in FL or KL might suggest that at least some component of enhanced CD122 gene expression via activation of these two type III RTKs is likely. However, we and others57 detect a low frequency of NK precursor cells in fresh CD34+ HPCs by LDA, so the IL-2/15Rβγcchains are likely to be constitutively expressed on some small number of CD34+ HPCs at a surface density that is below detection by flow cytometry. Indeed, others have failed to detect IL-2/15Rγc on the surface of mature NK cells that are known to functionally respond to IL-2 and IL-15.58 The same may be true for IL-15Rα expression on CD34+ HPCs. Our preliminary studies reported here suggest that FL and KL may upregulate this chain as well, although a quantitative analysis of the IL-15Rα gene product has not yet been performed. In addition to these scenarios, our data do not exclude the possibility that FL and/or KL may mediate some of their effects by inducing the expression of additional factors from the starting population of CD34+ HPCs.
The incorporation of the LDA assay for assessment of NK cell precursor frequency provided strong support for the CD34brightCD122+ cell as an NK cell intermediate. There was a striking positive correlation of CD56 expression with NKR expression, LGL morphology, cytotoxic activity, and cytokine production, as well as a notable absence of these features in the CD56− population. This allowed us to use CD56 expression as a simple and accurate readout for human NK cell outgrowth. We were then able to document a very high NK cell precursor frequency in the CD34brightCD122+ subset, in contrast to the CD34dim/negCD122− subset. Furthermore, we demonstrated that preincubation of CD34+HPCs in either KL or FL significantly increased the NK cell precursor frequency seen with IL-15, compared with fresh CD34+ cells plated in an LDA with IL-15. Because neither KL nor FL by itself induces NK cell differentiation, the mechanism likely involves the generation of the CD34brightCD122+ NK cell intermediate that is, in turn, responsive to IL-15. The observation that the NK cell precursor frequency was significantly higher with preincubation in FL versus KL is consistent with FL inducing over double the percentage of CD34brightCD122+ NK cell intermediates.
We also demonstrated that IL-15 has the ability to induce NKR and CD56 expression, LGL morphology, and cytotoxic properties as well as prepare the cell for potent cytokine and chemokine production after monokine stimulation. The CD56+ NK cells generated from human BM CD34+ HPCs with FL followed by IL-15 expressed both C-type lectin (NKG2A) and Ig-superfamily (CD158a, CD158b, and p70/CDw159 KIR) NKR. This appears to be in contrast to NK cell progenitors in the thymus cultured in IL-15 or IL-15 plus KL, in which only C-type lectin NKR were induced.59-61 The reason for variation in KIR expression between these two in vitro systems may relate to differences in BM versus thymic NK precursor cells, accessory cells in the cultures, or culture conditions. Studies are currently underway to further characterize the factors that may be critical for NKR induction during human NK cell development.
These findings, along with our earlier demonstration of IL-15 protein production within human BM stroma,11 provide compelling evidence for an important role for IL-15 in human NK cell development. Genetic disruption studies provide supporting evidence for this in the mouse. The interferon response factor-1 (IRF-1) knockout mouse lacks the ability to express IL-15 transcript in BM stroma, and lacks NK cells. Incubation of IRF-1−/− HPCs in IL-15 or transplantation of IRF-1−/− HPCs into lethally irradiated wild-type mice leads to the generation of NK cells, strongly suggesting that IL-15 is required in the process of NK cell development.19,20 Likewise, there is support for the role of FL in NK cell development, because mice genetically deficient in FL have low NK cell numbers.43 This would be consistent with the notion that absence of FL leads to fewer IL-15–responsive NK cell intermediates. The NK deficiency in FL−/− mice would suggest that KL is not fully redundant with FL in this regard. The Sld/Sld mouse, which lacks expression of c-kit, has not been noted as NK deficient, but it is unclear if a careful quantitative analysis of NK cells has been undertaken.21 Finally, Williams et al44 have recently demonstrated that, in the mouse, a combination of IL-6, IL-7, KL, and FL can induce CD122 on multipotential murine BM HPCs that, in turn, become responsive to IL-15 for NK cell development.
The data from the current study would suggest that both FL and KL have the capacity to induce CD34brightCD122+ NK cell precursors from CD34+ HPCs, but that FL may perform this function more efficiently. The reasons for this are not entirely clear, but may relate to differences in flt3 and c-kit expression on the earliest HPCs. Shah et al41 have reported that FL was able to induce significantly greater proliferation of quiescent CD34+CD38− cells than KL in long term culture. Haylock et al62 found that, in single cell assays, FL, but not KL, was able to recruit an additional subpopulation of HPCs representing approximately 12% of CD34+CD38− cells that were rhodaminedull and 4-hydroperoxycyclophosphamide (4-HC) resistant. Consistent with this, Miller et al63 have recently shown that NK cell progenitor frequency increases when FL is added to switch cultures of CD34+CD33−HPCs in IL-2, IL-7, and KL. In contrast to FL, KL has effects on mature CD56bright NK cells found in blood, promoting their survival64 and potentiating their IL-2/15–induced proliferation.33 KL does not enhance CD56brightNK cell cytotoxic activity in the presence or absence of IL-2/15,33 which is consistent with its inability to induce NK cell differentiation from HPCs. These differences highlight some distinct functional effects of FL and KL on NK cells due to the distribution of their respective receptors.
In the absence of FL or KL, IL-15 induces NK cell differentiation from CD34+ HPCs, but without significant expansion.11 44 This suggests that IL-15 itself promotes NK cell development via differentiation of committed precursors, not via proliferation. To address this possibility, we performed a kinetic analysis of CD56+ cells during NK cell development from CD34+ HPCs in the presence of IL-15 and either FL or KL. Cell cycle analysis during the substantial increase in CD56 expression proved that IL-15’s effect on CD56+ NK cell development could not be accounted for by induction of proliferation. Thus, IL-15 appears to be the factor most likely responsible for inducing the expression of genes that characterize NK cells.
Collectively, these data suggest that, in humans, both FL and KL participate in the generation of NK cell progenitors from CD34+ HPCs. Both induce an NK cell intermediate from HPCs and both are likely to enhance their expansion while IL-15 induces NK cell differentiation. In this capacity, these two RTK ligands are likely to be functionally redundant.65 The failure of a combination of KL and FL to enhance the absolute number of CD56+ NK cells obtained from CD34+ HPCs cultured with IL-15 plus either FL or KL alone supports this. However, evidence from our study and from others41,62 63 would suggest that FL might also perform its function on an additional subset of NK precursors, which are not responsive to KL. We also noted that CD34+ HPCs cultured in FL or KL can subsequently differentiate along different lineages in the absence of IL-15 and in the presence of other growth factors. Furthermore, we can introduce factors that negatively regulate human NK cell development. These observations remind us that the process of NK cell development is likely to be substantially more complex in vivo. Continued characterization of growth factors and their receptors on HPCs and their progeny will hopefully provide additional insight into this fascinating biological process.
ACKNOWLEDGMENT
The authors thank Dr Carlton Stewart for his advice and technical assistance in the cell cycle analysis by flow cytometry, Dr Jeffrey Miller for assistance with the LDA, Drs Stewart D. Lyman and Elaine Thomas for their critical review of the manuscript, Dr Vinay Kumar, and our anonymous reviewers for their constructive comments. We also thank Drs Alessandro Moretta and Lorenzo Moretta for kindly providing the anti-NKR antibodies.
H.Y. and T.A.F. contributed equally to this work.
Supported by Grants No. 5P30CA16058, CA68458, and CA65670 from the National Cancer Institute. T.A.F. is the recipient of the Howard Hughes Medical Institute Research Fellowship for Medical Students and the Bennett Fellowship from The Ohio State University College of Medicine.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Michael A. Caligiuri, MD, The Ohio State University, Division of Hematology/Oncology, 458A Starling Loving Hall, 320 W 10th Ave, Columbus, OH, 43210; e-mail:caligiuri-1@medctr.osu.edu.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal