The loss of body cell mass (bcm) in senescence and wasting is poorly understood. We now show that the plasma cystine/acid soluble thiol ratio, ie, an indicator of the redox state, is increased in old age and cancer patients and correlated with a decrease in bcm and plasma albumin. A cause/effect relationship was suggested by two independent studies with N-acetyl-cysteine (NAC). NAC caused an increase in the bcm of healthy persons with high plasma cystine/thiol ratios, and treatment of cancer patients with NAC plus interleukin-2 caused an increase in bcm, plasma albumin, and functional capacity. Albumin levels below 680 μmol/L were associated with an increase in body water. Our studies suggest that the shift in the redox state may contribute to the loss of bcm and may provide a quantitative guideline for therapeutic intervention. Treatment of cancer patients with thiol-containing antioxidants may improve the quality of life.
LIKE APOPTOTIC cell death, senescence and wasting are largely autonomous and biologically meaningful processes associated with an increased probability of death. The hallmarks of these processes include the massive loss of body cell mass (bcm) and muscle function, decreased resistance to infections, frailty (increased probability of disability), and organ failure.1-9 A biochemical correlate of senescence and a quantitative measure of cachexia is the decrease in the plasma albumin level.10-15Wasting is a common phenomenon in malignancies,2-5 sepsis, trauma,6 and certain infectious diseases including human immunodeficiency virus (HIV) infection.7 8
Wasting and death of an individual organism (ie, a competitor for food and space) may be advantageous for the species, just as apoptosis of an individual cell is typically advantageous for the organism. However, for the individual human subject the loss of bcm and muscle strength in senescence and wasting is often associated with psychological stress and financial burden. Its medical and social relevance may increase even further because the average lifespan is increasing progressively.16,17 Most of the years beyond age 70 are years of compromised physical and social function,16-18 and 45% of the people in the United States over the age of 85 years require assistance.16 The loss of muscle strength in the elderly is mostly related to loss of muscle mass.19
One of the prevailing hypotheses states that senescence may result from the accumulation of oxidative damage20-22 and that dietary antioxidants may slow the degenerative process.20,22,23 In support of this paradigm, vitamin E was shown to ameliorate age-related health problems,24 and certain age-related degenerative changes were even found to be reversed by antioxidant treatment.22 23 However, the available information fails to tell us what type and quantity of antioxidant we need and how we can monitor that we have received enough. In this report we now show (1) that senescence and wasting are associated with an easily demonstrable change in the redox state of the blood plasma, and (2) that important consequences of changes in the redox state can be shown in human subjects within a few weeks or months. In view of these relatively short observation periods, our findings may provide, for the first time, a quantitative guideline for a redox-oriented prophylactic therapy.
To increase the weight of the evidence, we performed several independent and complementary studies. To study the hypothetical role of the redox state in wasting, senescence, and albumin degradation, we determined in healthy elderly subjects and cancer patients the ratio of plasma cystine and acid-soluble sulfhydryl groups as a measure of the thiol/disulfide redox state. Because the acid-soluble sulfhydryl groups represent mostly cysteine, the cystine/thiol ratio is essentially an indicator of the equilibrium between cysteine disulfide (cystine) and cysteine, ie, of the redox state of the cysteine/cysteine disulfide redox couple in the plasma. This is analogous to the glutathione/glutathione disulfide (GSH/GSSG) ratio that is widely used as an indicator of the intracellular redox state. The effect of N-acetyl-cysteine (NAC) on the plasma albumin level and bcm has been studied to show a cause/effect relationship. Correlations between biochemical and biophysical parameters have been analyzed to test the strength of hypothetical linkages. The resulting pool of data illustrates the normal and pathological ranges of redox states and suggests in addition that certain redox states within the normal range might be associated with certain risks.
To determine the pathological role of the redox state in cancer patients, we investigated the hypothesis that treatment with the cysteine derivative NAC may have a positive effect on bcm and functional capacity of cancer patients. Prospectively defined secondary outcome measures were the survival time, the intracellular levels of glutathione (GSH) and glutathione disulfide (GSSG) in peripheral blood mononuclear cells (PBMC), and the plasma levels of amino acids and acid-soluble thiol. In addition, the plasma albumin level was determined because the albumin level of cachectic patients is a strong predictor of survival and cost of treatment,10 and because several previous attempts to increase the albumin level by nutritional therapy were not successful.10,25-27 Also, in elderly subjects a low albumin level was found to be correlated with a low 10-year survival rate12 and loss of muscle mass (sarcopenia).13 Human albumin and bovine albumin contain a single unpaired cysteine residue (Cys34) with antioxidative function.13,28-30 In the blood, albumin exists largely in its reduced form (mercaptalbumin, MA) and to a lesser extent in the oxidized form (nonmercaptalbumin, NA). The latter consists mainly of a mixed protein-cysteine disulfide or protein-glutathione disulfide and increases in proportion with age.30-32 It has been reported that redox processes mediate the conversion of albumin into an aged form with a threefold higher catabolic rate.29 The plasma glutamate level was of interest because the process of skeletal muscle wasting is commonly associated with a strong increase in the plasma glutamate level as a consequence of a decreased glutamate uptake capacity of the skeletal muscle tissue.33 Nitrate and nitrite levels were also included retrospectively to monitor the effects of interleukin-2 (IL-2) on nitric oxide production. High doses of IL-2 were previously found to induce an increase in nitric oxide production as manifested by increased plasma nitrate levels, hypotension, and a capillary leak syndrome associated with an increase in body water and body weight.34
SUBJECTS, MATERIALS, AND METHODS
Study on healthy human subjects (study A).
Plasma amino acids and acid-soluble thiols have been determined in the venous blood of 205 randomly selected healthy human subjects. The age distribution can be seen in Fig 1. Plasma albumin levels have been determined in 86 subjects and the bcm index in 93 subjects.
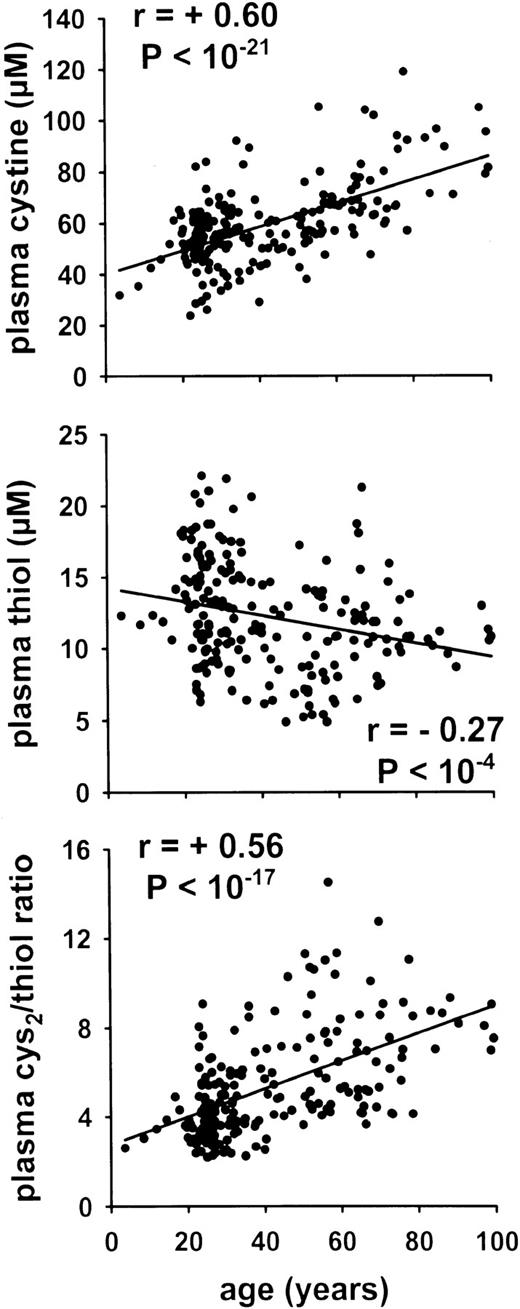
Correlation between plasma redox state and age in healthy persons (study A). Postabsorptive plasma amino acid and acid-soluble thiol levels have been determined in the plasma from the cubital vein of 205 randomly selected healthy human subjects of both sexes.
Correlation between plasma redox state and age in healthy persons (study A). Postabsorptive plasma amino acid and acid-soluble thiol levels have been determined in the plasma from the cubital vein of 205 randomly selected healthy human subjects of both sexes.
Prospectively designed randomized clinical trial for the comparison of the effect of NAC plus IL-2 versus IL-2 on cancer patients (study B).
Included were adult patients with different types of inoperable cancer who had previously failed to respond to standard therapy. Not eligible were patients with anorexia, a life expectancy of less than 2 months, and any type of cancer therapy during the preceding 6 weeks. Fifty patients were recruited initially. One patient who wanted a specific treatment was excluded. Twenty-seven patients were treated with IL-2 only (4 of these patients died and 1 left the study before the second examination) and 23 patients were treated with IL-2 plus NAC (3 of these patients died before the second examination). Randomization was performed by the attending physician (H.R.) (ie, by tossing a coin) and was stratified according to the type of tumor; treatment was known to both the physician and the patient (unblinded study). The sample size was estimated on the basis of preliminary information about intracellular GSH/GSSG ratios and plasma glutamate levels. It was estimated that 25 patients were needed for each treatment group to detect desirable changes with a power of at least 80% with a one-sidedt-test and 5% significance level.
An additional group of 20 patients was recruited subsequently for treatment with standard chemotherapy. Six of the 20 patients died and eight left the study before the second examination. This may be explained by their previous failure to respond to standard therapy.
IL-2 was administered at a dose of 6 × 106 IU subcutaneously twice a week. NAC was taken orally 3 times per day. To avoid excessive plasma concentrations of cystine and glutamine,35 NAC was used at variable doses ranging between 0.6 and 4.2 g depending on the latest measurements of plasma cystine and glutamine levels. A daily dose of 4.2 g NAC was assigned to patients with a plasma glutamine (gln) level <550 μmol/L and cystine (cys2) <60 μmol/L, 3.6 g NAC for patients with gln <550 μmol/L and cys2 > 60 μmol/L and for patients with gln 550 to 700 μmol/L and cys2 < 50 μmol/L, 2.4 g NAC for gln 550 to 700 μmol/L and cys2> 60 μmol/L, and 0.6 g NAC for patients with gln > 700 μmol/L. With these dose schedules, none of the patients under NAC therapy showed a plasma cystine level > 100 μmol/L and glutamine level > 910 μmol/L. On the basis of established knowledge, neither of the three treatment protocols, ie, low-dose IL-2 alone, IL-2 plus NAC, or standard therapy could be expected to have a priori an obvious advantage or disadvantage for these patients with respect to the sum of therapeutic benefits and potential side effects.
The primary and secondary outcome measures of study B have been described in the introduction. The functional capacity index as defined in Table 3, point 4 of ref 36 was taken as a measure of how the patient viewed her/his own quality of life (0 = normal, no limitations; 1 = not normal, but stable enough to be up with fairly normal activity; 2 = not feeling up to most things but in bed less than half the day; 3 = able to do little activity and most of the day in bed or chair; 4 = rarely out of bed).
Height, body mass, bcm, and body water were determined and blood samples were taken at baseline examination before the start of therapy. The second measurements were performed about 4 weeks after baseline examination and start of the therapy. Additional measurements followed at larger time intervals. Blood samples were taken from the cubital vein in the postabsorptive period. Acid-soluble thiol levels have been determined in all samples from the two groups with NAC plus IL-2 and IL-2 only, but only in one sample from the standard treatment group (n = 51).
The studies were conducted according to the principles of the Declaration of Helsinki. Before entering one of the treatment programs, each person was given a detailed explanation of all testing procedures and signed an informed consent. Each patient agreed to be assigned to the chosen treatment protocol.
Longitudinal study on a single healthy individual (study C).
Blood samples were obtained from a single healthy male subject in the sixth decade of life at randomly distributed time points over an observation period of 2 years.
Study on the effects of NAC on bcm of healthy subjects and its correlation with plasma amino acid levels (study D).
The study was designed as a randomized double-blind trial. Healthy and moderately well-trained men between 20 and 60 years old were recruited into the study and randomly assigned to the verum group (n = 18) and placebo group (n = 20). The dose of NAC was 2 × 200 mg orally per day on 3 days per week for 4 weeks. During this period both groups were also subjected to a program of anaerobic physical exercise. For details of this program see the report of Kinscherf et al.37
Determination of bcm, bcm index, and body water.
The bcm is defined as the sum of the oxygen-consuming, potassium-rich, and glucose-oxidizing cells. In practical terms it is the total body mass minus body fat and extracellular mass (bone and extracellular water). Bcm and body water were computed from the body weight and the electrical resistance and reactance of the body to weak alternating current (ie, by bioelectrical impedance analysis) with a commercial computer program as described previously.37 The biological and technical variability of longitudinal bcm measurements has been assessed previously.37 In analogy to the body mass index (body weight/height2), the bcm index was defined as the ratio of body cell mass/height2 (kg/m2). The relative total body water was defined as the ratio of total body water/bcm.
Determination of plasma levels of acid-soluble thiol, albumin, nitrate and nitrite, and amino acids including cystine, glutamine, and glutamate.
Plasma amino acid levels (including cystine, glutamine, and glutamate concentrations) were determined with the amino-acid analyzer, and acid-soluble thiol was determined with a photometric assay as described previously.37 Albumin was determined with a commercial kit (Sigma, Steinheim, Germany),38 and the sum of plasma nitrate and nitrite was measured colorimetrically by the Griess reaction.39
Statistical analysis.
The statistical evaluation of the individual changes between baseline examination and terminal examination was performed by the pairedt-test for dependent samples (two-tailed). The data from different treatment groups were compared statistically by the Kruskal Wallis or Wilcoxon rank-sum tests, or by Student's t-test for independent samples as indicated. The Trend test40 was used to compare the functional activity data. Arithmetic means and standard errors of the means were used as descriptive statistics. Correlations between parameters were described graphically by scatter plots and linear regression lines. The strength of relationship was assessed either by Spearman's rho or by Pearson's product correlation coefficient r as indicated. The result of the statistical test was judged by its P value. A P value <.05 was regarded as statistically significant.
RESULTS
Senescence is associated with a change in the redox state. Correlation between bcm index, redox state, and plasma albumin level in healthy human subjects (study A).
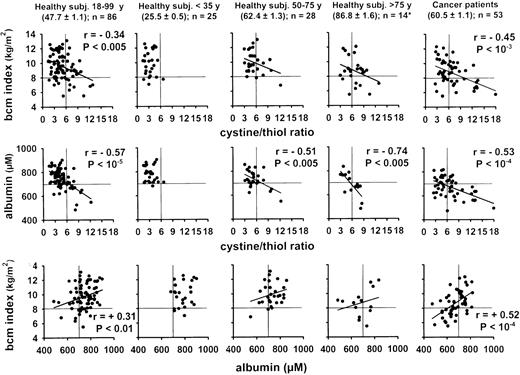
Our study on healthy human subjects (study A) showed a significant age-dependent increase of the plasma cystine level and a decrease of the plasma thiol level indicative of an age-dependent shift to a more oxidized condition (Fig 1). Our analysis also confirmed the negative correlation between plasma albumin concentration and age (r = .49, P < 10−5, see also Fig2). A possible linkage between the plasma cystine/thiol ratio, the plasma albumin level, and the bcm index was suggested by the strong correlation between these parameters (Fig 2, left panel).
Thiol redox state, plasma albumin, and bcm index of healthy subjects and cancer patients. Each point represents an individual person. The bcm index was defined as the bcm/height2 in analogy to the body mass index. Note that the bcm index and the plasma albumin level have not been determined for all subjects of study A and study B (see Subjects, Materials, and Methods). Horizontal and vertical lines indicate the window that contains most of the healthy young subjects less than 35 years old.
Thiol redox state, plasma albumin, and bcm index of healthy subjects and cancer patients. Each point represents an individual person. The bcm index was defined as the bcm/height2 in analogy to the body mass index. Note that the bcm index and the plasma albumin level have not been determined for all subjects of study A and study B (see Subjects, Materials, and Methods). Horizontal and vertical lines indicate the window that contains most of the healthy young subjects less than 35 years old.
Changes of the redox state, albumin level, and bcm in cancer patients. Baseline characteristics of the treatment groups in study B.
To determine the role of the redox state in cancer cachexia, we determined intracellular GSH and GSSG levels and plasma levels of cystine and thiol and studied the effect of NAC on bcm, functional capacity, and albumin level of cancer patients.
Seventy patients with inoperable cancer were recruited. Fifty patients were randomly assigned to treatment with IL-2 alone or IL-2 plus NAC. In addition, 20 patients received standard therapy. The most frequent tumors were carcinoma of the breast (3/5/3), pancreas (5/3/1), colon (3/2/1), prostate (2/2/1), and hematological tumors (4/2/0). (The data in brackets indicate the number of patients treated with IL-2 plus NAC, IL-2 alone, and conventional chemotherapy, respectively.) Primary outcome measures were changes of body cell mass and functional capacity. Secondary endpoints were survival time, intracellular GSH and GSSG levels, and plasma cystine/thiol ratios as indicators of pathological redox changes, the plasma glutamate level as an indicator of skeletal muscle function, plasma albumin, NO2− and NO3−.
The results showed first of all that cancer patients have, on the average, an altered redox state as manifested by increased plasma cystine/thiol ratios (Fig 2), decreased intracellular glutathione levels, and decreased intracellular GSH/GSSG ratios in the PBMC (Table1). In comparison with 82 healthy 40- to 70-year-old subjects from study A (mean age, 57.7 ± 1.1 years), the cancer patients (mean age, 60.5 ± 1.1 years) had at baseline examination significantly elevated plasma cystine/thiol ratios (6.99 ± 0.57 v 5.53 ± 0.22; P < .05), decreased plasma albumin levels (674 ± 9 v 760 ± 11 μmol/L; P < 10−7), and elevated plasma glutamate levels (47.7 ± 2.9 v 30.6 ± 1.8 μmol/L;P < 10−5). The bcm indices, albumin levels, and redox states of the cancer patients were again significantly correlated with each other and showed a pattern similar to that of healthy subjects with an age >75 years but different from that of young or age-matched subjects (Fig 2). Unexpectedly, the two studies B and A showed only a weak correlation between intracellular GSSG/GSH ratio and plasma cystine/thiol ratio (r = +.32 and +.23, respectively).
Effect of NAC and IL-2 on the Intracellular Glutathione Level in PBMC of Cancer Patients
| . | A Conventional Chemotherapy . | B IL-2 . | C NAC/IL-2 . | D Healthy Subjects . | Significance Between Groups (P values) . | |
|---|---|---|---|---|---|---|
| A-B . | A-C . | |||||
| n (F/M) | 6 (4/2) | 22 (9/13) | 20 (12/8) | 82 (34/48) | ||
| Age | 56.5 ± 2.5 | 59.7 ± 1.7 | 60.2 ± 2.4 | 57.5 ± 1 | ||
| Total intracellular glutathione (nmol/mg protein) | ||||||
| b | 11.6 ± 1.75 | 12.7 ± 1.2 | 13.5 ± 1.4 | 24.4 ± 1.1 | ||
| t | 10.0 ± 1.58 | 18.9 ± 1.9 | 19.8 ± 1.6 | 24.4 ± 1.1 | <.03 | <.005 |
| P < .01 | P < .005 | |||||
| GSH/GSSG ratio | ||||||
| b | 9.85 ± 2.80 | 8.7 ± 1.4 | 7.8 ± 1.6 | 17.7 ± 1.7 | ||
| t | 4.43 ± 0.93 | 17.5 ± 2.1 | 18.0 ± 2.2 | 17.7 ± 1.7 | <.005 | <.005 |
| P < .005 | P < .001 | |||||
| . | A Conventional Chemotherapy . | B IL-2 . | C NAC/IL-2 . | D Healthy Subjects . | Significance Between Groups (P values) . | |
|---|---|---|---|---|---|---|
| A-B . | A-C . | |||||
| n (F/M) | 6 (4/2) | 22 (9/13) | 20 (12/8) | 82 (34/48) | ||
| Age | 56.5 ± 2.5 | 59.7 ± 1.7 | 60.2 ± 2.4 | 57.5 ± 1 | ||
| Total intracellular glutathione (nmol/mg protein) | ||||||
| b | 11.6 ± 1.75 | 12.7 ± 1.2 | 13.5 ± 1.4 | 24.4 ± 1.1 | ||
| t | 10.0 ± 1.58 | 18.9 ± 1.9 | 19.8 ± 1.6 | 24.4 ± 1.1 | <.03 | <.005 |
| P < .01 | P < .005 | |||||
| GSH/GSSG ratio | ||||||
| b | 9.85 ± 2.80 | 8.7 ± 1.4 | 7.8 ± 1.6 | 17.7 ± 1.7 | ||
| t | 4.43 ± 0.93 | 17.5 ± 2.1 | 18.0 ± 2.2 | 17.7 ± 1.7 | <.005 | <.005 |
| P < .005 | P < .001 | |||||
Bold face indicates values for which significant changes occur.
Abbreviations: b, baseline examination; t, terminal examination.
Two of the prognostic factors, ie, plasma albumin and bcm index, showed an imbalance among the three treatment arms of study B in favor of the groups treated with IL-2 alone or with standard therapy if tested by the Kruskal Wallis rank-sum test (P < .002). The plasma cystine/thiol ratio, plasma glutamate level, and the functional capacity index, in contrast, were not significantly different between treatment groups if tested by the Wilcoxon and Kruskal Wallis rank-sum tests and the Trend test, respectively (P = .08,P = .37 and P = .09).
Bcm, plasma albumin, plasma glutamate, and the functional capacity of cancer patients are improved by treatment with NAC (study B).
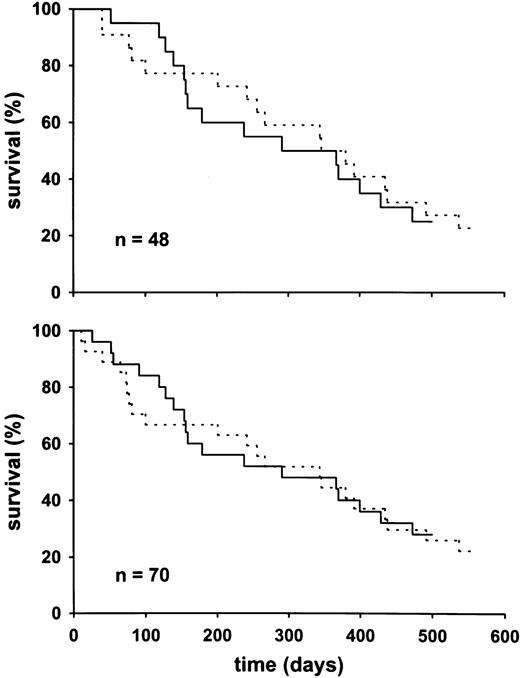
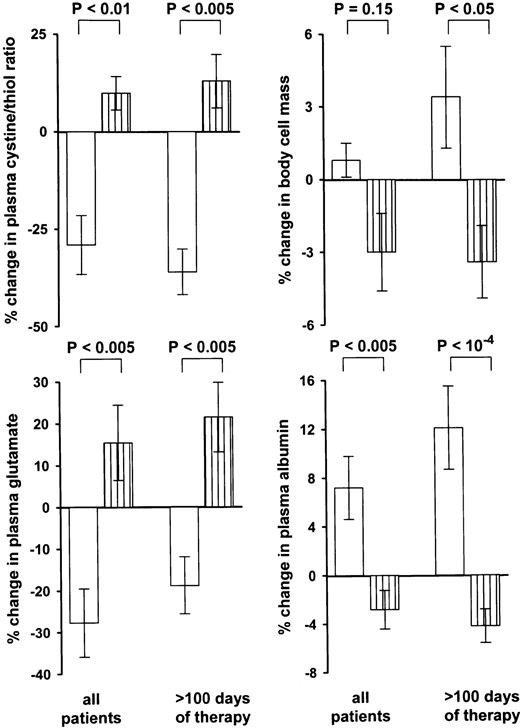
The treatment groups showed similar survival curves (Fig3). However, the IL-2 plus NAC–treated group showed a significant improvement of functional capacity, plasma albumin, plasma glutamate level, and cystine/thiol ratio, if compared with the other two treatment groups (Figs 4 and5). Also, in comparison with the individual baseline levels, the 20 IL-2 plus NAC–treated patients showed, on the average, a significant increase in plasma albumin (651 ± 13 to 696 ± 20 μmol/L;P < .03) and decrease in plasma glutamate (47.8. ± 5.1 to the essentially normal level 31.0 ± 3.6 μmol/L;P = .002) if tested by the paired t-test. The IL-2–treated group, in contrast, showed a decrease in plasma albumin (713 ± 15 to 685 ± 16;P < .02), and a slight increase in plasma glutamate (51.9 ± 5.5 to 53.2 ± 5.2). A significant increase in bcm was detectable in the IL-2 plus NAC–treated group after a lag phase. Therefore, we showed in Fig 5 the subgroup of patients with observation periods >100 days.
Survival time of the two groups treated with IL-2 plus NAC and IL-2 only (study B). (Upper panel) The survival time of the 48 patients with at least two examinations. (Lower panel) The survival curves of the entire group of 70 recruited patients. (—) The IL-2 plus NAC–treated group. (---) The combined groups treated with either IL-2 alone or standard therapy. Among the patients that were indicated as alive was one patient with the last observation point at 319 days (IL-2 plus NAC group) and one patient with the last observation at 291 days (control group).
Survival time of the two groups treated with IL-2 plus NAC and IL-2 only (study B). (Upper panel) The survival time of the 48 patients with at least two examinations. (Lower panel) The survival curves of the entire group of 70 recruited patients. (—) The IL-2 plus NAC–treated group. (---) The combined groups treated with either IL-2 alone or standard therapy. Among the patients that were indicated as alive was one patient with the last observation point at 319 days (IL-2 plus NAC group) and one patient with the last observation at 291 days (control group).
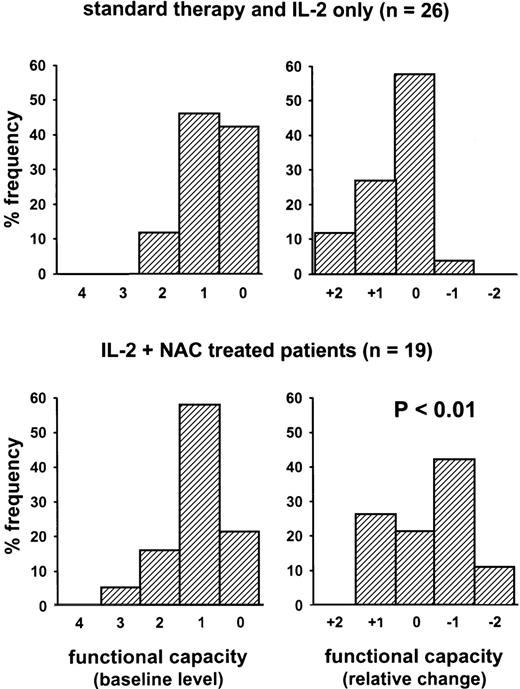
Functional capacity of cancer patients (study B). The figure shows frequency histograms. The scales on the x-axis have been reversed to account for the fact that a higher functional capacity index means a lower quality of life according to the definition by Ottery36: 0 = normal, no limitations; 1 = not normal, but able to be up with fairly normal activities; 2 = not feeling up to most things, but in bed less than half the day; 3 = able to do little activity and most of the day in bed or chair; 4 = rarely out of bed. Two patients in the IL-2–treated group did not complete the questionnaire. The changes of the functional capacity were different between the two treatment groups by the Trend test (P = .007).
Functional capacity of cancer patients (study B). The figure shows frequency histograms. The scales on the x-axis have been reversed to account for the fact that a higher functional capacity index means a lower quality of life according to the definition by Ottery36: 0 = normal, no limitations; 1 = not normal, but able to be up with fairly normal activities; 2 = not feeling up to most things, but in bed less than half the day; 3 = able to do little activity and most of the day in bed or chair; 4 = rarely out of bed. Two patients in the IL-2–treated group did not complete the questionnaire. The changes of the functional capacity were different between the two treatment groups by the Trend test (P = .007).
Effect of NAC treatment on the cystine/thiol ratio, bcm, plasma glutamate, and plasma albumin (study B). The changes in the IL-2 plus NAC–treated group (□) are shown in comparison with the combined patients of the other two treatment groups (▥). The change in bcm was statistically significant only if patients with observation periods >100 days are being compared. The changes were defined by the first and the last examination of each patient.
Effect of NAC treatment on the cystine/thiol ratio, bcm, plasma glutamate, and plasma albumin (study B). The changes in the IL-2 plus NAC–treated group (□) are shown in comparison with the combined patients of the other two treatment groups (▥). The change in bcm was statistically significant only if patients with observation periods >100 days are being compared. The changes were defined by the first and the last examination of each patient.
The data from all patients together showed a significant correlation of the change in functional capacity with the change in plasma albumin (P < .05) and the change in plasma glutamate (P < .05) if tested by Spearman's rho test. The change in albumin level, in turn, was correlated with the relative changes in bcm, plasma glutamate, and cystine/thiol ratio (r = +.61;P < .001, r = −.32; P = .05, andr = −.48, P < .02, respectively), if tested by Pearson's r-test.
The relatively low dose of IL-2 caused no increase in body water or hypotension (data not shown). The plasma levels of nitrate plus nitrite increased only in the group treated with IL-2 alone (21.5 ± 2.5v 32.6 ± 3.5 μmol/L; P < .01) but not in the group with IL-2 plus NAC (22.3 ± 3.1 v 24.6 ± 3.5 μmol/L). The analysis of 20 healthy subjects showed a concentration of 23.1 ± 1.5 μmol/L. This effect of NAC on NO2− and NO3− may be useful for any type of IL-2 therapy. A significant increase in the glutathione levels and GSH/GSSG ratios of the PBMC was found in both IL-2–treated groups but was not further increased by the additional treatment with NAC (Table 1).
Most of the adverse events in study B could be explained by the underlying malignant disease and to a lesser extent by the treatment. Mild fever has been observed about 6 to 8 hours after IL-2 injection, and patients with a daily dose of >3 g NAC complained frequently about heartburn and nausea. These symptoms disappeared after treatment with OMEPRAZOL (Astra Chemicals, D 22876, Wedel, Germany; oral doses of 20 to 40 mg/d).
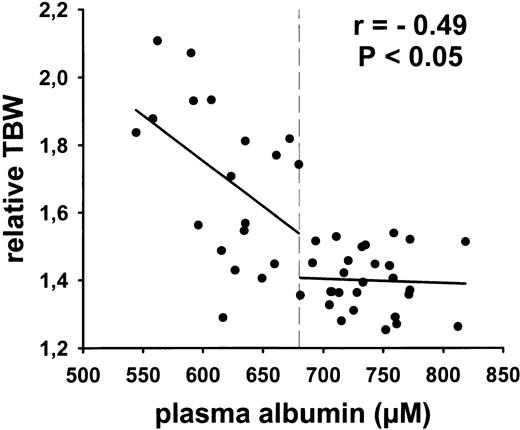
The decrease in plasma albumin below 680 μmol/L is associated with an increase in relative body water.
Because albumin plays an important role in the control of the onco-osmotic pressure and prevention of edema,10 41 we determined also the relative body water of the cancer patients as defined by the ratio of total body water per bcm. The analysis showed that the decrease in plasma albumin levels below 680 μmol/L was associated with an increase in relative total body water (Fig6).
Correlation between relative body water and plasma albumin in cancer patients (study B). Each point indicates a single person at baseline examination. The albumin cutoff level of 679.6 μmol/L (P < .001) has been computed by maximally selected two-sample tests designed to search for possible structural changes and to select an optimal cutoff value as described by Lausen et al.46 The figure indicates also the correlation coefficientr and the corresponding P value for the group of persons with albumin <680 μmol/L.
Correlation between relative body water and plasma albumin in cancer patients (study B). Each point indicates a single person at baseline examination. The albumin cutoff level of 679.6 μmol/L (P < .001) has been computed by maximally selected two-sample tests designed to search for possible structural changes and to select an optimal cutoff value as described by Lausen et al.46 The figure indicates also the correlation coefficientr and the corresponding P value for the group of persons with albumin <680 μmol/L.
Longitudinal changes in albumin are correlated with changes in the plasma cystine/thiol ratio during short observation periods (study C).
In view of the relatively slow age-dependent changes between the third and tenth decade of life (see Figs 1 and 2), we performed a longitudinal study on a single healthy individual in the sixth decade of life to determine whether a correlation between longitudinal changes in plasma albumin level and plasma cystine/thiol ratio may be demonstrable also in a healthy person within a relatively short observation period. Plasma cystine/thiol ratios, albumin levels, and bcm were determined at 39 randomly chosen time points during a 2-year observation period. The resulting data (not shown in detail) showed considerable variations in cystine/thiol ratios (3.3 to 9.4) and albumin levels (684 to 884 μmol/L) and showed significant correlations (1) between the albumin level and plasma cystine/thiol ratio (r = −.61; P < 10−4) and (2) between the changes in the albumin level and corresponding changes in the cystine/thiol ratio (r = −.53;P < 10−3). However, neither of the two was significantly correlated with the corresponding changes in bcm in this study.
NAC causes an increase in bcm but not in intracellular glutathione of healthy volunteers with high plasma cystine/thiol ratio (study D).
To obtain additional evidence for a cause/effect relationship between redox state and bcm in healthy subjects, we investigated in a placebo-controlled study the effect of NAC treatment in the context of the endogenous cystine/thiol ratio on the change in bcm. The healthy volunteers were additionally subjected to a program of anaerobic physical exercise to generate a condition similar to that of cancer patients who are known to express a high rate of glycolytic activity in muscle tissue.42 (Another reason for this study design was that physical exercise has been considered as a therapeutic tool to increase body cell mass17,43 and that strong physical exercise was shown to cause the oxidation of glutathione in the blood.44,45 This oxidation was previously shown to be ameliorated by treatment with NAC.45)
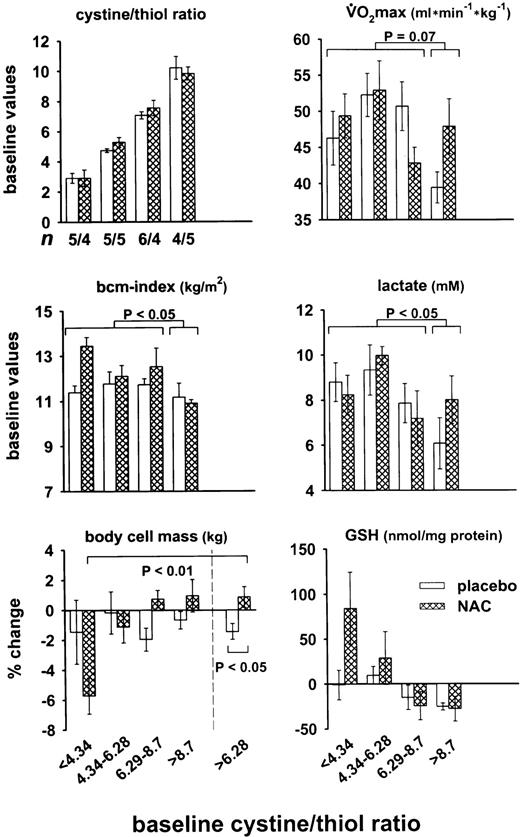
When the group of 38 volunteers of study D was divided into quartiles according to their cystine/thiol ratio at baseline examination, it was seen that persons with a cystine/thiol ratio > 8.7 had a significantly lower VO2max, lower lactate-producing capacity, and, in line with the correlation in Fig 2, a lower bcm index than the rest of the group (Fig 7, upper panels). Using maximally selected two-sample tests to search for possible structural changes and to select an optimal cutoff value46 we obtained a cystine/thiol cutoff ratio of 8.2 (P = .07) for the correlation with VO2max, again 8.2 (P = .03) for the correlation with the bcm index, and 7.2 (P < .02) for the correlation with the lactate level. Treatment with NAC induced in comparison with the placebo group a relative increase in bcm in persons with baseline cystine/thiol ratio >6.28 (ie, >median). For this group, ie, for the upper two quartiles together, the relative increase in bcm was statistically significant (P < .05). However, in line with the results of Table 1, NAC caused in persons with cystine/thiol ratios > 6.28, on the average, no increase in the intracellular GSH level of PBMC.
Baseline performance and response to NAC treatment of healthy subjects with different plasma redox states (study D). The design of this study has been described in detail.37 The total group of 38 volunteers has been subdivided into quartiles according to their baseline cystine/thiol ratio (upper left panel). The data for VO2max, bcm index, and plasma lactate after exercise to exhaustion (see ref 37) indicate the mean ± SEM of the two treatment groups in the four quartiles. The lower two panels show the relative changes of bcm and total intracellular glutathione in PBMC during the 5-week observation period including 4-week anaerobic physical exercise and treatment with NAC or placebo. The significance between the indicated groups has been determined by the t-test for independent samples.
Baseline performance and response to NAC treatment of healthy subjects with different plasma redox states (study D). The design of this study has been described in detail.37 The total group of 38 volunteers has been subdivided into quartiles according to their baseline cystine/thiol ratio (upper left panel). The data for VO2max, bcm index, and plasma lactate after exercise to exhaustion (see ref 37) indicate the mean ± SEM of the two treatment groups in the four quartiles. The lower two panels show the relative changes of bcm and total intracellular glutathione in PBMC during the 5-week observation period including 4-week anaerobic physical exercise and treatment with NAC or placebo. The significance between the indicated groups has been determined by the t-test for independent samples.
DISCUSSION
Taken together, our studies show a substantial change in the plasma thiol/disulfide redox state in human senescence and wasting and suggest strongly that this change may be a causative factor and a potential target for therapeutic intervention. The weight of the evidence for a cause/effect relationship is mainly based on the effects of NAC on the bcm in the two independent studies B and D. It is additionally supported by the correlation between bcm index and redox state in the two independent studies A and B. Treatment of the cancer patients with IL-2 plus NAC improved the functional capacity, bcm, albumin level, and the glutamate level. It must be emphasized that the changes in bcm in Figs 5 and 7 are based on the differences between longitudinal impedance measurements and body mass data of individual subjects. In view of the relative constant geometry of the individual subject, intraindividual differences in bcm can be determined with much greater precision than interindividual differences.37 Moreover, the oxidative metabolic capacity (VO2max) and the capacity to produce lactate (which is linked to VO2max47) were significantly lower in healthy persons with high cystine/thiol ratio than in persons with lower cystine/thiol ratio (Fig 7). Our data suggest (1) that a ratio >8.2 which corresponds, according to Fig 2, to an albumin level of about 680 μmol/L may be a risk factor for loss of bcm and muscle function even among otherwise healthy persons, and (2) that persons with a ratio >6.3 may already benefit from treatment with a thiol-containing antioxidant. Persons with a ratio <6.3, in contrast, did not appear to benefit from NAC-treatment, and persons with a ratio <4.34 showed even a negative effect of NAC. This negative effect was not statistically significant, but in combination with the profile in the upper right panel of Fig 7, it suggested the possibility that the cystine/thiol ratio of approximately 4.3 to 6.3 may be superior to both higher and lower cystine/thiol ratios. These findings may provide a guideline for prophylactic redox-oriented therapy.
The strong correlation between the plasma cystine/thiol ratio and albumin level in three independent studies, and the effect of NAC on the albumin level (Fig 5) are again strong indications for a cause/effect relationship. It may be noted that the cystine/thiol ratio of the young and old healthy subjects is approximately 4 and 8, respectively (Fig 1), whereas the ratio of oxidized/reduced albumin was reported to be approximately 0.3 and 1, respectively,31indicating that the plasma cystine/thiol ratio is approximately 10-fold higher than the ratio of oxidized albumin/reduced albumin. The plasma albumin level may be limited, in principle, either by the rate of albumin degradation or by the rate of hepatic albumin biosynthesis. NAC was previously shown to improve liver cell functions in patients with paracetamol intoxication, ie, in persons with a strong hepatic glutathione deficiency,48 but there is no evidence that the patients and healthy subjects in our present studies had a priori abnormal hepatic glutathione levels. Nevertheless, the possibility that NAC may increase the albumin level by enhancing the rate of hepatic albumin biosynthesis rather than by decreasing its rate of degradation has not been formally excluded.
The effect of NAC treatment on the albumin level is particularly important in view of earlier unsuccessful attempts to improve the albumin level by nutritional therapy14,25-27 and because the albumin level is a strong predictor of hospital survival and cost of hospitalization.14 One of the important functions of albumin is the maintenance of the colloid oncotic pressure and prevention of edema.10,14,26,41 We found that a decrease in plasma albumin below 680 μmol/L was associated with a strong increase in body water. The biphasic concentration dependency (Fig 6) resembles the correlation between blood pressure and albumin concentration.49 However, it may be an oversimplification to assume that the linkage between redox state and bcm or functional capacity is entirely mediated by changes in the albumin level. Numerous proteins appear to be tagged for degradation by oxidation,50 and changes of the extracellular redox state may also induce intracellular changes, including the induction of immediate/early genes.51
Unexpectedly, the albumin level, bcm, and functional capacity were significantly increased by NAC in the absence of detectable changes in the glutathione status (studies B and D). The increase in the intracellular glutathione level in response to IL-2 treatment alone (Table 1) was in line with earlier studies in vitro,52 but was not accompanied by an improvement of other biochemical or clinical parameters. The additional treatment with NAC, in contrast, caused an improvement of several clinically relevant parameters but no additional increase in the glutathione level or GSH/GSSG ratio (Table 1). The observation that NAC failed to increase the intracellular glutathione level or GSH/GSSG ratio despite its significant effects on the thiol redox state in the plasma (Fig 5, upper left panel) is puzzling but is consistent with earlier studies from other laboratories. An increase in intracellular glutathione levels by NAC was previously seen only in the liver of patients with paracetamol intoxication, ie, in persons with a strong hepatic glutathione deficiency,48 but not in other cell types and/or other conditions (see Fig7).53 54
NAC has been proposed as a drug for cancer prevention.55 A direct antitumoral activity has been shown in mice56 but not in humans. NAC has also been proposed for the treatment of HIV infection with the aim to reconstitute the abnormally low plasma cystine, glutamine, and arginine levels.35,57,58 In contrast to cancer patients and elderly subjects, HIV-infected persons frequently have cystine/thiol ratios that are lower than normal (H.P. Eck, R. Breitkreutz, W. Dröge, unpublished observation, 1988-1998). These relatively reducing conditions in the plasma of HIV-infected persons may result from the increase in the plasma level of thioredoxin that has been reported by others and which may be induced by IL-6.59
The positive effect of NAC on the bcm and functional activity of the cancer patients (study B) was not associated with improved survival (Fig 3). However, cachexia is often a limiting factor in standard chemotherapy, and any treatment that ameliorates the wasting syndrome may possibly enable the physician to apply a more aggressive chemotherapy. Therefore, this report may be useful for the design of new clinical trials. However, it may be important to design individually guided doses of NAC (or related drugs) according to the individual needs. NAC is generally described as a relatively safe drug,52 55 but should be used with caution.
ACKNOWLEDGMENT
The statistical advice of Dr L. Edler for the design of the randomized trials, and the technical assistance of N. Erbe and M. Schykowski and the assistance of I. Fryson in the preparation of this manuscript are gratefully acknowledged.
Address reprint requests to Wulf Dröge, PhD, Division of Immunochemistry, Deutsches Krebsforschungszentrum, Im Neuenheimer Feld 280, D-69120 Heidelberg, Germany.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal