A human herpesvirus-8 (HHV-8) enzyme-linked immunosorbent assay (ELISA) with a whole virus lysate as antigen was developed and used to measure the seroprevalence rate and levels of IgG antibodies to HHV-8 in sera/plasma of various patient groups and blood donors. The virus antigen was prepared from the KS-1 cell line, which produces lytic virus, and therefore contains a broad array of viral proteins. Seroprevalence studies using this ELISA showed the following: 10 of 91 blood donors (11%) had an average HHV-8 antibody titer of 118; 67 of 72 (93%) classic Kaposi's sarcoma (KS) patients were positive with an average titer of 14,111; and 57 of 62 (92%) KS/human immunodeficiency virus (HIV) patients were positive with an average titer of 4,000. A study on a very limited number of serial serum samples from patients before and after diagnosis with KS showed highly elevated antibody titers to HHV-8 virus after KS lesions developed. Preliminary data show that 50% of the sera from HIV-1+ homosexual patients contain IgG antibodies to HHV-8 suggesting that this population is at high risk for developing KS. Antibody results correlated well with the confirmatory immunofluorescent assays (IFA) using KS-1 cells as the substrate. This HHV-8 IgG antibody detection ELISA is sensitive and specific and does not cross-react with Epstein-Barr virus (EBV) or other human herpesviruses. The results of this HHV-8 antibody survey suggest that this rapid ELISA assay can be used to screen large numbers of sera to find those at risk for developing KS.
KAPOSI'S SARCOMA-associated herpesvirus (KSHV) or human herpesvirus-8 (HHV-8) was first detected in KS lesions by representational difference analysis by Chang et al.1 The virus has also been found in body cavity-based lymphomas (BCL or BCBL), also called primary effusion lymphoma (PEL).2-4 The KS-1 cell line was established from the pleural cavity-based lymphoma of a KSHV positive, human immunodeficiency virus (HIV)-1 and Epstein-Barr virus (EBV) negative patient.4 Intracellular viral particles have been detected in the KS-1 cell line,4 and sufficient quantities of enveloped and nonenveloped virus (1.8 × 109particles/L) can be pelleted from culture supernatants.5
Seroprevalence reports for HHV-8 antibodies in the general population have been described as ranging from 0% to 25% based on immunofluorescent assays (IFA) to latent and lytic proteins,6-10 as well as single peptide or recombinant antigen enzyme-linked immunosorbent assay (ELISA), and immunoblot assays.10-13 This study describes the development and use of an ELISA for the detection of HHV-8 IgG antibodies in clinical samples using whole virus lysate as antigen containing most, if not all, of the viral structural and nonstructural proteins. The ELISA is free of cross-reactivity to other human herpesviruses.
MATERIALS AND METHODS
Sera/plasma panels.
All samples tested for this study were from adults. Blood donor sera were from a well-characterized panel used for control reagents and comparison studies at Advanced Biotechnologies Inc (Columbia, MD). HIV-1 sera/plasma samples with or without diagnosis of KS were obtained from the North Shore University Hospital (Manhasset, NY) and the University of Southern California School of Medicine. The HIV-1/KS samples were received either coded or uncoded. The uncoded group was presented along with dates of diagnosis of KS and samples of KS patients' sera before KS diagnosis are identified in this report as “pre-KS.” Coded KS sera samples from classical KS patients without HIV-1 coinfection were obtained from the New York University Medical Center and the University of Southern California School of Medicine. HIV-1/non–KS samples from North Shore University Hospital were from a characterized group of sera from the late 1980s and early 1990s that did not develop KS on follow-up; these samples are referred to as retrospective. Follow-up on other HIV-1/non–KS patients was not known when the samples were submitted. All sera/plasma panels from these sources contained coded controls from patients without HIV-1 or KS. Assay results on coded samples were identified after testing and these identifications are reported in the Results section and Table 1.
Percentages of HHV-8 IgG Antibody in Donors and Various Patient Groups
| Patient Sample . | No. Tested . | No. Positive . | Percent Positive . |
|---|---|---|---|
| Blood donor | 91 | 10 | 11 |
| Classic KS (HIV-1 neg) | 72 | 67 | 93 |
| KS (HIV-1+) individuals | 62 | 57 | 92 |
| KS (HIV-1+) total sera-150 | 66 | 61 | 92 |
| Pre-KS | 31 | 26 | 84 |
| HIV-1 (non-KS) retrospective-151 | 43 | 11 | 26 |
| HIV-1 (non-KS) homosexuals-152 | 14 | 7 | 50 |
| Hodgkin's cases | 42 | 0 | 0 |
| Heterosexual (non-HIV/non-KS) | 19 | 1 | 5 |
| Homosexual (non-HIV/non-KS) | 14 | 2 | 14 |
| Patient Sample . | No. Tested . | No. Positive . | Percent Positive . |
|---|---|---|---|
| Blood donor | 91 | 10 | 11 |
| Classic KS (HIV-1 neg) | 72 | 67 | 93 |
| KS (HIV-1+) individuals | 62 | 57 | 92 |
| KS (HIV-1+) total sera-150 | 66 | 61 | 92 |
| Pre-KS | 31 | 26 | 84 |
| HIV-1 (non-KS) retrospective-151 | 43 | 11 | 26 |
| HIV-1 (non-KS) homosexuals-152 | 14 | 7 | 50 |
| Hodgkin's cases | 42 | 0 | 0 |
| Heterosexual (non-HIV/non-KS) | 19 | 1 | 5 |
| Homosexual (non-HIV/non-KS) | 14 | 2 | 14 |
Sera with a titer of 1:80 or higher are listed as positive.
“KS (HIV-1+) total sera” includes data from multiple draws of several patients.
Sera from patients later diagnosed with KS were removed from this group and placed in the pre-KS group.
KS outcome of this group not known at time of testing.
Development of ELISA using HHV-8 viral lysate.
The KS-1 cell line was tested to insure freedom from adventitious agents such as mycoplasma and other herpesviruses. Supernatant fluids from KS-1 cultures were clarified by low speed (200xg) centrifugation before pelleting in a high-speed centrifuge (19,000 rpm in a Beckman type 19 rotor, Beckman Instruments Inc, Palo Alto, CA). Generally, virus pellets contained approximately 2 × 109 viral particles per liter when quantified by electron microscopy using negatively stained preparations. The average protein concentration of these virus pellet preparations was 1.58 mg/mL. The pellets were resuspended to a concentration 200× that of the supernate. For purified virus preparations, the virus was subjected to isopynic centrifugation on a 20% to 50% sucrose density gradient, and the peak fractions collected and pooled after ELISA detection using a reference HHV-8 antibody. The virus was lysed with Triton X-100 and diluted 200× for direct pelleted virus and 300× for purified virus, to give 3 to 5 μg/mL preparation of protein for coating, depending on the preparation. This viral preparation was coated onto ELISA plates (Nunc polysorb #446140; Roskilde, Denmark). Sera or plasma at various dilutions were allowed to react for 30 minutes with the viral lysate. The unbound serum was then rinsed away with a washing buffer containing Tween-20. In the next step, a goat antihuman IgG horseradish peroxidase conjugate was added. After a final rinsing, TMB (tetramethyl benzidine) was used to develop the color. To assess the cross-reactivity of the HHV-8 virus preparation from KS-1 cells with EBV, an EBV ELISA performed with same serum using the same ELISA format except that direct pelleted EBV from the B95-8 cell line (assayed to assure it was free of type C simian virus) was used for coating the plates instead of the HHV-8.
Development of IFA to HHV-8 late antigens using KS-1 cells.
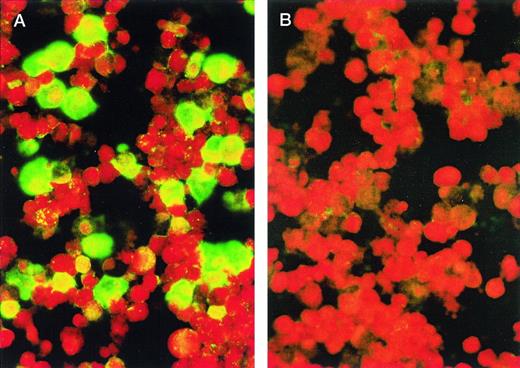
For the IFA IgG (Advanced Biotechnologies Inc, Cat. No. 15-330-000), KS-1 cells were washed twice in phosphate-buffered saline (PBS), placed on slides, air dried, and fixed for 15 minutes with cold acetone. Such preparations generally expressed between 30% to 50% late antigen positive cells by IFA, using HHV-8 positive polyclonal human sera from a classical KS (HIV-1 negative) patient (Fig 1). The reactivity of this serum was also confirmed by Western blot using purified HHV-8 antigen. Test sera or plasma at various dilutions, starting with 1:20, were placed on the fixed cells and incubated at 37°C for 0.5 hour. After the slides were washed with PBS, a goat antihuman IgG fluorescein isothiocyanate (FITC) conjugate containing Evans blue as a counter stain was placed on the cells and the slides were again incubated at 37°C for 0.5 hour. After washing in PBS, a coverslip was added with mounting solution and the slides were observed with a fluorescence microscope. All sera or plasma were tested at a starting dilution of 1:20. Figure 1A shows staining of antigen positive cells by antibody positive serum and Fig1B is a preparation stained with a serum from a healthy donor lacking such antibodies.
KS-1 cells (p39) were air-dried onto slides and fixed with acetone. Plasmas were diluted 1:20 with PBS. In (A), the cells were stained with a plasma from a classic KS patient; in (B), with plasma from a blood donor. FITC-conjugated goat antihuman IgG was used as the secondary antibody. Cells were photographed at original magnification × 400. Green staining cells indicate presence of HHV-8 antigens.
KS-1 cells (p39) were air-dried onto slides and fixed with acetone. Plasmas were diluted 1:20 with PBS. In (A), the cells were stained with a plasma from a classic KS patient; in (B), with plasma from a blood donor. FITC-conjugated goat antihuman IgG was used as the secondary antibody. Cells were photographed at original magnification × 400. Green staining cells indicate presence of HHV-8 antigens.
Criteria for positive samples.
Based on our negative control sera and KS patient surveys, an ELISA titer of 1:80 was considered positive. Blood donor, KS/HIV-1, and classic KS samples with low ELISA titers were also tested by IFA. Samples with ELISA titers greater than 1:80 were also positive by IFA; but samples with ELISA titers lower than 1:80 were negative by IFA (at 1:20). The majority of the samples with ELISA titers of 1:80 were positive by IFA (at 1:20), and we therefore have selected this dilution as the cut-off value to evaluate the results of the ELISA. Also we found that sera assayed at concentrations less than 1:80 by ELISA and 1:20 by IFA displayed some nonspecific reactivity especially with Fc receptors. Therefore, samples with ELISA titers below 80 and IFA titers below 1:20 are reported as negative or zero.
Cross-reactivity with EBV.
EBV IgG antibody was detected by IFA using slides coated with P3HR-1 cells expressing EBV-viral capsid antigen (VCA) (Stellar Biosystems, Columbia, MD). A KS serum exhibiting antibody to EBV-VCA (titer of ≥1:200) was adsorbed with live P3HR-1 cells (EBV producer cell line) overnight at 4°C to remove EBV antibodies and to test for cross-reactions with HHV-8 by IFA; significant loss in HHV-8 antibody titer would be indicative of a cross-reaction of EBV antibody with HHV-8.
RESULTS
Antigen evaluation.
Table 2 shows that the serum antibody titers of samples tested on ELISA plates coated with direct pelleted virus varied very little from the titers obtained with plates coated with sucrose purified virus. No antibody titer on the same serum varied by more than one dilution, regardless of the preparation used for coating the plate as is shown in Table 1. Neither preparation showed consistently higher titers than the other; therefore, we chose to use direct pelleted viral lysate as the source of antigen for the ELISA.
Comparison of Lysates from DPV or SPV for Use as ELISA Antigen
| Sample . | Titer of DPV . | SPV . | Sample . | Titer of DPV . | SPV . |
|---|---|---|---|---|---|
| Blood donor 1 | 80 | 160 | Non-KS patient | 80 | 80 |
| Blood donor 2 | 80 | 80 | Non-KS patient | 40 | 40 |
| Blood donor 3 | 20 | 0 | KS/HIV | 16,000 | 16,000 |
| Blood donor 4 | 40 | 80 | KS/HIV | 10,000 | 10,000 |
| Blood donor 5 | 160 | 80 | KS/HIV | 6,400 | 6,400 |
| Blood donor 6 | 320 | 320 | KS/HIV | 3,200 | 3,200 |
| Blood donor 7 | 320 | 160 | KS/HIV | 25,600 | 32,000 |
| Blood donor 8 | 80 | 80 | KS/HIV | 25,600 | 32,000 |
| Blood donor 9 | 320 | 160 | KS/HIV | 25,600 | 16,000 |
| Blood donor 10 | 160 | 80 | KS/HIV | 25,600 | 32,000 |
| Blood donor 11 | 0 | 0 | KS/HIV | 25,600 | 16,000 |
| Blood donor 12 | 0 | 20 | KS/HIV | 12,800 | 16,000 |
| Blood donor 13 | 20 | 40 | KS/HIV | 12,800 | 12,800 |
| Blood donor 14 | 0 | 0 | Classic KS | 16,000 | 16,000 |
| Blood donor 15 | 20 | 40 | Classic KS | 32,000 | 64,000 |
| Blood donor 16 | 0 | 20 |
| Sample . | Titer of DPV . | SPV . | Sample . | Titer of DPV . | SPV . |
|---|---|---|---|---|---|
| Blood donor 1 | 80 | 160 | Non-KS patient | 80 | 80 |
| Blood donor 2 | 80 | 80 | Non-KS patient | 40 | 40 |
| Blood donor 3 | 20 | 0 | KS/HIV | 16,000 | 16,000 |
| Blood donor 4 | 40 | 80 | KS/HIV | 10,000 | 10,000 |
| Blood donor 5 | 160 | 80 | KS/HIV | 6,400 | 6,400 |
| Blood donor 6 | 320 | 320 | KS/HIV | 3,200 | 3,200 |
| Blood donor 7 | 320 | 160 | KS/HIV | 25,600 | 32,000 |
| Blood donor 8 | 80 | 80 | KS/HIV | 25,600 | 32,000 |
| Blood donor 9 | 320 | 160 | KS/HIV | 25,600 | 16,000 |
| Blood donor 10 | 160 | 80 | KS/HIV | 25,600 | 32,000 |
| Blood donor 11 | 0 | 0 | KS/HIV | 25,600 | 16,000 |
| Blood donor 12 | 0 | 20 | KS/HIV | 12,800 | 16,000 |
| Blood donor 13 | 20 | 40 | KS/HIV | 12,800 | 12,800 |
| Blood donor 14 | 0 | 0 | Classic KS | 16,000 | 16,000 |
| Blood donor 15 | 20 | 40 | Classic KS | 32,000 | 64,000 |
| Blood donor 16 | 0 | 20 |
Abbreviations: DPV, direct pelleted virus; SPV, sucrose purified virus.
Cross-reactions with other herpesviruses.
Human blood donor sera specifically tested and found to have high antibody titers to other human herpesviruses (EBV, 10 sera; cytomegalovirus [CMV], 6 sera; herpes simplex virus-1 (HSV-1), 10 sera; HSV-2, 10 sera; HHV-6, 9 sera; and HHV-7, 1 serum) and several monkey sera including squirrel monkeys, seropositive for herpesvirus saimiri (HVS) antibody, were assayed by HHV-8 ELISA and all were found to give no signal on our HHV-8 IgG antibody ELISA, showing the lack of antibody cross-reactivity with these other herpesviruses. To confirm this finding, a serum from a KS/HIV patient with an HHV-8 antibody titer of 10,000, was tested for EBV antibodies by IFA and found to have an EBV-VCA antibody titer of ≥200. Aliquots of this serum were then treated with various dilutions of EBV antigen to absorb out the antibodies. These aliquots were then assayed with an EBV antibody ELISA and an HHV-8 antibody ELISA. The optical density (OD) for the HHV-8 ELISA remained the same, regardless of how much EBV antibody had been used for the absorption; and with the EBV ELISA the OD decreased as increasing amounts of EBV antibody was absorbed out of the serum.
In the reciprocal ELISA experiment, HHV-8 purified virus was added at various dilutions to aliquots of the HHV-8 and EBV antibody positive serum. In this experiment, the OD remained constant for the EBV ELISA, but decreased for the HHV-8 ELISA as increasing amounts of HHV-8 antibodies were adsorbed out of the serum. This indicated that no cross-reactivity occurred between antibodies to EBV and HHV-8 antigen or between the HHV-8 antibodies and EBV antigen.
Similar results were obtained by IFA. The EBV absorbed serum showed no signal for EBV-VCA at a 1:10 dilution; but on KS-1 cells, it was strongly reactive up to a dilution of ≥1:200. The signal did not weaken on the KS-1 cells after the EBV had been absorbed.
Furthermore, 91 normal donors were tested and only 11% were found to give a positive signal on this ELISA. Because antibodies to EBV are prevalent in approximately 85% of the normal population, one would expect to find higher percentages of positive signal of this HHV-8 IgG antibody ELISA if it had cross-reactivity for EBV.
Correlation of HHV-8 IgG antibody as detected by ELISA and IFA.
The titers obtained by IFA correlated very well (P < .0001, r = 0.915) with the antibody ELISA for the same samples, although the titers by IFA were consistently lower (range, 1:20 to ≥1:5,120) than those obtained with the ELISA, but they were proportional with the ELISA. Because approximately 40% of the cells in the prepared IFA slides were antigen positive, the remaining reddish colored negative cells in the preparation could be used as an internal control for nonspecific staining. Nonspecific staining tends to show up as a general yellow-green haze, but as can be seen in Fig 1, the negative cells only fluoresce red with the counter stain, Evan's blue. The majority of the antibody positive samples exhibited intense diffuse staining of the entire cell, which is typical for the expression of late antigens. However, some sera from KS patients showed strong reactivity at the cell margin, which is typical of antibodies to envelope proteins. Some cells also showed intense nuclear staining, which is associated with nonstructural proteins.
Seroprevalence.
The data in Table 1 show that 93% of 72 sera tested from classic KS and 92% of the 62 sera or plasma of KS/HIV-1 patients had IgG antibody to HHV-8. Eighty-four percent of 31 sera tested before patients manifest symptoms of KS (pre-KS) were positive for HHV-8 antibodies. Antibodies to the virus could be found in the plasma of 11% of 91 blood donors tested. The group labeled “HIV-1(non-KS)” contained sera from patients who did not subsequently develop KS; in contrast, the follow-up in the group labeled “HIV-1 (non-KS) homosexuals” had not been done on the patients at the time the samples were sent to us.
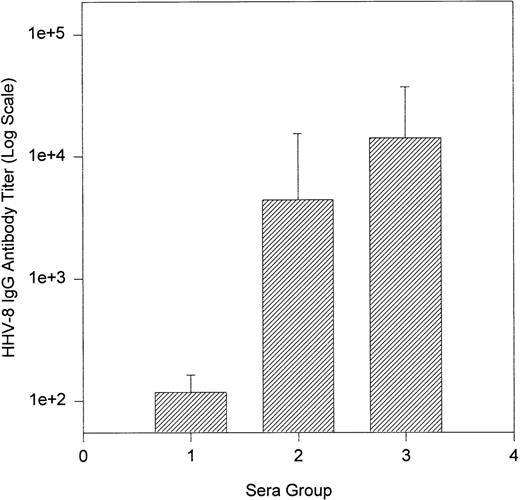
Figure 2 shows that the average and median antibody titer of classic KS patients was higher (titer range, 0 to 128,000; average, 14,111; median, 5,120) than that of KS/HIV patients (titer range, 0 to 64,000; average, 4,000; median, 400). In contrast, the average titers of the positive blood donors were lower than those of KS patients (titer range, 80 to 200; average, 118; median, 100) (Fig2). The average titer of all blood donors tested was 14 and the median was 0.
Bar graph of Log10 average HHV-8 IgG antibody titers: blood donors and KS patients. Group 1, positive blood donors; group 2, KS/HIV-1 patients; group 3, classic KS patients.
Bar graph of Log10 average HHV-8 IgG antibody titers: blood donors and KS patients. Group 1, positive blood donors; group 2, KS/HIV-1 patients; group 3, classic KS patients.
Longitudinal study on KS/HIV patients' sera.
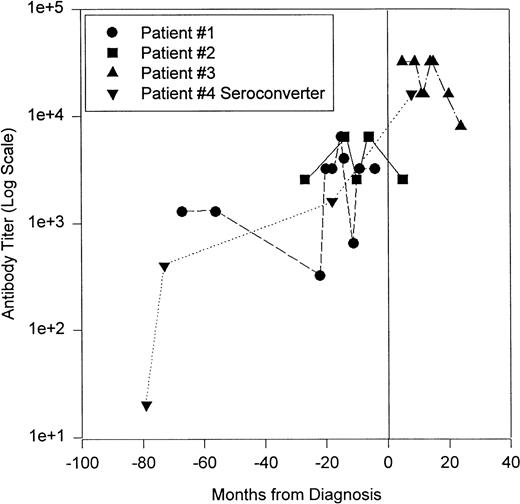
Figure 3 shows the HHV-8 antibody serotiters of four KS/HIV patients followed over time. Patient no. 1 had several serum samples before KS was diagnosed, but all samples had a positive titer. This patient developed symptoms of KS 4 months after the last sample and died a month later; while the CD4 count of this patient dropped from 103 to 2/mm3 from the first sample to the last. The second patient (no. 2) was also positive for three samples before diagnosis and showed a similar pattern of falling CD4 counts, from 218 to 47/mm3. Patient no. 3 samples were all drawn after the patient developed KS and these sera had the highest HHV-8 antibody titers. The last patient (no. 4) was a seroconverter. CD4 counts on patients 3 and 4 were not available. These preliminary samples seem to indicate that the titer increases slowly on sequential bleedings before KS was diagnosed, and after diagnosis, the titer increases sharply. However, to use the antibody titer to viral proteins to predict the development of KS, many more patients will have to be evaluated to see if this pattern holds true.
Longitudinal follow-up of KS/HIV-1 patients for IgG antibody to HHV-8. Patient no. 1 (•) was diagnosed with KS a month after the last serum sample and died a month after diagnosis. On autopsy, KS was found in his internal organs. Patient no. 2 (▪) was diagnosed with KS between his fourth and fifth samples. Patient no. 3 (▴) was diagnosed with KS before the first sample. Patient no. 4 (▾) was a seroconverter, his first sample had no HHV-8 IgG titer, but all of his subsequent samples did; he was diagnosed with KS between his third and fourth serum samples. Vertical line represents diagnosis of KS. See text for more details.
Longitudinal follow-up of KS/HIV-1 patients for IgG antibody to HHV-8. Patient no. 1 (•) was diagnosed with KS a month after the last serum sample and died a month after diagnosis. On autopsy, KS was found in his internal organs. Patient no. 2 (▪) was diagnosed with KS between his fourth and fifth samples. Patient no. 3 (▴) was diagnosed with KS before the first sample. Patient no. 4 (▾) was a seroconverter, his first sample had no HHV-8 IgG titer, but all of his subsequent samples did; he was diagnosed with KS between his third and fourth serum samples. Vertical line represents diagnosis of KS. See text for more details.
DISCUSSION
Many reports have now appeared in the literature on the seroprevalence of HHV-8,6-15 using a variety of methods, including ELISA or immunoblot techniques using peptides of minor capsid protein, IFA to latency-associated nuclear antigen (LANA), and lytic antibody IFA. Generally, the methods using the peptides and the LANA-IFA7,11 gave lower overall results than those using the lytic antibody IFA, which includes a step with mouse monoclonal antihuman IgG123 (American Type Culture Collection HF6508).6 This is probably due to the fact that the lytic antibody IFA method presents a wider array of antigens with which the serum antibodies may react. The results reported here using our whole virus ELISA fall roughly between the LANA IFA7,11 and the lytic antibody IFA6 for both blood donors and KS patients. Lennette et al,6 using the BCBL-1 cell line as a substrate for IFA, reported on antibodies to both lytic and latent proteins of HHV-8 and found that nearly 25% of the general adult population has HHV-8 antibodies, which is higher than the 11% positive blood donors found by ELISA in our study. Their higher value may be due to nonspecific cross-reactivity. We have also obtained cross-reactivity in our samples if they were insufficiently diluted before testing (less than 1:20 for IFA and less than 1:80 for ELISA). Other specific cross-reactivities with cellular antigens, such as those of B cells, may also be responsible for the higher findings among blood donors. However, both the present study and Lennette's find nearly all classic or endemic KS patients without HIV were positive for HHV-8 antibodies. Lennette et al reported 100% of her classic cases positive, and we have found only five of the 72 samples reported to us as classic KS with no antibody. Their finding for KS/HIV seroprevalence is higher (96%) than our finding of 92%; perhaps this difference is due to their larger study population.
Twenty-six of our HIV-1/non–KS sera were HHV-8 antibody positive, which is more than double the general population (11%), but lower than the pre-KS patients (84%). The samples in this HIV-1/non–KS category are sera from the late 1980s and early 1990s, with known histories, including both homosexuals and intravenous drug abusers. Any patients who were diagnosed later with KS were not included in this category, but were included in the pre-KS group. If we did not have this retrospective data on the sera, the percentage of positive HIV-1/non–KS samples would have undoubtedly been higher. This is demonstrated in the “HIV-1/non-KS homosexual” category where the HHV-8 prevalence is 50% when the KS status was not known at the time of testing. Subsequent follow-up found that four of the seven homosexual patients who had antibody to HHV-8 were diagnosed with KS within 5 years of the time the tested sera were drawn. If later serum samples from these patients can be obtained, they will be assayed to see if they show the same pattern of elevated antibody titers after the development of KS as seen in Fig 3. The percentage seropositivity in heterosexual and perhaps homosexual non–HIV-1/non-KS groups suggest they would probably approach the same percentages as the blood donors if larger numbers of samples were to be tested. The absence of any HHV-8 antibody in Hodgkin's patients suggests that HHV-8 plays no role in this disease.
The peptides used for ELISA and blot techniques by other investigators8,11-13 were carefully chosen so they did not contain sequences that might cross-react with EBV. However, by using discrete peptides, the assays may well miss antibodies to other immunogenic viral proteins in the virus, especially because it is not yet known which viral proteins are immunodominant. Despite the fact that HHV-8 shows some sequence homology with EBV,16 the data presented here indicates that the antibodies to HHV-8 detected by the ELISA to viral lysate are specific and do not cross-react with EBV or other human herpesviruses. To assess the applicability of various immunologic assays for HHV-8, a well-designed study is needed where the same sera panel are tested by the various available assays.
The fact that classic KS patients have much higher average titers of HHV-8 than do KS patients with HIV-1 would indicate that conditions that allow KS to manifest itself are more likely to occur in HIV-1–infected patients. Biggar17,18 has traced the explosive increase in the number of KS cases in acquired immunodeficiency syndrome (AIDS) patients. Before 1980, the relative risk of KS for all men was 0.29/100,000.17 In 1990, the relative risk of developing KS for homosexual/bisexual men with AIDS had risen to 106,000 times more than the non-AIDS population and for nonhomosexual/bisexual men with AIDS, the relative risk was 13,000 above the non-AIDS population.18 Some of this increased risk is certainly due to the impaired immune system of HIV patients and increased exposure through risk behaviors; but a growing body of evidence points to the increased expression of cytokines and growth factors found in HIV-1 patients that contributes to initiation or maintenance of KS lesions, as well as the presence of the HIV-1 Tat protein.19-21 In general, it can be stated that those patients coinfected with both HIV-1 and HHV-8 are at high risk for developing KS.
The five classic KS patients and the five HIV-1/KS patients without antibodies to HHV-8 hold open the possibility that some agent(s) other than HHV-8 can also initiate the cascade of cytokines and growth factors that leads to KS lesions. However, HHV-8 DNA is certainly found by polymerase chain reaction (PCR) in nearly all classical KS lesions,22 and perhaps these patients were not able to mount an IgG antibody response to the virus. We have not yet addressed the question of the presence of IgM antibodies to HHV-8, but it should prove to be a very interesting study. It is also a possibility that these samples were misidentified or misdiagnosed. Indeed, we received one sample, not included in our data, that was identified as a classic KS and had no HHV-8 antibody titer. A biopsy of this patient was subsequently reexamined and was found to have been misinterpreted and the diagnosis of KS was removed.
The limited preliminary data from the longitudinal studies seems to show a pattern of HHV-8 IgG antibody titers that appears similar to that of EBV-VCA where significantly elevated antibody titers are found in Burkitt's lymphoma (BL) and nasopharyngeal carcinoma (NPC). Elevated EBV-VCA titers have been attributed to the pathogenesis of BL and NPC.23 24 Therefore, a significant increase in IgG antibody to structural viral proteins suggests that HHV-8 plays a role in the pathogenesis of KS. Consistent follow-up of HHV-8–infected individuals for IgG antibody would add to the knowledge of its role in HHV-8–associated malignancies.
Our HHV-8 whole virus ELISA showed elevated HHV-8 IgG antibody in the sera of KS patients and is able to detect antibodies to HHV-8 before patients develop KS symptoms. Because we used a whole virus lysate, most if not all, of the latent and lytic proteins are represented, and therefore the assay is more likely to detect most sera with antibodies to HHV-8. The data we obtained from the HHV-8 IgG antibody whole virus ELISA correlated very well with our IFA data. Our ELISA is specific, reproducible, and ideal for large-scale seroepidemiologic studies, as well as for longitudinal follow-up of KS patients and those at risk for KS, such as HIV-1 positive homosexuals. The ease of running these assays allows not only a qualitative positive or negative reading, but also the ability to easily titrate samples.
Based on the data presented here, we concluded that in comparison to other alpha, beta, and gamma human herpesviruses (HSV-1, CMV, HHV-6, HHV-7, and EBV), the prevalence of HHV-8 in the general population is substantial (11%), but not as widespread as other human herpesviruses, which have prevalency rates of over 80% in the general population. Elevated IgG antibody to HHV-8 in KS patients adds to the growing body of evidence that HHV-8 plays a role in the etiology and pathogenesis of KS.
Address reprint requests to Louise G. Chatlynne, PhD, Advanced Biotechnologies Inc, 9108 Guilford Rd, Columbia, MD 21046.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal