The chemokine receptor CCR5 can function as a coreceptor for human immunodeficiency virus-1 (HIV-1) entry into CD4+ T cells and macrophages, especially during the early stages of HIV-1 infection. The regulation of CCR5 expression may affect not only leukocyte migration, but also infectivity by HIV-1 and, therefore, acquired immunodeficiency syndrome (AIDS) pathogenesis. We report here that agents which increase intracellular concentrations of cyclic adenosine monophosphate (cAMP) rapidly downregulate CCR5 gene expression, with consequent loss of CCR5 expression and function in monocytes/macrophages. Chemotaxis and intracellular Ca2+mobilization in monocytes pretreated with prostaglandin E2or dibutyryl-cAMP for 24 hours were significantly reduced in response to the CCR5 ligand, MIP-1β. Moreover, HIV-1 entry into monocyte-derived macrophages pretreated with dibutyryl-cAMP or prostaglandin E2 was markedly decreased. Our findings suggest that resistance to HIV-1 can be induced by agents which increase cellular levels of cAMP and that this may suggest additional therapeutic strategies to limit infection by HIV-1.
SEVERAL CHEMOKINE RECEPTORS have been shown to act as coreceptors for human immunodeficiency virus-1 (HIV-1) entry into cells of different lineages.1-6 CCR5, the receptor for RANTES, MIP-1α, and MIP-1β, is expressed in primary monocytes and macrophages, primary T cells, and granulocyte precursors.2,4 Entry of macrophage-tropic (M-tropic) HIV-1 isolates is primarily mediated by CCR5, although CCR3 and CCR2b can also participate in some cells, but to a lesser extent.2-6Individuals with mutations of CCR5 that affect cell-surface expression show resistance to HIV-1 infection.7,8 In this context, modulation of CCR5 expression could affect not only leukocyte migration, but also infectivity by HIV and the pathogenesis of acquired immunodeficiency syndrome (AIDS). This postulate is supported by in vitro studies showing that levels of CCR5 expression correlate with susceptibility to infection with M-tropic isolates of HIV-1.9
Cyclic adenosine monophosphate (cAMP)-mediated regulation of receptor function has been investigated in various receptor systems and was shown to differentially modulate the expression of receptors for the chemotactic factors PAF, C5a, and formyl-methionyl-leucyl-phenylalanine (fMLP). Consequently, cell-permeable cAMP analogs and others cAMP agonists upregulate the expression of the chemotactic factor C5a receptor,10 11 whereas they downregulate the PAF12 receptor and minimally inhibit the FMLP receptor (our unpublished observations, 1997). In the present study, we investigated the regulation of CCR5 surface expression in human monocytes by cAMP-elevating agents.
MATERIALS AND METHODS
Isolation and Stimulation of Monocytes
Monocytes were obtained from peripheral blood (PB) of healthy volunteers. PB mononuclear leukocytes were enriched by dextran sedimentation and collected at the interface of a Ficoll-Hypaque cushion (Pharmacia, Baie d'Urfe, QC, Canada). Mononuclear cells at the Ficoll interface were collected and monocytes were then purified by adherence on plastic Petri dishes coated with defibrinized autologous serum. Cells were resuspended in RPMI 1640 medium (GIBCO-BRL, Burlington, Ontario, Canada) containing 10% fetal bovine serum (FBS). For RNA studies only, they were allowed to rest overnight before stimulation with the appropriate stimuli. Mono-Mac-1 cells were obtained from the German Collection of Microorganisms and Cell Cultures (Braunschweig, Germany) and maintainted in RPMI 1640 medium, 10% FBS, nonessential amino acids, and 1 mmol/L Na-pyruvate.
Northern Blot Analysis
Total RNA was isolated by acid guanidium thiocyanate-phenol-chloroform extraction,13 separated by electrophoresis on 1% agarose and transferred onto a Hybond-N (Amersham, Oakville, Ontario, Canada) membrane for Northern analysis. The cDNA corresponding to whole coding sequence of human CCR5 was amplified by polymerase chain reaction from genomic DNA of Raji cells using the primers 5′-GCTCTAGAGATTATCAAGTGTC-3′ (sense), and 5′-GGGGTACCTCACAAGCCCACAGATATTTCCTGCTCCCC-3′ (antisense). Control hybridizations were performed with the human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) cDNA probe obtained from the American Type Culture Collection (Rockville, MD). The probes were labeled with a multiprime DNA labeling system (Amersham) using [α32P] dCTP (specific activity, >3,000 Ci/mmol; Amersham). Membranes were prehybridized for 4 hours in a mixture containing 120 mmol/L Tris, 600 mmol/L NaCl, 8 mmol/L EDTA, 0.1% sodium pyrophosphate (NaPP), 0.2% sodium dodecyl sulfate (SDS), and 100 μg/mL heparin; hybridization was performed overnight at 68°C in the same mixture in which the concentration of heparin was increased to 625 μg/mL and dextran sulfate at 10% was added. The membranes were then washed once at room temperature for 20 minutes in 2× SSC (1× SSC: 0.15 mol/L NaCl, 0.15 mol/L sodium citrate, pH 7); once with 0.1× SSC, 0.1% SDS at 68°C for 60 minutes, and then rinsed at room temperature with 0.1× SSC. The membranes were exposed to Kodak XAR-5 film (Eastman Kodak, Rochester, NY) with intensifying screens for 24 hours at −80°C. Densitometric analysis of the autoradiograms was performed and expressed as ratios of CCR5/GAPDH.
Calcium fluorimetry.
For Ca2+-mobilization assays, 3 × 106cells were loaded in Hanks' balanced salt solution (HBSS) (GIBCO-BRL) containing 350 mg/L NaHCO3 and 10 mmol/L HEPES, pH 7.0, with the calcium indicator Fura-2 AM (Molecular Probes, Eugene, OR) for 30 minutes at room temperature. Loaded cells were washed twice, suspended in fresh loading buffer, and added to a constantly stirred cuvette in a SLM/Aminco spectrofluorimeter (SLM Instruments, Urbana, IL). The concentration of Ca2+ was brought to 1.5 mmol/L by adding a solution of CaCl2 into the cuvette 10 minutes before recordings. Maximal fluorescence (Fmax) was obtained by adding Triton X-100 (Sigma, St Louis, MO) to a final concentration of 0.5%. Minimal fluorescence (Fmin) was determined by subsequent addition of EGTA, in Tris.HCl buffer (100 mmol/L, pH 9.0) to 125 mmol/L. Stimuli consisted of MIP-1β (Pepro Tech Inc, Rocky Hill, NJ) and fMLP (Sigma).
Chemotaxis assay.
Monocyte chemotactic activity was performed with Boyden chambers using a modified Boyden-chamber chemotaxis assay. MIP-1β or control medium were added to the lower chamber and 200 μL of monocytes (6 × 105) in Gey's BSS (GIBCO-BRL) suplemented with 2% bovine serum albumin was added to the upper chamber. The two chambers were separated by a 5-μm pore size polycarbonate filter (Neuroprobe, Cabin John, MD). After incubation for 2 hours, the filter was disassembled and the upper side of the filter was scraped free of cells. Cells on the lower side were removed with EDTA 5 mmol/L and centrifuged before counting by FACScan (Becton Dickinson, San Jose, CA) analysis with scatter-gating on monocytes. The results were then converted to a chemotaxis index (mean number of cells migrating to a specific stimulus/mean number of cells migrating to control medium). The statistical significance of the chemotactic indices of cells migrating in response to MIP-1β versus medium control was evaluated using the Student's t-test.
Flow cytometry.
The expression of CCR5 on the surface of monocytes was monitored with the monoclonal anti-CCR5 antibody (2D7) provided by the AIDS Research and Reference Reagent Program (Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD). In brief, 3 × 105 treated cells were washed twice with phosphate-buffered saline (PBS) and labeled 30 minutes at 4°C with the antibody. Cells were then washed with cold PBS and incubated for 30 minutes at 4°C with fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgG (Bio/Can Scientific, Mississauga, Onatario, Canada). Finally, cells were washed in PBS and resuspended in PBS before flow cytometry analysis with a FACScan flow cytometer (Becton Dickinson).
Confocal microscopy.
Monocytes pretreated with medium or prostaglandin E2(PGE2) for 48 hours were treated for 30 minutes with either medium or MIP-1β (100 ng/mL) and fixed with 4% paraformaldehyde (15 minutes at room temperature [RT]). They were then placed in 0.1% Triton (20 minutes at RT), then sequentially incubated with 5% milk (30 minutes at RT) and 0.1% glycine (60 minutes at RT). The cells were then incubated with monoclonal anti-CCR5 antibody (2D7) followed with rhodamine-conjugated goat anti-mouse antibodies (Bio/Can Scientific). The cells were then analyzed on a Molecular Dynamics (Sunnyvale, CA) Multi Probe 2001 confocal argon laser scanning system equipped with a Nikon (Molecular Dynamics) Diaphot epifluorescence inverted microscope. Scanned images were transferred onto a Silicon Graphics (Molecular Dynamics) Indy 4000 workstation equipped with Molecular Dynamics' Imagespace analysis software.
HIV-1 infection of cells.
Monocytes (5 × 105) were maintained in culture in 48-well plate (Costar, Cambridge, MA) to allow differentiation into macrophages. On day 5 of culture, cells (monocytes and Mono Mac-1) were treated for 48 hours with PGE2, dibutyryl-cAMP (dBcAMP; Sigma), or medium before exposure to the M-tropic strains HIV-1Ada-M and HIV-1JR-FL (25 ng of p24). HIV-1 isolates were allowed to adsorb for 2 hours at 37°C before complete aspiration of medium, washing, and addition of fresh medium. After a 96-hour incubation period, luciferase activity was monitored as described previously.14 15 Virus stocks were generated by transfecting 293T cells with 5 μg each of pNL4-3-Luc-R+E− and pCDNA-I/Amp-based expression vectors encoding Ada-M and JR-FL. Both plasmids were kind gifts from N.R. Landau (Aaron Diamond AIDS Research Center, New York, NY).
RESULTS
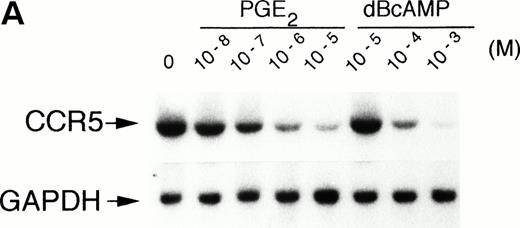
We examined the expression of CCR5 mRNA in monocytes exposed 4 hours to graded concentrations of the cell-permeable cAMP analog, dBcAMP. As shown in Fig 1A, primary human monocytes constitutively expressed high levels of CCR5 mRNA, which was significantly reduced by more than 90% with concentrations of dBcAMP as low as 10−4 mol/L. Many autacoids and hormones affect cellular functions by modulating intracellular levels of cAMP. Among them, prostaglandins (PG) of the E series (ie, PGE2) increase [cAMP]i by activating adenylate cyclase, following their interaction with EP4 receptors on leukocytes.16 A similar effect is seen after interaction of β2 adrenergic agonists with their respective receptors.17 Interestingly, PGE2-treatment had an important downregulatory effect on the steady-state level of CCR5 mRNA (Fig 1A). Accumulation of the transcripts was downregulated in a concentration-dependent manner being evident at 10−8 mol/L (25% reduction) and almost completely inhibited at 10−5 mol/L (>95% reduction). Kinetic studies showed that the PGE2-induced reduction of CCR5 expression was time-dependent and rapid, being evident as early as 2 hours of incubation (Fig 1B). This downregulation with PGE2 was still noticeable more than 48 hours posttreatment (data not shown). The mRNA half-life of CCR5 in human monocytes is approximately 80 minutes and PGE2 reduced it by 50% (Fig 1C), suggesting that at least part of CCR5 mRNA downregulation occurred at a posttranscriptional level.
Effect of PGE2 and dBcAMP on CCR5 mRNA expression. (A) Human monocytes were incubated for 4 hours with graded concentrations of PGE2 or dBcAMP and total RNA was extracted, separated, and analyzed by Northern blotting for CCR5 mRNA expression. (B) Kinetics of PGE2 downregulation of CCR5 gene expression. Cells were incubated in the absence or presence of PGE2 10−5 mol/L for the indicated time periods followed by Northern blot analysis of CCR5 mRNA expression. (C) Effect of PGE2 on CCR5 mRNA stability. Monocytes were incubated 1 hour with medium or PGE2 10−5mol/L before mRNA synthesis was stopped by the addition of actinomycin D (5 μg/mL). At the indicated times thereafter, total RNA was prepared and analyzed by Northern blot. The figure illustrates the autoradiogram of one representative experiment with percentages of remaining CCR5 mRNA relative to the values at time 0, corrected for corresponding GAPDH values. n = 2 to 4 independent experiments.
Effect of PGE2 and dBcAMP on CCR5 mRNA expression. (A) Human monocytes were incubated for 4 hours with graded concentrations of PGE2 or dBcAMP and total RNA was extracted, separated, and analyzed by Northern blotting for CCR5 mRNA expression. (B) Kinetics of PGE2 downregulation of CCR5 gene expression. Cells were incubated in the absence or presence of PGE2 10−5 mol/L for the indicated time periods followed by Northern blot analysis of CCR5 mRNA expression. (C) Effect of PGE2 on CCR5 mRNA stability. Monocytes were incubated 1 hour with medium or PGE2 10−5mol/L before mRNA synthesis was stopped by the addition of actinomycin D (5 μg/mL). At the indicated times thereafter, total RNA was prepared and analyzed by Northern blot. The figure illustrates the autoradiogram of one representative experiment with percentages of remaining CCR5 mRNA relative to the values at time 0, corrected for corresponding GAPDH values. n = 2 to 4 independent experiments.
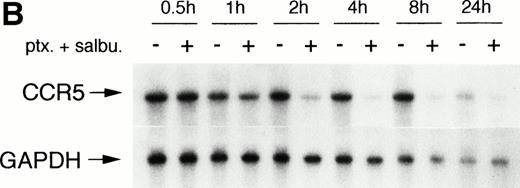
Increasing intracellular cAMP levels with the xanthine phosphodiesterase inhibitor pentoxifylline was also effective in reducing CCR5 mRNA expression (Fig 2A). Whereas the β2 adrenergic agonist, salbutamol, was only marginally effective by itself, it could synergize with pentoxifylline and abrogate CCR5 mRNA expression by greater than 90%. Again the effect was time-dependent, being maximal at 4 hours and persisting for at least 24 hours (Fig 2B).
Modulation of CCR5 mRNA expression by salbutamol and/or pentoxifylline. (A) Monocytes were incubated for 4 hours with medium, salbutamol, pentoxifylline, or both drugs. Northern blot analysis of CCR5 mRNA expression is illustrated. (B) Cells were incubated with medium or the combination of pentoxifylline (ptx) and salbutamol (salbu) for the indicated time periods and CCR5 mRNA expression was analyzed by Northern blot. n = 2 to 4 independent experiments.
Modulation of CCR5 mRNA expression by salbutamol and/or pentoxifylline. (A) Monocytes were incubated for 4 hours with medium, salbutamol, pentoxifylline, or both drugs. Northern blot analysis of CCR5 mRNA expression is illustrated. (B) Cells were incubated with medium or the combination of pentoxifylline (ptx) and salbutamol (salbu) for the indicated time periods and CCR5 mRNA expression was analyzed by Northern blot. n = 2 to 4 independent experiments.
To examine whether the reduction of CCR5 mRNA expression induced by cAMP elevating agents was paralleled by a concomitant downregulation of surface CCR5 expression, we performed flow cytometry studies using a monoclonal anti-CCR5 antibody (Fig 3A). Pretreatment of monocytes with PGE2 for 48 hours showed more than 55% reduction of CCR5 protein expression (mean fluorescence PGE2-treated: 56, v untreated cells: 123). Treatment with dBcAMP or pentoxifylline in combination with salbutamol showed a similar decrease of CCR5 surface expression (data not shown). In contrast, treatment of CD4+ T cells with dBcAMP or PGE2 had no significant effect on their expression of CCR5 (control: 9.6% ± 4.2%; dBcAMP: 8.4% ± 2.1%; PGE2: 10.6% ± 2.7%; data not illustrated). To distinguish between reduction in total CCR5 protein expression and potential receptor internalization, cells were examined using laser confocal microscopy (Fig 3B). Pretreatment of monocytes with PGE2 for 48 hours markedly reduced CCR5 expression, which remained nevertheless at the plasma membrane level (left and middle panels); in comparison, monocytes incubated for 48 hours in medium and then stimulated for 30 minutes with MIP-1β (100 ng/mL) showed evident internalization of CCR5 (right panel).
CCR5 protein expression on monocytes. (A) Flow cytometry analysis: Monocytes were incubated for 48 hours with medium or PGE2 (10−5 mol/L) before labeling either with FITC-conjugated goat anti-mouse antibody alone (dotted line) or with anti-CCR5 (2D7) antibody followed by FITC-conjugated goat anti-mouse antibody (untreated cells: thick line; PGE2-treated cells: thin line). Representative experiment of eight independent experiments. (B) Laser confocal microscopy: Monocytes were incubated for 48 hours with medium (left) or PGE2 (10−5 mol/L) (middle) before permeabilization and labeling with anti-CCR5 (2D7) antibody followed by rhodamine-conjugated goat anti-mouse antibody. For comparison, monocytes incubated with medium for 48 hours were stimulated with MIP-1β (100 ng/mL) for 30 minutes before labeling, to illustrate CCR5 internalization (right).
CCR5 protein expression on monocytes. (A) Flow cytometry analysis: Monocytes were incubated for 48 hours with medium or PGE2 (10−5 mol/L) before labeling either with FITC-conjugated goat anti-mouse antibody alone (dotted line) or with anti-CCR5 (2D7) antibody followed by FITC-conjugated goat anti-mouse antibody (untreated cells: thick line; PGE2-treated cells: thin line). Representative experiment of eight independent experiments. (B) Laser confocal microscopy: Monocytes were incubated for 48 hours with medium (left) or PGE2 (10−5 mol/L) (middle) before permeabilization and labeling with anti-CCR5 (2D7) antibody followed by rhodamine-conjugated goat anti-mouse antibody. For comparison, monocytes incubated with medium for 48 hours were stimulated with MIP-1β (100 ng/mL) for 30 minutes before labeling, to illustrate CCR5 internalization (right).
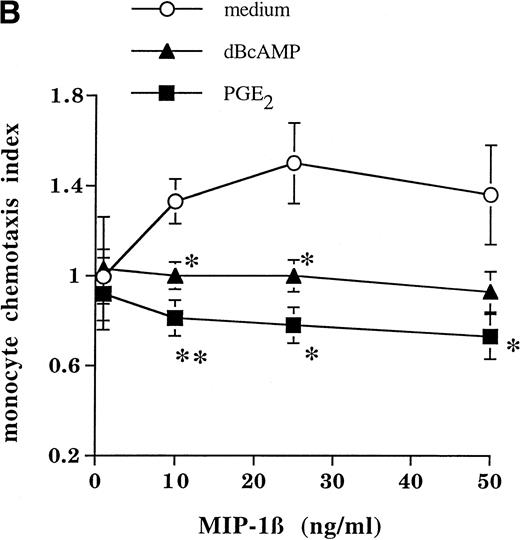
Functional downregulation of CCR5 by cAMP-augmenting agents was also observed in monocytes in terms of their responses to the CCR5 ligand MIP-1β. As shown in Fig 4, pretreatment of monocytes with PGE2 or dBcAMP for 24 hours almost totally abrogated their responsiveness to MIP-1β (but not to the chemotactic tripeptide fMLP) in terms of [Ca2+]i flux (Fig 4A). Chemotaxis of monocytes in response to MIP-1β was also significantly reduced by preexposure to PGE2 or dBcAMP (Fig 4B).
Mobilization of Ca2+ and chemotaxis of monocytes. (A) Cells were incubated for 24 hours in the absence or presence of PGE2 (10−5 mol/L) or dBcAMP (10−-4 mol/L), washed, and loaded with Fura-2 AM; increases in intracellular Ca2+ upon addition of MIP-1β (100 ng/mL) were measured with a fluorescence spectrophotometer. (B) Chemotactic activity of MIP-1β in a modified Boyden-chamber chemotaxis assay. Data are expressed as means ± SEM of three or more experiments. Statistically significant differences between control and treated cells are indicated (*P < .05, ** P < .02, unpaired t-test; n = 3 to 6 independent experiments).
Mobilization of Ca2+ and chemotaxis of monocytes. (A) Cells were incubated for 24 hours in the absence or presence of PGE2 (10−5 mol/L) or dBcAMP (10−-4 mol/L), washed, and loaded with Fura-2 AM; increases in intracellular Ca2+ upon addition of MIP-1β (100 ng/mL) were measured with a fluorescence spectrophotometer. (B) Chemotactic activity of MIP-1β in a modified Boyden-chamber chemotaxis assay. Data are expressed as means ± SEM of three or more experiments. Statistically significant differences between control and treated cells are indicated (*P < .05, ** P < .02, unpaired t-test; n = 3 to 6 independent experiments).
Finally, we tested whether CCR5 downregulation with cAMP-elevating agents results in a concomitant diminished susceptibility to HIV-1 infection. Monocyte-derived macrophages (Fig 5A) and Mono-Mac-1 cells (Fig 5B) treated for 48 hours with PGE2 (10−5mol/L) or dBcAMP (10−4 mol/L) before virus inoculation showed a drastic reduction in their susceptibility to infection with recombinant luciferase-encoding virions pseudotyped with Env proteins from M-tropic strains HIV-1Ada-M and HIV-1JR-FL. PGE2 and dBcAMP reduced macrophage infectivity by HIV-1Ada-M by 92% and 88%, respectively, and MonoMac 1 cell infectivity by 94% and 71%, respectively. Infectivity by HIV-1JR-FL was reduced by 91% and 48%, respectively, in macrophages, and by 97% and 86%, respectively, in MonoMac 1 cells.
Susceptibility of cells to infection by M-tropic HIV-1 isolates. Recombinant luciferase-encoding HIV-1 particles pseudotyped with Ada-M and JR-FL Env proteins were used to infect monocyte-derived macrophages (A) or Mono-Mac-1 cells (B) after a 48-hour pretreatment with PGE2 (10−5 mol/L) or dBcAMP (10−4 mol/L). Luciferase activity was quantitated 96 hours after initiation of virus infection.
Susceptibility of cells to infection by M-tropic HIV-1 isolates. Recombinant luciferase-encoding HIV-1 particles pseudotyped with Ada-M and JR-FL Env proteins were used to infect monocyte-derived macrophages (A) or Mono-Mac-1 cells (B) after a 48-hour pretreatment with PGE2 (10−5 mol/L) or dBcAMP (10−4 mol/L). Luciferase activity was quantitated 96 hours after initiation of virus infection.
DISCUSSION
The fact that cAMP-augmenting agents can markedly reduce CCR5 expression on monocytes/macrophages has potential implications in inflammation as well as in susceptibility to HIV-1 infection. Firstly, chemotaxis of monocytes in response to MIP-1β, and possibly to other less selective CCR5 agonists, is significantly impaired in cells exposed to a number of cAMP-augmenting agents. However, this is not a universal phenomenon because receptors for other chemoattractants, such as C5a or fMLP, are not affected in the same manner. In fact, C5aR expression is upregulated by elevation of [cAMP]i, whereas fMLPR expression is minimally affected by cAMP levels. In contrast, PAFR expression is affected in the same direction as that of CCR5, but its downregulation is predominantly transcriptional.12
Secondly, the ability of cAMP-augmenting agents to downregulate CCR5 expression in monocyte-derived macrophages was also found to markedly impair the capacity of M-tropic HIV-1 isolates to infect treated cells. Such a downregulation of an HIV coreceptor expression appears to reproduce the resistance to infection by HIV-1 found in cells from “naturally” resistant individuals bearing mutations in the CCR5 molecule. Whether our in vitro findings can be transposed in vivo remains to be tested. It should be noted that elevated levels of PGE2 have been reported in individuals infected with HIV-1, which is of interest in this regard.18 19
Although blocking of HIV infectivity by chemokines and chemokine antagonists is being intensely investigated, the potential for inhibition of HIV-1 infection by downregulation of its coreceptor(s) remains to be explored. Understanding the regulation of HIV-1 coreceptors will help to elucidate the interplay between these receptors and HIV-1 in the pathogenesis of AIDS. In a recent study, downregulation of CCR5 expression was shown in CD4+ T cells after stimulation with anti-CD28 antibody, with associated resistance to M-tropic HIV-1 infection.20 In contrast, activation of T cells21 and natural killer cells22 with interleukin-2 (IL-2) led to enhanced CCR5 and CCR2b expression, respectively. On the other hand, the expression of CCR2 in human monocytes23 and in the THP-1 monocytic cell line24 was found to be downregulated by lipopolysaccharide and the cytokines interferon-γ, tumor necrosis factor-α, and IL-1β. Thus, it appears of interest to target chemokine receptor expression, as an alternative to receptor occupancy, to inhibit HIV infection as well as to modulate inflammatory processes.
Approximately 10% of CCR5 alleles among European and North American whites contain a 32-bp deletion (ccr5Δ32) which prevents normal expression of CCR5.7 8 The observed protection from HIV-1 infection in individuals homozygous for the deletion provides support for the potential value of therapeutic strategies that target CCR5-HIV interactions. Furthermore, because the ccr5Δ32 genotype is not associated with any obvious phenotype affecting fitness or survival in the population, therapeutic strategies designed to interfere with CCR5 expression are likely not to have deleterious effects on the host.
ACKNOWLEDGMENT
We thank S. Turcotte and D. Gingras for technical assistance.
Supported by grants to M.J.T., J.S., and M.R.-P. from the Medical Research Council of Canada. M.J.T. holds a scholarship award from the Fonds de la Recherche en Santé du Québec.
Address reprint requests to Marek Rola-Pleszczynski, MD, Immunology Division, Department of Pediatrics, Faculty of Medicine, Université de Sherbrooke, 3001, N 12th Ave, Sherbrooke (QC), Canada, J1H 5N4; e-mail: mrolaple@courrier.usherb.ca.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.








This feature is available to Subscribers Only
Sign In or Create an Account Close Modal