Ferrochelatase catalyzes the chelation of ferrous iron and protoporphyrin to form heme. It is expressed as a housekeeping gene in all cells, but is upregulated during erythropoiesis. Ferrochelatase activity is deficient in the inherited disease protoporphyria as a result of heterogeneous mutations. Although human ferrochelatase is transcribed from a single promoter in both nonerythroid and erythroid cells, previous studies using transient transfection assays failed to demonstrate erythroid-specific increased expression from 4.0 kb of the human ferrochelatase promoter containing the erythroidcis-elements, GATA and NF-E2. The present study analyzes the in vivo regulation of the ferrochelatase gene to provide insights into the mechanism of its erythroid-specific enhancement. Transgenic (TG) mouse lines were generated in which the luciferase reporter gene was driven by either a 150-bp ferrochelatase minimal promoter (−0.15 TG) or by a 4.0 kb extended 5′ upstream region (−4.0 TG). Expression of the −4.0 TG transgene was generally consistent with the endogenous gene during embryonic development and in nonerythroid and erythroid tissues as demonstrated by Northern blotting and mRNA in situ hybridization. The −4.0 TG was expressed at a higher level than the −0.15 TG in nonerythroid and erythroid tissues, including during extramedullary erythropoiesis induced by n-acetylphenylhydrazine injection. The enhanced erythroid expression of the −4.0 TG correlates with the appearance of a DNase I hypersensitive site in the 5′ flanking region of the transgene. Therefore, in the context of chromosomal integration, the 5′ flanking region of the ferrochelatase gene is necessary and sufficient to confer high levels of transgene expression in erythroid tissue.
FERROCHELATASE (protoheme ferro-lyase, EC 4.99.1.1), the last enzyme in the heme biosynthetic pathway, catalyzes the chelation of ferrous iron and protoporphyrin to form heme. Ferrochelatase activity is required in all cells to synthesize heme used as a cofactor in a variety of proteins, including respiratory cytochromes,1 and is required at higher levels during erythropoiesis for hemoglobin synthesis.2
Protoporphyria is an autosomal dominant disease characterized clinically by photosensitivity3 and hepatobiliary injury,4 which is due to elevated protoporphyrin levels. The protoporphyrin accumulates as the result of decreased ferrochelatase activity (about 15% to 30% of normal),5-7which is caused by a variety of mutations in the ferrochelatase gene.8-14 A decrease in the mRNA levels of the normal ferrochelatase allele combined with an inactivating mutation in the diseased allele has been proposed to be responsible for the decreased enzymatic activity observed in patients (<50% of normal).15
Human ferrochelatase is transcribed from a single promoter in both erythroid and nonerythroid cells.16,17 Transient transfection assays show that the minimal Sp1-driven promoter is required for ferrochelatase expression in several cell lines.17 However, the presence of the upstream GATA and NF-E2 cis-elements is not sufficient to drive erythroid-specific enhanced expression.18 Further analysis shows that increased transcription in erythroid cell lines is achieved through the deletion of a repressor region located between the minimal promoter and the upstream GATA and NF-E2 elements.17
In this study, we analyze the regulation of the ferrochelatase gene in vivo in an attempt to provide insights into the mechanism of erythroid specific enhancement of the intact ferrochelatase promoter. Understanding these regulatory mechanisms may provide insight into the deficient ferrochelatase expression in protoporphyria. Specifically, we generate and analyze transgenic (TG) mouse lines in which the luciferase reporter gene is driven by either the ferrochelatase minimal promoter or by an extended 5′ upstream region, and demonstrate that in the context of normal chromatin structure, the intact 5′ upstream region of the ferrochelatase gene confers appropriate erythroid-enhanced, developmental and cell-specific expression. Our data also suggest that appropriate expression of the transgene correlates with the appearance of DNase I hypersensitivity site in the 5′ flanking region of the transgene.
MATERIALS AND METHODS
Vector construction.
For generation of the transgenic mouse lines, two constructs containing either −0.15 kb (−0.15 TG) or −4.0 kb (−4.0 TG) of the human ferrochelatase promoter driving expression of the P pyralis luciferase (Luc) cDNA were used. Both reporter gene constructs have been described previously.17 For injection, DNA fragments encompassing the promoter fragments, the luciferase coding sequences and the SV40 polyadenylation sequences were released by digestion with BamHI. Because the long (−4.0 TG) promoter fragment contained an internal BamHI site at −263 bp, a triple helix formation19 was induced with an oligonucleotide at this site to protect it from BamHI cleavage. Fragments were purified on an 0.8% Tris-Acetate-EDTA (TAE) agarose gel, electroeluted, and microinjected into C3H/C57B6 single cell embryos. Positive transgene founder mice were back crossed to the C57B6 strain and F1 progeny used for subsequent experiments.
Transgene screening.
Transgene positive mice were determined by detecting luciferase cDNA in the mouse genome or by measuring luciferase activity in whole blood. Genomic DNA from −0.15 TG mice was isolated from 0.5 cm resected tails by standard methods,20 and 10 μg of tail DNA was digested with EcoRI, separated on a 0.7% TAE-agarose gel, followed by transfer to MagnaNT membrane (MSI, Westboro, MA). Southern blots were performed as described21 and probed using a 32P-deoxycytidine triphosphate (dCTP) random-primed labeled fragment of the P pyralis luciferase cDNA. Blots were washed twice in 2X SSC/0.1% sodium dodecyl sulfate (SDS) at 55°C, twice in 0.1X SSC/0.1% SDS at 65°C, and exposed to X-OMAT AR film (Eastman-Kodak, Rochester, NY) for 18 hours at −80°C with intensifying screens. Mice containing the −4.0 TG construct were screened for the transgene by adding approximately 5 μL of tail vein blood to 500 μL of luciferase buffer (Analytical Bioluminescence Laboratory, Ann Arbor, MI), and measuring the luciferase activity in 20 μL of the lysate using a Monolight 2010 luminometer (Analytical Bioluminescence). Luciferase activities above 10,000 light units/10 seconds were scored as transgene positive. The luciferase activity of control mice was below 300 light units/10 second measurement (background measurement for luminometer was 200 light units/10 seconds).
Northern blot analysis/luciferase assays.
Adult, transgene positive mice were injected with 200 μL of a 0.1% (wt/vol) solution of n-acetylphenylhydrazine (PHZ) to induce hemolysis,22,23 or phosphate-buffered saline (PBS) as the control for 7 consecutive days, and then euthanized by cervical dislocation on day 9. Total RNA was isolated from brain, kidney, liver, skeletal muscle, testis, and spleen from PHZ- and PBS-treated mice by centrifugation through a CsCl gradient,21 and 15 μg of total RNA was analyzed by separation on a 0.7% agarose/2.2 mol/L formaldehyde gel. RNA was transferred to Magnagraph membrane (MSI), ultraviolet (UV) cross-linked, and probed with a random-primed 32P-dCTP–labeled mouse ferrochelatase1 or human EKLF cDNA.24 The membranes were hybridized for 2 hours at 55°C in Quick-Hyb Buffer (Amersham, Arlington Heights,IL) containing 2 × 106 cpm/mL of ferrochelatase probe, followed by two washes in 2X SSC/0.1% SDS at 55°C for 15 minutes and two washes in 0.1X SSC/0.1% SDS at 60°C for 15 minutes. Blots were exposed to X-OMAT AR film with intensifying screen at −80°C for 12 hours. For EKLF detection, the blot was stripped of the ferrochelatase probe by boiling in 0.1X SSC/0.1% SDS and reprobed with the EKLF probe. The blot was hybridized and washed identically to the ferrochelatase blot and exposed to X-OMAT-AR film for 22 hours at −80°C with intensifying screens. Equal loading and quality of RNA was assessed by ethidium bromide staining of 18S and 28S ribosomal RNAs.
For the luciferase assays, approximately 3.0 mm3 fragments of brain, kidney, liver, skeletal muscle, testis, spleen, bone marrow and 20 μL of blood were homogenized in 500 μL of luciferase assay buffer, and allowed to sit on ice for 20 minutes. Cellular debris was removed by centrifugation, and 20 to 200 μL of lysate was assessed for luciferase activity as previously described. Total protein from the lysates was quantitated with Protein Assay Reagent (Bio-Rad, Hercules, CA) using bovine serum albumin as a standard.
In situ hybridization.
Mouse embryos at 15.5 days postcoitum (d.p.c.) were collected in PBS and fixed overnight in 4% paraformaldehyde (PFA) in PBT (PBS, 0.1% Tween-20). After fixation, embryos were sequentially dehydrated though a methanol series and stored at −20°C in absolute methanol. Whole mount in situ hybridization was performed on sequentially rehydrated embryos essentially as described25 with digoxygenin (DIG)-labeled antisense RNA probes generated as recommended by the manufacturer (Boehringer-Mannheim, Mannheim, Germany). For luciferase detection, a BamHI fragment containing the full-length luciferase gene, the small t antigen splice site, and the SV40 polyadenylation sequences, was cloned from p19 Luc26 into pBluescript SK+(Stratagene, La Jolla, CA). The resulting template was digested usingSal I and transcribed with T3 RNA polymerase to yield an antisense probe, or alternatively digested with Not I and transcribed with T7 RNA polymerase as a sense control RNA. For the detection of murine ferrochelatase, clone 6A1 containing the full coding sequence of the murine ferrochelatase cDNA was shortened by deletion of a Pst I fragment containing the 3′ end. Antisense RNA probes were generated from this construct by using SP6 RNA polymerase on EcoRI linearized templates. After whole mount in situ hybridization, selected embryos were cleared in sucrose, embedded in gelatin, and sectioned (60 μm) using a cryomicrotome. Tissue sections were then washed, dehydrated, and mounted with DPX (Fluka, Buchs, Switzerland). Slides were scanned in a SprintScan film scanner (Polaroid, Cambridge, MA).
For spleens, −4.0 TG mice were treated with the 7-day regimen of PBS or PHZ and killed on day 9. Spleens were resected and fixed in 4% paraformaldehyde/PBS at 4°C for 12 hours, then transferred to 70% ethanol and stored at 4°C until sectioning. The fixed spleens were paraffin embedded and 5.0-μm sections were placed on glass slides. Sections from control and PHZ-treated animals were stained with hematoxylin/eosin27 or subjected to in situ hybridization with the same probes described above, essentially as described.28 For detection, the AP substrate Western Blue (Promega, Madison, WI) was applied and allowed to develop for approximately 18 hours. Sections were then washed in water, counterstained with eosin,27 and mounted in DPX (Fluka).
DNase I hypersensitivity assays.
Nuclei were obtained from both normal and PHZ-treated spleens as described29 and stored in nuclear storage buffer at −80°C. For DNase I digestion, aliquots of nuclei were thawed on ice, 10 vol of Buffer A (15 mmol/L Tris-HCl, pH 7.5, 15 mmol/L NaCl, 60 mmol/L KCl, 0.15 mmol/L spermine, 0.5 mmol/L spermidine, 1 mmol/L dithiothreitol [DTT]) was added, the suspension was centrifuged at 500xg for 5 minutes. The nuclei were then resuspended at a concentration of 5 × 107 nuclei/mL in nuclear digestion buffer (NDB, Buffer A supplemented with 0.25 mol/L sucrose and 2 mmol/L CaCl2) and 100-μL aliquots were digested with DNase I (Boehringer-Mannheim) (concentration ranging from 0 to 0.2 mg/mL) at room temperature for 20 minutes. Next, 200 μL of 2X PK buffer (200 mmol/L Tris-HCl, pH 7.5, 25 mmol/L EDTA, 300 mmol/L NaCl, 2% SDS), 100 μL of TE buffer (10 mmol/L Tris-HCl, pH 7.6, 1 mmol/L EDTA), and proteinase K (Boehringer-Mannheim) (final concentration of 200 μg/mL) were added. After overnight incubation at 37°C, samples were extracted twice with 2 vol of phenol:chloroform:isoamyl alcohol (25:24:1) and precipitated with 2 vol of ethanol. The DNA samples were resuspended in water, digested withBamHI, electrophoresed through 0.8% agarose-1X TBE gels, transferred to Hybond N+ membranes (Amersham), and analyzed by Southern blot analysis using a 32P-labeled gene-specific probe (−1.1 kb Xba I site to −250 bpBamHI). Hybridization and detection was performed as described above for Northern blots.
RESULTS
Generation and characterization of transgenic mice.
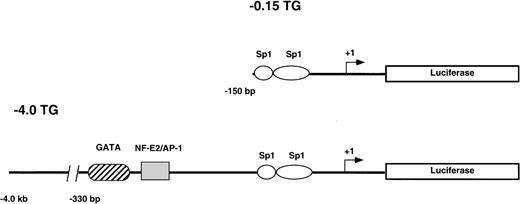
Two reporter gene constructs (−0.15 TG and −4.0 TG) containing −0.15 kb or −4.0 kb, respectively, of the human ferrochelatase promoter linked to the luciferase reporter gene were injected into CH3/C57B6 single cell mouse embryos. The −0.15 kb construct consists of the minimal TATA-less human ferrochelatase promoter containing two Sp1-like cis-acting elements. Both Sp1 consensus binding sites have been previously shown to functionally bind recombinant Sp1 in DNase I footprinting and transient transfection assays.17 The −4.0 kb fragment includes the minimal promoter and a region from −260 bp to −330 bp that harbors two erythroid cis-acting elements that have been previously demonstrated to bind GATA-1 and NF-E217(Fig 1).
Reporter gene constructs used to generate transgenic mouse lines. The structure of the constructs used to generate the −0.15 TG and −4.0 TG lines are shown. Construct −0.15 TG contains the minimal human ferrochelatase promoter containing two Sp1-like elements, and construct −4.0 TG contains the minimal promoter, the erythroid enhancer elements GATA, and NF-E2 and approximately 3.5 kb of upstream sequence. Both promoter constructs drive the expression of the luciferase reporter gene.
Reporter gene constructs used to generate transgenic mouse lines. The structure of the constructs used to generate the −0.15 TG and −4.0 TG lines are shown. Construct −0.15 TG contains the minimal human ferrochelatase promoter containing two Sp1-like elements, and construct −4.0 TG contains the minimal promoter, the erythroid enhancer elements GATA, and NF-E2 and approximately 3.5 kb of upstream sequence. Both promoter constructs drive the expression of the luciferase reporter gene.
The transgene status of −0.15 and −4.0 TG founders and F1 mice was assessed by Southern blotting or luciferase activity. Low luciferase activity in whole blood lysates of −0.15 TG mice required the use of Southern blotting to confirm the presence of the transgene. In contrast, high luciferase activity in whole blood lysates from −4.0 TG mice was sufficient to confirm the presence of the transgene. We obtained five founder mice that carried the −0.15 kb transgene and two founder mice that carried the −4.0 kb transgene. All founder lines expressed functional luciferase. The relative copy number of the luciferase transgene was determined by dot blot and ranged from 1 to 10 in the −0.15 TG mice and was 1 in the −4.0 TG mice. We observed that luciferase activity in the −4.0 TG mice continued and remained constant over many generations. However, the luciferase activity in the −0.15 TG mice decreased or was completely absent after approximately 1 year in the founder mice and their progeny.
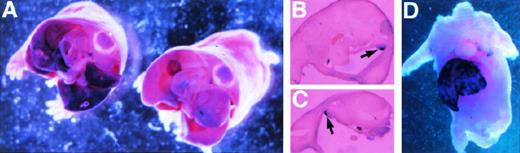
To analyze the transgene for appropriate developmental expression, we studied the −4.0 kb transgene and endogenous ferrochelatase gene in the fetus. The endogenous and reporter gene mRNA species were detected using mouse ferrochelatase and luciferase antisense DIG-labeled RNA probes, respectively (Fig2, see page 323). This analysis demonstrates localization of both the ferrochelatase and transgene mRNA in the fetal liver at 15.5 days postcoitus. This expression is consistent with the colonization of the fetal liver by hematopoietic precursors at this stage of fetal development.29 Interestingly, transgene expression is also detected in the gonads, while the endogenous gene is absent (Fig 2B and C).
Whole mount in situ hybridization detects ferrochelatase and luciferase expression in −4.0 TG mouse embryos. Whole-mount in situ hybridization was performed on 15.5 d.p.c. embryos by using antisense DIG-labeled RNA probes. (A) Detection of the luciferase transgene in the fetal liver of luciferase positive (left) and luciferase negative (right) transgenic litter mates. Transversal sections are shown, and the view is from posterior to anterior. (B) and (C) indicate staining in the gonads (left and right arose from different transversal microtome sections) of the luciferase positive mouse shown in (A). (D) Shows the localization of the endogenous murine ferrochelatase gene to the fetal liver at the same developmental stage. In all cases, hybridization to sense RNA probes gave no detectable signal (not shown).
Whole mount in situ hybridization detects ferrochelatase and luciferase expression in −4.0 TG mouse embryos. Whole-mount in situ hybridization was performed on 15.5 d.p.c. embryos by using antisense DIG-labeled RNA probes. (A) Detection of the luciferase transgene in the fetal liver of luciferase positive (left) and luciferase negative (right) transgenic litter mates. Transversal sections are shown, and the view is from posterior to anterior. (B) and (C) indicate staining in the gonads (left and right arose from different transversal microtome sections) of the luciferase positive mouse shown in (A). (D) Shows the localization of the endogenous murine ferrochelatase gene to the fetal liver at the same developmental stage. In all cases, hybridization to sense RNA probes gave no detectable signal (not shown).
Transcription from the human ferrochelatase promoter in transgenic mice is similar to the endogenous mouse promoter.
To compare the level of expression of the −4.0 kb transgene to the endogenous gene in nonerythroid and erythroid tissues of an adult mouse, we assessed the levels of ferrochelatase mRNA from tissues of normal mice and compared this with the luciferase activity in the same tissues of −4.0 TG mice. Total RNA was analyzed from various nonerythroid and erythroid tissues in normal adult mice. Samples from the same tissues of −4.0 TG mice, including bone marrow and blood, were assessed for luciferase activity. We further analyzed erythroid expression in the mouse spleen, which is able to switch from a primarily nonerythroid organ to an erythroid organ when treated with the hemolytic agent n-acetylphenylhydrazine (PHZ).22 23Adult transgene negative and −4.0 TG mice were injected with PHZ or PBS as a control for 7 consecutive days and samples were collected on day 9 after the first injection.
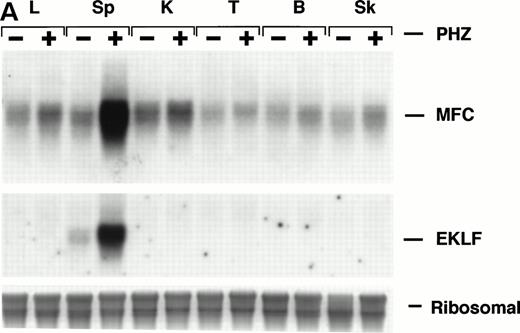
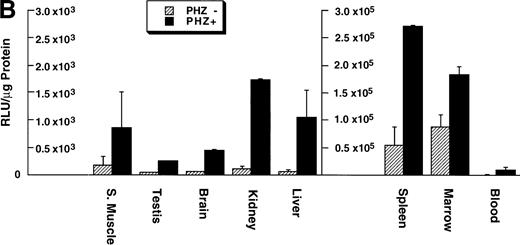
Northern blot analysis shows equivalent low expression levels of the endogenous ferrochelatase mRNA in nonerythroid tissues (Fig 3A, PHZ-). Similar low basal levels of luciferase activities were observed in the corresponding nonerythroid tissues of −4.0 TG mice, which is consistent with the endogenous gene expression (Fig 3B, PHZ-). Interestingly, there was a notable difference in the mouse ferrochelatase mRNA levels compared with the transgene luciferase activity in the control spleens (Fig 3A, spleen, PHZ-; and Fig 3B, spleen, PHZ-). The ferrochelatase mRNA levels in the spleens of the normal mice are low and equivalent to the other nonerythroid tissues, whereas the luciferase activity in the spleens of −4.0 TG mice was 300-fold to 1,300-fold higher than the activities in other nonerythroid tissues.
Mouse ferrochelatase and reporter gene expression in various nonerythroid and erythroid tissues. (A) Total RNA was isolated from liver (L), spleen (Sp), kidney (K), testis (T), brain (B), and skeletal muscle (Sk) from control (PBS-treated) or PHZ-treated mice. A total of 15 μg of total RNA was analyzed by Northern blot. RNA was probed for mouse ferrochelatase mRNA or EKLF mRNA with radiolabeled mouse ferrochelatase or human EKLF cDNA probes. Equal loading of RNA was assessed by ethidium bromide staining of 18S and 28S ribosomal RNA. (B) 3.0-mm3 fragments of various tissue described above plus marrow from control-treated (PHZ-, hatched bars) or PHZ-treated (solid bars) −4.0 TG mice were homogenized in luciferase assay buffer. Luciferase activity of tissue extracts was measured for 10 seconds in a luminometer and expressed as relative light units per μg of protein. Luciferase activity in erythroid tissues is expressed on a scale 100 times that of nonerythroid tissues. Each luciferase activity data point represents an average of two to three mice.
Mouse ferrochelatase and reporter gene expression in various nonerythroid and erythroid tissues. (A) Total RNA was isolated from liver (L), spleen (Sp), kidney (K), testis (T), brain (B), and skeletal muscle (Sk) from control (PBS-treated) or PHZ-treated mice. A total of 15 μg of total RNA was analyzed by Northern blot. RNA was probed for mouse ferrochelatase mRNA or EKLF mRNA with radiolabeled mouse ferrochelatase or human EKLF cDNA probes. Equal loading of RNA was assessed by ethidium bromide staining of 18S and 28S ribosomal RNA. (B) 3.0-mm3 fragments of various tissue described above plus marrow from control-treated (PHZ-, hatched bars) or PHZ-treated (solid bars) −4.0 TG mice were homogenized in luciferase assay buffer. Luciferase activity of tissue extracts was measured for 10 seconds in a luminometer and expressed as relative light units per μg of protein. Luciferase activity in erythroid tissues is expressed on a scale 100 times that of nonerythroid tissues. Each luciferase activity data point represents an average of two to three mice.
Treatment of mice with PHZ induces hemolytic anemia, splenomegaly, and extramedullary erythropoiesis.22,23 Under such conditions, we observed an increase in the number of immature erythroid precursors. Staining sections of normal or PHZ-treated livers for heme with 3,3'-diaminobenzidine tetrahydrochloride (DAB)27 showed that there were 10-fold more DAB positive, nucleated erythroid cells in PHZ-treated livers compared with control livers (average of DAB positive cells for 10 20X fields; control, x = 0.6 ± 0.7 and PHZ+, x = 6.2 ± 3.4, P < .001; data not shown).
Upregulation of an erythroid specific mRNA, erythroidKrüppel-like factor24 30 (EKLF, Fig 3A) and an increased population of erythroid precursors confirms extramedullary erythropoiesis occurring in the spleen (Fig4A, see page 323). Northern blot analysis and luciferase activity show an approximate fivefold increase in both ferrochelatase mRNA and luciferase expression, respectively, in PHZ-treated spleens compared with control spleens (Fig 3A and B).
Cellular expression of the luciferase reporter gene in the spleen. Sections of spleen from control-treated or PHZ-treated −4.0 TG mice were (A) stained with hematoxylin/eosin to assess cell lineage or (B) probed for luciferase expression with an antisense DIG-labeled luciferase RNA probe and counterstained with eosin. Lymphatic nodules are noted with a white arrow (A and B) and erythroid precursor cell clusters are noted with a black arrow (A and B, PHZ+). Hybridization to sense RNA probes gave no detectable signal (not shown).
Cellular expression of the luciferase reporter gene in the spleen. Sections of spleen from control-treated or PHZ-treated −4.0 TG mice were (A) stained with hematoxylin/eosin to assess cell lineage or (B) probed for luciferase expression with an antisense DIG-labeled luciferase RNA probe and counterstained with eosin. Lymphatic nodules are noted with a white arrow (A and B) and erythroid precursor cell clusters are noted with a black arrow (A and B, PHZ+). Hybridization to sense RNA probes gave no detectable signal (not shown).
Expression of the −4.0 TG in the spleens of control and PHZ-treated spleens was determined by in situ hybridization using an antisense luciferase DIG-labeled riboprobe. The antisense luciferase probe detects high level expression in the deeply hematoxylin-stained erythroid precursor cells in the pulp region, but not in the myeloid cells, in the lymphatic nodules of the PHZ-treated spleens (Fig 4A and B). Transgene expression was below the level of detection in the control spleens (PHZ-).
Cis-acting elements in −4.0 kb of the human ferrochelatase gene are needed for a full erythroid transcriptional response in adult mice.
Our previous studies demonstrate little difference in the expression of the −0.15 TG or −4.0 TG reporter gene constructs in nonerythroid and erythroid cells using transient transfection assays, despite the presence of GATA and NF-E2 erythroid elements in the −4.0 TG construct.17 Both GATA-1 and NF-E2 from erythroid cell extracts have been shown to bind their consensus sites in the −260 bp to −330 bp region of the human ferrochelatase promoter.17 To assess the functional contributions of the minimal promoter and upstream elements, we compared expression of the −0.15 TG construct to the −4.0 TG construct in the context of genomic integration in the nonerythroid and erythroid tissues of transgenic mice.
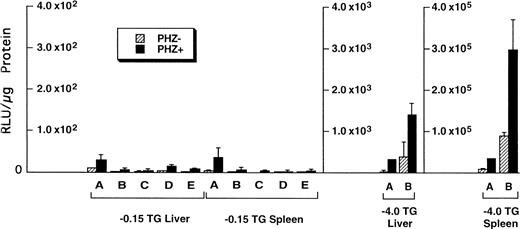
Of the tissues tested, only the livers and spleens from the −0.15 TG mice had luciferase activity above background levels. The luciferase activities in the liver and spleen of F1 progeny from five different −0.15 TG founders were compared with activities in F1 progeny of two different −4.0 TG founders (Fig5). We found that in control mice (PHZ-) there was an overall higher luciferase activity in the −4.0 TG mice compared with the −0.15 TG mice. The luciferase activity in the livers of control mice was 10-fold to 100-fold higher in the −4.0 TG mice compared with the −0.15 TG mice. The spleen luciferase activities in control mice were 1,000-fold to 10,000-fold higher in the −4.0 TG mice compared with the −0.15 TG mice. Therefore, the markedly higher luciferase activity in the −4.0 TG line over the −0.15 TG line in the spleens suggests the presence of functional erythroid cis-acting elements upstream of the minimal human ferrochelatase promoter, and that these elements are necessary for the maximal erythroid response.
Luciferase activities in −0.15 TG and −4.0 TG mice suggest elements 5′ of the proximal promoter are necessary for enhanced erythroid expression. Mice containing the −0.15 TG and −4.0 TG constructs were treated with a 7-day regimen of PBS (PHZ-, hatched bars) or PHZ (solid bars). On day 9, fragments of liver or spleen were homogenized in luciferase assay buffer and luciferase activity was assessed as described previously. Letters below each set of bars represent individual founder mice. Each bar represents an average luciferase activity of two to three mice from each founder. Three scales are necessary to accurately display the range of data obtained for the two transgenic lines.
Luciferase activities in −0.15 TG and −4.0 TG mice suggest elements 5′ of the proximal promoter are necessary for enhanced erythroid expression. Mice containing the −0.15 TG and −4.0 TG constructs were treated with a 7-day regimen of PBS (PHZ-, hatched bars) or PHZ (solid bars). On day 9, fragments of liver or spleen were homogenized in luciferase assay buffer and luciferase activity was assessed as described previously. Letters below each set of bars represent individual founder mice. Each bar represents an average luciferase activity of two to three mice from each founder. Three scales are necessary to accurately display the range of data obtained for the two transgenic lines.
A DNase I hypersensitive site appears at −2.0 kb of the human ferrochelatase promoter in transgenic mouse spleens during induced extramedullary erythropoiesis.
Our data comparing liver and spleen luciferase activities in −0.15 TG and −4.0 TG mice suggest the presence ofcis-elements located 5′ of the minimal promoter that are capable of enhancing transcription of the ferrochelatase gene during erythropoiesis. To further analyze the region upstream of the minimal promoter, we performed DNase I hypersensitivity (HS) analysis to detect upstream sequences that could account for the enhanced expression in the −4.0 TG lines.
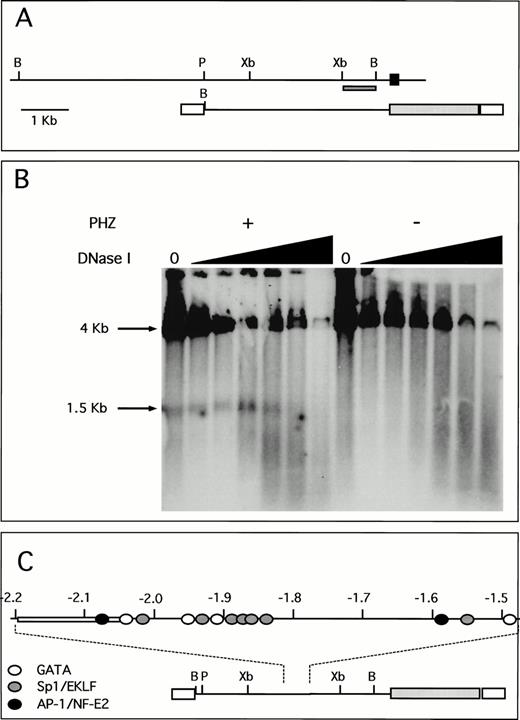
A unique fragment of the human ferrochelatase gene was selected by its ability to detect single bands in Southern blot analysis. This probe and its location within both the human genome and the transgene are shown (Fig 6A). DNase I hypersensitivity analysis indicates a strong HS site located approximately 1.9 to 2.0 kb upstream from the transcription start site (Fig 6B). This DNase I HS site is only present in chromatin isolated from the spleens of PHZ-treated animals, indicating a strong association between the appearance of the HS site and the induction of transgene expression. Moreover, this HS site is not present in nuclear chromatin isolated from the human erythroid cell lines K562 and HEL that were analyzed using these methods (data not shown). Analysis of the DNA sequence of this region shows multiple putative binding sites for GATA-1 and Sp1-related factors, as well as a single AP-1/NF-E2 binding site. Although other similar consensus cis-elements are found throughout this region, there is a greater number of these sites clustered around the HS region (Fig 6C), just downstream of an AT-rich region. Binding of nuclear proteins to these elements is currently under investigation.
A unique HS site appears 5′ of the proximal promoter in PHZ-treated −4.0 TG mouse spleen nuclei. (A) Location of the probe used for the detection of DNase I HS sites is indicated (dark hatched box) in the context of both the human ferrochelatase genomic locus (top) and the luciferase reporter transgene (bottom). The black box represents exon 1, open boxes represent SV40 sequences, and the light hatched box represents the luciferase reporter gene from p19 Luc (B, BamHI; P, PstI; and Xb, Xba I). (B) Nuclei were isolated from both normal and PHZ-treated spleens from −4.0 TG mice. After DNase I digestion of intact nuclei, DNA was isolated, digested to completion withBamHI, and the resulting fragments were identified by using the indicated probe in Southern blot analysis. The resulting fragments and their sizes are indicated. (C) Location of putativecis-elements as deduced from the analysis of the primary structure of the hypersensitive region and surrounding sequences (top). The open box (at −2.05 kb to −2.2 kb) indicates the AT-rich region. The location of this HS region and surrounding sequences within the transgene is indicated (bottom).
A unique HS site appears 5′ of the proximal promoter in PHZ-treated −4.0 TG mouse spleen nuclei. (A) Location of the probe used for the detection of DNase I HS sites is indicated (dark hatched box) in the context of both the human ferrochelatase genomic locus (top) and the luciferase reporter transgene (bottom). The black box represents exon 1, open boxes represent SV40 sequences, and the light hatched box represents the luciferase reporter gene from p19 Luc (B, BamHI; P, PstI; and Xb, Xba I). (B) Nuclei were isolated from both normal and PHZ-treated spleens from −4.0 TG mice. After DNase I digestion of intact nuclei, DNA was isolated, digested to completion withBamHI, and the resulting fragments were identified by using the indicated probe in Southern blot analysis. The resulting fragments and their sizes are indicated. (C) Location of putativecis-elements as deduced from the analysis of the primary structure of the hypersensitive region and surrounding sequences (top). The open box (at −2.05 kb to −2.2 kb) indicates the AT-rich region. The location of this HS region and surrounding sequences within the transgene is indicated (bottom).
DISCUSSION
Although ferrochelatase is synthesized by all cell types at a housekeeping level, the gene is upregulated during erythropoiesis to increase the synthesis of heme. Our study suggests that appropriate erythroid-enhanced expression of the human ferrochelatase promoter requires a chromatin environment. In a transgenic mouse model, −4.0 kb of the human ferrochelatase 5′ flanking region contains elements that provide both basal expression levels of the transgene and enhanced transcriptional activity in erythroid tissues of embryonic and adult mice.
In our previous studies using transient transfections into erythroid and nonerythroid cell lines, the −4.0 kb ferrochelatase reporter gene was not expressed at higher levels than the −0.15 kb reporter gene.17 The presence of upstream GATA and NF-E2cis-elements resulted in higher levels of expression in erythroid cell lines only when a repressor sequence located between the minimal promoter and the erythroid elements was deleted.17We propose that the different results in these two studies reflect the contribution of chromatin structure in the transgenic mice, but not in the transient transfections.
In general, the −4.0 kb human ferrochelatase promoter transgene responds appropriately to housekeeping and erythroid developmental signals in a manner analogous to the endogenous gene. In nonerythroid tissues, low basal levels of endogenous ferrochelatase mRNA and luciferase activities in −4.0 TG mice were observed. Upon induction of extramedullary erythropoiesis, both endogenous ferrochelatase mRNA levels and luciferase activities increased at least fivefold. There was an unexpected increase in ferrochelatase mRNA and luciferase activity in nonerythroid tissues of PHZ-treated animals, and additionally, there was a greater induction by PHZ of the transgene (4- to 9-fold) compared with the endogenous gene (1.5-fold to 2-fold) in nonerythroid tissues.
We propose that the observed enhanced transcriptional activity in the nonerythroid tissues of PHZ-treated animals is due to contaminating erythroid cells from circulating blood present in these tissues and not from enhanced expression in the nonerythroid tissues. A 13-fold increased luciferase activity in the peripheral blood (Fig 3B) and 10-fold more DAB positive erythroid cells in the livers of PHZ-treated −4.0 TG mice supports this concept.
An alternative hypothesis to explain the enhanced transcriptional activity observed in PHZ-treated nonerythroid tissues is that PHZ, an oxidative-type drug,31 32 is activating oxidative stress elements in the ferrochelatase promoter. However, the treatment of three nonerythroid cell lines, 293 (kidney epithelial), HepG2 (hepatocarcinoma), and HeLa (cervical carcinoma) with concentrations of PHZ comparable to those given to the mice (a range of 1 μmol/L to 500 μmol/L), did not stimulate expression of the −0.15 TG or −4.0 TG human ferrochelatase reporter gene in transient transfection assays (data not shown).
In untreated spleens of −4.0 TG mice, the level of transgene expression is higher than the transgene expression in other nonerythroid tissues from the same mice (Fig 3A and B). This expression pattern in the −4.0 TG mice differs from the endogenous ferrochelatase expression, which is equivalent in the nonerythroid spleen and other nonerythroid tissues. This observation is consistent with the idea that the endogenous promoter may contain negative regulatory elements that are absent in the −4.0 kb transgene. The lack of a functional negative regulatory mechanism in the −4.0 kb transgene may also explain the higher induction of the transgene compared with the endogenous gene in nonerythroid tissues of PHZ-treated animals, and the observed luciferase expression in the gonads of −4.0 TG embryonic mice, where no endogenous ferrochelatase expression is detected (Fig 2C). Alternatively, it is possible that GATA-1 expressed in Sertoli cells33 is able to productively interact with the 4.0 kb human promoter transgene, but not with the endogenous mouse promoter.
Comparison of luciferase activities in the liver and spleen of −0.15 TG and −4.0 TG mice suggests there arecis-elements upstream of the minimal promoter that are necessary for a full erythroid-enhanced transcriptional response from the ferrochelatase promoter. Although the luciferase activities in the −0.15 TG mice are orders of magnitude lower than in the −4.0 TG mice, there is a similar fold-induction of the transgene in the minimal promoter −0.15 TG mice and the −4.0 TG mice treated with PHZ. It is possible that the induction observed in the PHZ-treated −0.15 TG mice may be the result of erythroid-specific transcription factors activating the minimal promoter at the Sp1-like elements located between −83 and −150 bp. A recent report also suggests the presence of an additional erythroid-specific cis-element located between −80 and −72 in the ferrochelatase promoter that confers upregulation of a reporter gene in transient transfection assays34 and which may be active in the erythroid tissue of −0.15 TG mice. However, in light of our results in this study, transient transfection assays may not accurately reflect physiologic use of enhancing elements in the ferrochelatase promoter.
In addition to the previously described erythroidcis-elements,16,17 GATA and NF-E2, a new DNase I HS site appears approximately 2.0 kb upstream from the transcriptional start site that harbors a cluster of putative erythroid elements, GATA, NF-E2, and CACCC, whose presence appears to be a general feature of erythroid-specific control sequences.18 The presence of a HS site in nuclei from PHZ-treated spleens not treated with exogenous DNase indicates the maintenance of an open chromatin region, which is highly accessible to endogenous nucleases. HS sites usually indicate the presence of functionally relevant sequences involved in transcriptional control and might be involved in a variety of activities such as increasing the rate of transcription, providing insulator function, or allowing accessibility of the promoter to the basal transcription machinery.35 36
Transient transfection experiments performed on both erythroid and nonerythroid cell lines using a human ferrochelatase promoter construct containing the −2.0 kb HS site did not show increased transcriptional activation when compared with constructs lacking the site17 suggesting that this site does not act as a classical enhancer. However, linking a −375 bp ferrochelatase promoter fragment to the β-globin HS2 resulted in transcriptional enhancement (unpublished data). These data are consistent with the view that the newly identified human ferrochelatase HS site might be involved in promoter accessibility and that the function of this element can only be assessed in a native chromatin configuration achieved after genomic integration of the transgene. In this regard, the presence of multiple erythroid-specific binding sites, the susceptibility to nucleases, and the presence of a long AT-rich stretch suggests a structural similarity between the human ferrochelatase HS site and matrix attachment regions (MARs) and locus control regions (LCRs).36,37 The weak intensity of the HS band in the human ferrochelatase promoter compared with the intact BamHI fragment may reflect that only a few cells, probably those at the correct developmental stage, are able to poise the human ferrochelatase promoter for high levels of expression, as has been proposed for the β-globin LCR.35 Chromatin disruption may not be sufficient on its own for transcriptional activation, and therefore must rely on local interaction of trans-acting factors with the proximal promoter.38,39 In the case of the human ferrochelatase promoter, it is tempting to speculate that the distal control element is involved in alleviating repression, thus allowing the interaction of the erythroid-specific elements (GATA and NF-E2) with the proximal promoter to render high levels of erythroid-specific expression. In this regard, cooperative effects on transcription have been described between GATA-1 and Sp1/EKLF,40-42 and NF-E2 is a strong candidate for the task of maintaining an active chromatin configuration due to its chromatin remodeling capabilities.43
There is an increasing number of other erythroid genes that also contain HS sites with various activities 5′ upstream at similar locations. These include the mouse glycophorin gene, which displays the CACCC, NF-E2 and GATA cis-elements,44,45 the mouse porphobilinogen deaminase gene,46 the mouse ferritin H subunit gene,47 the mouse erythropoietin receptor,48 and the human carbonic anhydrase gene.49 The presence of these types of cis-elements in many erythroid-specific genes suggests the general necessity of maintaining open chromatin domains through the final steps of erythroid differentiation, during which there is a progressive condensation of chromatin that is accompanied by silencing of transcription.50
ACKNOWLEDGMENT
We thank Dr Michael Breindl for fruitful discussions and support during the course of this work, Dr Anne Bang for assistance with some of the figures, Jennifer Armstrong for critically reading the manuscript, Dr C. Camerini-Otero for the triple helix restriction endonuclease digestion, and Dr Stewart Orkin for the human EKLF cDNA. Embryo injections were performed by the UNC-CH Transgenic Mice facility. Image scanning and in situ hybridization assistance was provided by the molecular biology core facility of the Center for Gastrointestinal Biology and Disease, UNC-CH.
Supported by the National Institutes of Health Grants No. DK47361 and DK34987 (to D.A.B.). A.T. has been supported by a fellowship from the Fundación Dr Manuel Morales (Tazacorte, Isla de La Palma, Canary Islands, Spain).
Address reprint requests to David A. Brenner, MD, University of North Carolina at Chapel Hill, CB#7038, Department of Medicine, Chapel Hill, NC 27599-7038; e-mail: dab@med.unc.edu.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal