Polymorphonuclear neutrophils (PMN) contain multiple distinct secretory compartments that are sequentially mobilized during cell activation. Complement receptor type 1 (CR1) is a marker for a readily mobilizable secretory vesicle compartment, which can undergo exocytic fusion with the plasma membrane independently of secretion of traditional granule contents. The basis for the formation of these distinct compartments is incompletely understood. Primary and secondary granules are generated directly from the Golgi complex during different stages of development of the cell, obviating the need for sorting signals for proper packaging of their constituents. To determine whether the secretory vesicles are formed in a similar manner, we studied a stable rat basophilic leukemia cell line (RBL-CR1) transfected with a plasmid containing the cDNA of human CR1 driven by a viral promoter. The CR1 was present primarily intracellularly in small vesicles resembling the CR1 storage pools in resting PMN. Activation of RBL-CR1 resulted in translocation of intracellular CR1 to the plasma membrane, with mobilization requirements different from those of the classical RBL granules. Thus, in RBL-CR1, continuously synthesized CR1 is stored and upregulated in much the same way as in PMN. This suggests that differential timing of gene expression is not essential for proper storage of CR1 and that other sorting mechanisms are involved, which can be studied in RBL-transfectants.
ACTIVATION OF polymorphonuclear neutrophil granulocytes (PMN)1 is accompanied by sequential exocytosis of distinct intracellular compartments in response to various stimuli.1-3 As this occurs, functionally important membrane proteins, stored intracellularly in the walls of these compartments, are translocated to the plasma membrane during exocytic fusion. The order in which these compartments are mobilized and in which their membrane proteins appear on the cell surface is important for the functioning of the cell in vivo. PMNs respond to chemoattractants by exiting from the circulation and migrating into the tissues at sites of infection or inflammation. Receptors and adhesion molecules are expressed first to increase responsiveness to chemoattractants and adherence to the endothelial cells.1,3,4 This is followed by the secretion of gelatinase,5 which facilitates movement of the cell through basement membranes and matrix. Finally the components of the microbicidal oxidase are assembled and activated, and the contents of the primary and secondary granules are released.2
Complement receptor type I (C3b/C4b receptor, CD35, CR1) is of particular interest because it is not found in the traditional granules, but rather, serves as a marker of a unique class of readily mobilizable “secretory vesicles.”1,6 In resting PMNs, most of the CR1 is present intracellularly in these small vesicles, while only 10% is present on the cell surface.6,7Treatment of PMNs with the chemotactic peptide f-MLP, the ionophore A23187 in the presence of Ca++, and/or other stimuli, leads to increased cytosolic free Ca++, which results in the translocation of the CR1-containing vesicles to the cell surface and thus to increased plasma membrane expression of CR1.8,9 The requirements for mobilization of the CR1-containing vesicles are distinct from those of the traditional granules, and maximal upregulation of CR1 may occur with only minimal release of primary and secondary granule constituents.1,10As the PMNs are activated and the receptors are translocated to the cell surface, the endocytic activity of the cells increases and substantial internalization of CR1 occurs, even in the absence of ligand.7,11 This internalization can be further enhanced by treating the cells with phorbol myristate acetate (PMA).12 13
The intracellular localization of CR1 in resting and activated cells has been described at the ultrastructural level using immunoelectron microscopy.6,11 In resting cells, CR1 is stored primarily in small vesicles, which appear distinct from the traditional granules.6 During activation, PMNs substantially increase fluid-phase endocytosis and develop vacuoles and multivesicular bodies, which are not seen in resting cells.11 Immunoelectron microscopy has shown that markers of fluid-phase endocytosis, as well as internalized CR1, are directed to these newly forming compartments.11
The sorting mechanism(s) that PMNs use to selectively store CR1 in the “secretory vesicles” remains unclear. The finding that the primary and secondary granules derive directly from the Golgi complex14 and are generated during different stages of development of the cell14,15 suggests that their components are selectively packaged according to their order of synthesis. It is not known whether this mechanism is also responsible for the formation of the secretory vesicles and for the storage of CR1 in their walls, or whether the proteins present in secretory vesicles contain sequences that serve as trafficking signals, which specifically determine their sorting into these readily mobilizable vesicles. This latter mechanism has been shown to be used for proteins whose plasma membrane expression can be rapidly upregulated in other types of cells.16-18
As CR1 contains none of the described sequences18 that could potentially target the receptor to the secretory vesicles, we investigated whether the order of its synthesis during cell development determines its intracellular localization, or whether the necessary information was contained in the sequence of the CR1 protein itself. We therefore used heterologous expression systems to study the trafficking and storage of human CR1 that was synthesized continuously under the control of a viral promoter.19 When CR1 is expressed in nonsecretory COS cells, it is present only on the plasma membrane. By contrast, when we expressed human CR1 in rat basophilic leukemia (RBL) cells, which, like PMN, are of granulocytic origin and contain well described exocytic compartments,20 we found that most of the receptors are stored intracellularly in structures resembling those in which it is found in PMN. In addition, we found that the cell surface expression increases rapidly in response to stimulation, exactly as described for PMN. CR1 in RBL cells is also reinternalized during endocytosis, again mimicking its trafficking in PMN.
Thus, continuously synthesized human CR1 in stably transfected RBL cells is stored and translocated in much the same way as in PMN, suggesting that the sorting mechanisms used by CR1 are similar in both cell types. The structure of the protein rather than the precise order of gene expression is thus likely to be the major determinant of the packaging of CR1 in the secretory vesicles.
MATERIALS AND METHODS
Cell lines and antibodies.
The COS-1 monkey kidney fibroblast cell line and RBL cells were both obtained from the American Type Culture Collection (Rockville, MD). Antibodies used included three mouse monoclonal antibodies that recognize different epitopes on human CR1: 3D9, C543, and YZ1, which have all been described previously9,12; W6/32, an antibody directed against human major histocompatability complex (MHC) class I, which cross-reacts with rat MHC class I (American Type Culture Collection); antirat CD71, directed against the transferrin receptor (TfR) (PharMingen, San Diego, CA); and YMC1019, a rat antibody directed against serotonin (Accurate Chemical & Scientific Corp, Westbury, NY). MOPC 21, an IgG1 (Sigma, St Louis, MO) was used as an isotype matched nonimmune control for 3D9 in fluorescence-activated cell sorting (FACS) and immunofluorescence microscopy analyses. Affinity isolated, fluorescein isothiocyanate (FITC)-conjugated goat F(ab')2antimouse IgG (Biosource, Camarillo, CA) was used as a second antibody in immunofluorescent microscopy and flow cytometry studies. FITC-conjugated goat F(ab′)2 antirat IgG (Cappel, Durham, NC) was used as a second antibody to detect the rat antibody YMC1019.
Cell lines expressing human CR1.
Cells were grown in Eagle's minimal essential medium with Earle's salts supplemented with nonessential amino acids, 1 mmol/L pyruvate and 20% heat inactivated fetal calf serum (FCS) (EMEMsup). RBL cells (1 × 106/mL) were transfected by electroporation at 300 V and 960 μF in 1 mL of ice cold phosphate-buffered saline (PBS) in the presence of 30 μg of pABCD and 0.3 μg PBSneo, which had been linearized with Sfi I and Xmn I, respectively. The plasmid pABCD contains the cDNA encoding full-length CR1 of the F allotype inserted into pAprM8, a plasmid that was originally derived from CDM8, and which uses a cytomegalovirus (CMV) promoter to drive the inserted cDNA.19,21 The plasmid pBSneo, carrying the gene for resistance to the antibiotic G418, was prepared by ligation of the 1.9-kb Sal I fragment of pMT.neo.I22 into the Sal I site of pBSKS+. Control cells were transfected in parallel with pAprM8, the plasmid without the CR1 cDNA insert, and pBSneo. Two days after electroporation, transfected cells were selected in EMEMsup containing 0.75 mg/mL G418. Clones that expressed human CR1 were identified by indirect immunofluorescence using the anti-CR1 antibody YZ1 after limiting dilution cloning and these results were confirmed using 3D9, which was used throughout the remainder of these experiments. We will refer to RBL cells expressing human CR1 as RBL-CR1. Once this cell line was established, cells were maintained in RPMI-1640 supplemented with 2 mmol/L L-glutamine, 100 U/mL each of penicillin and streptomycin, 10% heat inactivated FCS, and 0.25 mg/mL G418. All cell culture supplies were from GIBCO-BRL (Gaithersburg, MD), except for FCS, which was from HyClone (Logan, UT). COS cells were transiently transfected with pABCD using diethyl aminoethyl (DEAE)-dextran23 and analyzed 48 to 72 hours after transfection.
To confirm that 3D9 recognized only human CR1 in the transfected cell lines, we performed Western blots of lysates from both the transfected and control cell lines. The anti-CR1 antibody 3D9 detected a single band of approximately 200 kD, corresponding to CR1, only in those cell lines transfected with the plasmid containing the human CR1 cDNA. This band was absent from control cell lines, indicating that 3D9 does not recognize any intrinsic COS or RBL cell proteins.
Enzyme-linked immunosorbent assay (ELISA) and Western blot assays for CR1 and subcellular fractionation.
ELISA to quantitate total cellular CR1 and extracellular soluble CR1 was performed using the monoclonal antibodies 3D9 and C543 as previously described.1,24 Western blots were done using 3D9 and goat antimouse alkaline phosphatase conjugate.24Preparation of detergent lysates and nitrogen cavitates and subcellular fractionation on Percoll density gradients was performed as previously described.1 24
Immunofluorescence microscopy.
Cells were grown overnight on sterile coverslips in 24-well plates, then washed with PBS, and subsequently fixed in 2% paraformaldehyde in 0.1 mol/L phosphate buffer pH 7.4 for 30 minutes. Coverslips were then washed twice in PBS and permeabilized for 30 minutes with 0.15% saponin in blocking buffer consisting of PBS containing 0.1% bovine serum albumin (BSA), 20 mmol/L glycine and 1% cold water fish gelatin (Sigma). The coverslips were subsequently removed from the 24-well plates and preincubated with blocking buffer supplemented with 10% normal goat serum and 0.015% saponin (blocking buffer++) for 30 minutes, then transferred to blocking buffer++containing excess first antibody and incubated for 45 minutes at room temperature. Excess antibody was removed by six washes with blocking buffer containing 0.015% saponin. Coverslips were then incubated for 45 minutes with blocking buffer++ containing the second antibody. Excess second antibody was also removed by extensive washing. Cells were then washed one time in PBS, one time in water, and subsequently dehydrated in 70% alcohol and 100% alcohol. Coverslips were then allowed to dry and mounted on glass slides using mowiol25 and studied using an inverted Nikon microscope (Nikon Instrument Group, Melville, NY). Nonpermeabilized cells were stained using the same protocol except that saponin was excluded from all the buffers used.
Activation of RBL-CR1.
RBL were released from tissue culture flasks by a brief incubation with versene 1:5,000 (GIBCO-BRL). Flasks were tapped to dislodge the cells, which were then resuspended in complete culture medium to replace cations chelated by the versene. Cells were subsequently washed twice in Hanks' balanced salt solution (HBSS) without Ca++, Mg++, or Phenol red (GIBCO-BRL) but supplemented with 0.1% gelatin (Sigma) pH 7.4, (HBSS/g). Activation experiments were done exactly as previously described for PMNs.9 Basal medium for all experiments was HBSS/g and experiments were performed with 1 × 106cells/mL. Cells were activated by a 1-hour incubation at 37°C in HBSS/g containing 1.2 mmol/L Ca++and 1 μmol/L A23187 (Calbiochem, La Jolla, CA). Controls included cells incubated in HBSS/g alone, HBSS/g containing 1 μmol/L A23187, but no Ca++, and the ionophore with both Ca++and 5 mmol/L EDTA. In some experiments, cells were preincubated for 5 minutes at 37°C with 50 nmol/L PMA in HBSS/g, after which A23187 and Ca++ were added and incubation was continued for 1 hour. Cells were subsequently spun down and resuspended in Ca++ free HBSS containing 0.1% BSA, 0.05% NaN3 and 10-4 mol/L phenylmethyl sulfonyl fluoride (FACS buffer), and washed twice in this buffer. These cells were subsequently subjected to indirect immunofluorescent labeling (see below). Some experiments were performed in the presence of inhibitors of protein synthesis: puromycin or cycloheximide (10 μg/mL) were added to the cells 10 minutes before the addition of the ionophore and remained present throughout the experiment. A few experiments were performed in PIPES buffer that was formulated as follows: 25 mmol/L 1,4-piperazinediethanesulfonate (PIPES), 119 mmol/L NaCl, 5 mmol/L KCL, 5.6 mmol/L glucose 0.4 mmol/L MgCl2, and 0.1% BSA, pH 7.2.
Flow cytometry.
Cells (1 × 106 cells per tube) were incubated for 45 minutes at 4°C with a saturating amount of antibody in FACS buffer and were then washed and labeled with FITC-labeled second antibody as previously described.9 Fluorescence intensity was determined using a Becton Dickinson FACScan (Mountain View, CA) and data were analyzed using Consort 30 software (Becton Dickinson). Mean fluorescence values were determined by subtracting the background mean fluorescence of control samples that had been incubated with an irrelevant first antibody and the same FITC-labeled second antibody.
When comparing the CR1 distribution on intact cells with that in permeabilized cells, we first fixed the cells in ice cold periodate/lysine/paraformaldehyde26 for 20 minutes, then washed in blocking buffer, as described for immunofluorescence microscopy, and permeabilized the cells in blocking buffer containing 0.4 mg/mL saponin. Cells were then immunostained in the presence of excess human IgG to prevent nonspecific staining, and analyzed as described.
Degranulation as measured by the release of β-hexosaminidase.
Cells were activated as described above and a 0.5-mL aliquot was removed at the end of the incubation time. Cells were spun down, the supernatant was collected, and both supernatant and cell pellet were frozen at −80°C until the β-hexosaminidase assay was performed.27 The cell pellet was lysed in 0.5 mL of assay buffer, consisting of 0.1 mol/L citric acid and 0.05% Triton X-100. The procedure was done in a 96-well plate, each well containing 200 μL of the substrate 4-methylumbelliferyl-N-acetyl-β-D-glucoseaminide (Sigma) at 0.3 mg/mL in assay buffer. Samples (5 μL, 10 μL, 25 μL) of the supernatants and cell lysates were added and incubated for 60 minutes at 37°C, after which the reaction was stopped by transferring 100 μL of the reaction mixture to 1 mL of glycine stop buffer consisting of 133 mmol/L glycine, 83 mmol/L Na2CO3, 67 mmol/L NaCl pH 10.6. Samples were immediately read at 448 nm on a filter fluorimeter. β-Hexosaminidase activities measured were corrected for the sample volume, and the release into the supernatant was expressed as a percentage of the total activity measured in the supernatant and cell pellet.
Immunoelectron microscopy.
Preparation of ultrathin cryosections for immunoelectron microscopy28 and the staining of CR1 in the sections has been previously described.11 As an endocytic tracer, BSA was coupled to 10 nm gold as described previously29 and added to RBL cells at a final protein concentration of 0.5mg/mL and incubated for 1 hour at 37°C. Cells were subsequently processed for immunoelectron microscopy as previously described.11
RESULTS
Constitutive expression of CR1 in cell lines.
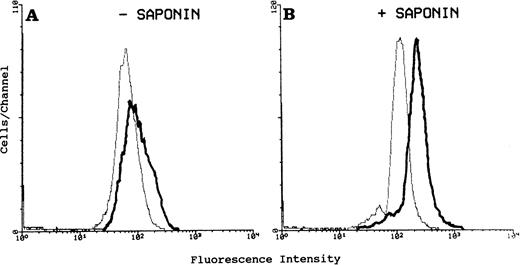
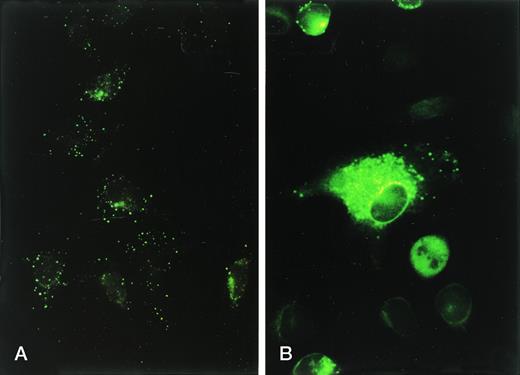
We expressed CR1 in COS-1 cells, which are derived from monkey kidney fibroblasts,23 and in RBL cells, which like PMNs, are of granulocytic lineage and exhibit well-defined exocytosis.27 29 In the COS cells, we found that CR1 was expressed exclusively on the plasma membrane: FACS analysis showed little difference between the amount of CR1 detectable on the surface of intact cells and the total detectable in saponin permeabilized cells, indicating that little or no CR1 was stored inside the COS cells (not shown). Furthermore, the cell surface expression did not change in response to stimulation with A23187 and Ca++ (not shown). By contrast, when studying RBL cells in which the same plasmid was used to direct expression of human CR1 (RBL-CR1), we found that the total cellular content of CR1, as measured by FACS analysis of saponin-permeabilized cells, was much greater than the amount of CR1 measured on the cell surface of nonpermeabilized cells (Fig 1A and B). The percentage of total CR1 present on the cell surface in four different experiments was only 24.7% + 4.8% of the total CR1 present in the permeabilized cells. Fluorescence microscopy showed very little labeling for CR1 on the surface of the nonpermeabilized cells (Fig2A). In the permeabilized cells, CR1 could be readily detected and was found in punctate structures scattered diffusely throughout the cytoplasm (Fig 2B). The labeling intensity varied considerably between cells, with some cells showing very bright staining and others showing only minimal labeling. Overall, different preparations of RBL-CR1 contained from 25% to 90% as much total CR1 per cell as mature human peripheral blood PMN, and more than 60% of the total CR1 produced by RBL-CR1 cultures was in intact cells versus in the culture media.
FACS analysis of human CR1 in intact versus permeabilized RBL-CR1 cells. Cells were fixed in periodate/lysine/paraformaldehyde with (B) and without (A) permeabilization with saponin. Cells were then stained with anti-CR1 monoclonal antibody 3D9 or control antibody MOPC 21; followed by FITC-conjugated antimouse antibody. A total of 10,000 cells from each preparation was analyzed. Mean fluorescence values are: (A) background (gray line) 66; CR1 (black line) 99. (B) Background (gray line) 109; CR1 (black line) 232.
FACS analysis of human CR1 in intact versus permeabilized RBL-CR1 cells. Cells were fixed in periodate/lysine/paraformaldehyde with (B) and without (A) permeabilization with saponin. Cells were then stained with anti-CR1 monoclonal antibody 3D9 or control antibody MOPC 21; followed by FITC-conjugated antimouse antibody. A total of 10,000 cells from each preparation was analyzed. Mean fluorescence values are: (A) background (gray line) 66; CR1 (black line) 99. (B) Background (gray line) 109; CR1 (black line) 232.
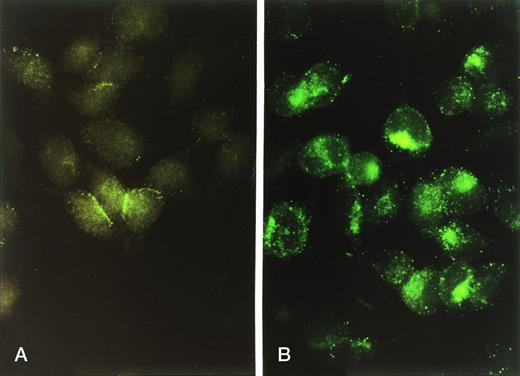
Indirect immunofluorescent staining of human CR1 in intact (A) versus permeabilized (B) RBL-CR1 cells. Cells were stained as in Fig 1A and B, respectively, and prepared for microscopy as described in Materials and Methods.
Indirect immunofluorescent staining of human CR1 in intact (A) versus permeabilized (B) RBL-CR1 cells. Cells were stained as in Fig 1A and B, respectively, and prepared for microscopy as described in Materials and Methods.
CR1 expression on the plasma membrane of RBL transfectants is upregulated in response to the influx of extracellular Ca++.
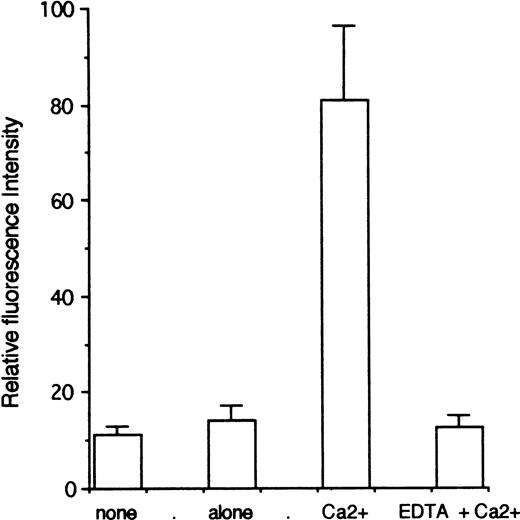
Because the immunofluorescence data suggested that a substantial fraction of the CR1 in the RBL transfectants was stored inside the cell, we wished to determine whether RBL-CR1 cells were capable of translocating the intracellular CR1 to the cell surface. In PMN, the influx of extracellular Ca++ induced by the ionophore A23187 leads to a substantial increase in plasma membrane expression of CR1 as a result of the translocation of CR1 storage vesicles to the cell surface.9 To establish whether Ca++ influx results in increased surface expression of CR1 in RBL-CR1 as well, we incubated the cells with ionophore A23187 and Ca++ and quantified the plasma membrane CR1 expression by FACS analysis, as shown in Fig 3. Baseline CR1 expression was measured on cells that were incubated in media without ionophore (Fig 3, none). In these experiments, the mean CR1 expression increased about eightfold on the addition of A23187 and Ca++ (Fig 3, Ca2+), and this increase could be completely inhibited by the addition of the chelator EDTA. When the ionophore was added in the absence of Ca++ (Fig 3, alone) the expression also remained at baseline level. Puromycin or cycloheximide (10 μg/mL), which inhibit protein synthesis, had no effect on the upregulation of CR1 (data not shown) suggesting that new protein synthesis is not required for this CR1 upregulation.
Ionophore-activation of RBL-CR1 results in increased cell surface expression of CR1. Cells were incubated for 60 minutes at 37°C in HBBS/g containing l μmol/L A23187 to which 1.2 mmol/L CaCl2 was added (Ca2+). Controls included: cells incubated in HBSS without A23187 (none); only A23187, without CaCl2 (alone); and A23187 with CaCl2 plus 5 mmol/L EDTA. Cells were stained as described and the mean fluorescence of 5,000 cells from each condition was determined by FACS analysis. Results are the mean ± standard error of mean (SEM) for four to six experiments.
Ionophore-activation of RBL-CR1 results in increased cell surface expression of CR1. Cells were incubated for 60 minutes at 37°C in HBBS/g containing l μmol/L A23187 to which 1.2 mmol/L CaCl2 was added (Ca2+). Controls included: cells incubated in HBSS without A23187 (none); only A23187, without CaCl2 (alone); and A23187 with CaCl2 plus 5 mmol/L EDTA. Cells were stained as described and the mean fluorescence of 5,000 cells from each condition was determined by FACS analysis. Results are the mean ± standard error of mean (SEM) for four to six experiments.
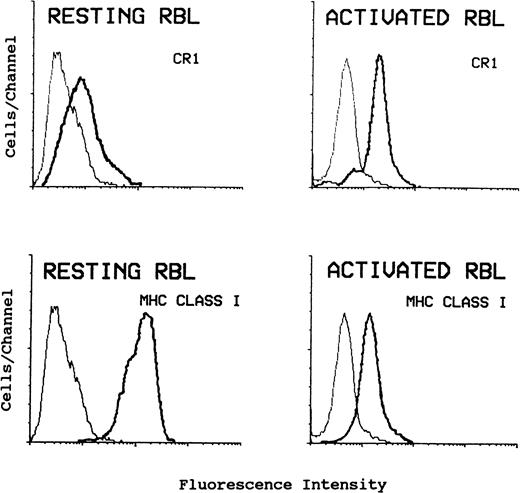
To establish whether CR1 upregulation results from a general membrane perturbation or represents a specific translocation of CR1 containing structures from inside the cell, we also studied the MHC class I expression on resting and ionophore-activated cells. Surface expression of CR1 and MHC class I was determined by FACS analysis as shown in Fig 4. MHC class I expression is high on resting RBL cells (Fig 4, lower left panel). On activation of RBL-CR1 with A23187 and Ca++, the increased surface expression of CR1 (Fig 4, upper right panel) is actually accompanied by decreased expression of MHC class I (Fig4, lower right panel). The transferrin receptor present on RBL is also downregulated on ionophore activated cells (data not shown). These results suggest that there is specificity in the movement of CR1 and that endocytosis, as well as exocytosis, increases when RBL are activated, as is the case with PMN.
Flow cytometric analysis of CR1 and MHC class I on resting and activated RBL-CR1 shows that the increased cell surface expression of CR1 on activated cells coincides with decreased expression of MHC class I. Cells were either incubated in HBSS/g (resting) or in HBBS/g containing 1 μmol/L A23187 and 1.2 mmol/L CaCl2 (activated) for 1 hour at 37°C. Cells were stained using anti-MHC class I, W6/32 (lower panels), or anti-CR1, 3D9 (upper panels) as described, and analyzed by FACS analysis. Light lines are for isotype-matched control as first antibody.
Flow cytometric analysis of CR1 and MHC class I on resting and activated RBL-CR1 shows that the increased cell surface expression of CR1 on activated cells coincides with decreased expression of MHC class I. Cells were either incubated in HBSS/g (resting) or in HBBS/g containing 1 μmol/L A23187 and 1.2 mmol/L CaCl2 (activated) for 1 hour at 37°C. Cells were stained using anti-MHC class I, W6/32 (lower panels), or anti-CR1, 3D9 (upper panels) as described, and analyzed by FACS analysis. Light lines are for isotype-matched control as first antibody.
CR1 is not present in the classical granules of RBL-CR1.
In resting PMN, CR1 is stored in vesicles that are distinct from the primary and secondary granules, which contain the cells' major secretory products.6 In RBL cells, the large dense granules characteristic of basophils and mast cells are absent, but instead these cells have numerous pleiomorphic granules that vary in size.20 To identify the intracellular compartment in which the CR1 is stored, we first performed subcellular fractionation experiments in which nitrogen cavitates of RBL-CR1 cells were subjected to Percoll density gradients as described by Borregaard.31Cavitates of PMN were run in parallel. Similar results were found for both cell types, with 84.5% of the total CR1 in the RBL cells sedimenting in the γ-band or light membrane fraction and 86.5% of the CR1 in PMN sedimenting in this fraction. With RBL-CR1, most of the remaining CR1 was in lighter fractions and less than 1% was in heavier fractions corresponding to denser granules; while with PMN, 5% was in lighter fractions and 8.4% was in heavier fractions.
To further characterize the intracellular storage sites of CR1 in the RBL-CR1 cells we used immunofluorescent staining to localize serotonin, a major secretory product that is stored in the granules of these cells.32 In Fig 5B (see page 303), we show that serotonin is stored in granular structures that are located in a cluster in the vicinity of the nucleus. The appearance and juxtanuclear localization of the large serotonin-containing granules contrasts sharply with the cytoplasmic distribution of the small punctate CR1-containing structures (Fig 5A), suggesting that CR1 and serotonin are not present in the same structures.
Indirect immunofluorescent staining of CR1 versus serotonin in RBL-CR1. Cells were grown on glass coverslips, fixed, and saponin permeabilized and stained using anti-CR1 and FITC-antimouse immunoglobulin (A) or antiserotonin YMC1019 (rat monoclonal antiserotonin) followed by FITC-antirat immunoglobulin (B).
Indirect immunofluorescent staining of CR1 versus serotonin in RBL-CR1. Cells were grown on glass coverslips, fixed, and saponin permeabilized and stained using anti-CR1 and FITC-antimouse immunoglobulin (A) or antiserotonin YMC1019 (rat monoclonal antiserotonin) followed by FITC-antirat immunoglobulin (B).
Upregulation of CR1 in RBL-CR1 does not coincide with secretion of β-hexosaminidase.
In PMNs, the requirements for mobilization of the CR1-containing secretory vesicles differ considerably from those of the traditional granules, and as a result, plasma membrane expression of CR1 can be upregulated without secretion of the traditional granules' contents.1,10 β-hexosaminidase, like serotonin, is a component of the RBL granules whose release has been used as a measure of exocytosis.27 To establish whether CR1 upregulation in RBL-CR1 cells coincides with secretion of β-hexosaminidase, we activated the cells and evaluated both CR1 expression and β-hexosaminidase release. In Fig 6, comparison of bars labeled B with those labeled A shows that ionophore activation leads to eightfold upregulation of surface CR1 (Fig 6, left panel), but to scarcely any release of β-hexosaminidase above the baseline level (Fig 6, right panel). Exocytosis and the resulting release of preformed mediators such as β-hexosaminidase and serotonin from RBL granules has been extensively studied and it has been reported that pretreatment of RBL cells with PMA before ionophore treatment significantly enhances secretion.30,33 34 Therefore, we also assessed CR1 expression and degranulation in PMA pretreated cells, as shown in the bars labeled C. This treatment did not alter CR1 expression from that observed with ionophore and Ca++ alone (Fig 6, left panel), but the release of β-hexosaminidase was significantly increased, amounting to 30% of the total β-hexosaminidase content of the cell (Fig 6, right panel). These data suggest that the compartments in which CR1 is stored in these RBL transfectants are distinct from the classically described RBL granules and have different mobilization requirements.
Ionophore activation of RBL-CR1 results in increased cell surface expression of CR1, but not in the release of the granule constituent β-hexosaminidase. (A) Resting cells in HBSS/g; (B) cells activated with A23187 and Ca++ as described; (C) cells pretreated for 5 minutes with 50 nmol/L PMA, then activated with A23187 and Ca++ as in (B). CR1 expression was examined by FACS analysis (left panel). Degranulation was assessed by determining the β-hexosaminidase content of both the cell pellet and the supernatant and calculating the percent β-hexosaminidase that was released (right panel). Results are given as the mean ± SEM for four experiments.
Ionophore activation of RBL-CR1 results in increased cell surface expression of CR1, but not in the release of the granule constituent β-hexosaminidase. (A) Resting cells in HBSS/g; (B) cells activated with A23187 and Ca++ as described; (C) cells pretreated for 5 minutes with 50 nmol/L PMA, then activated with A23187 and Ca++ as in (B). CR1 expression was examined by FACS analysis (left panel). Degranulation was assessed by determining the β-hexosaminidase content of both the cell pellet and the supernatant and calculating the percent β-hexosaminidase that was released (right panel). Results are given as the mean ± SEM for four experiments.
The fact that we did not detect β-hexosaminidase release from RBL-CR1 treated with A23187 and Ca++ was surprising, as ionophore activation is generally reported to result in the secretion of RBL granule contents.33,34 We postulated that this might be due to our use of the same experimental conditions as used for studies of CR1 in PMN, with HBSS as the incubation buffer. We therefore performed ionophore stimulation in PIPES buffer, which has been more commonly used in this type of study in RBL cells. β-hexosaminidase release in response to A23187 plus Ca++ under these conditions amounted to 25% of the total β-hexosaminidase content (not shown) and pretreatment with PMA before activation in PIPES buffer increased the release to about 60% of the total (not shown), in agreement with previous studies.34 Thus, while secretion of β-hexosaminidase by RBL-CR1 in HBSS differs from that generally reported in PIPES buffer, the use of the former did allow us to delineate the differences in mobilization requirements of the CR1 containing vesicles versus the granules.
Ultrastructural localization of CR1 in RBL-CR1.
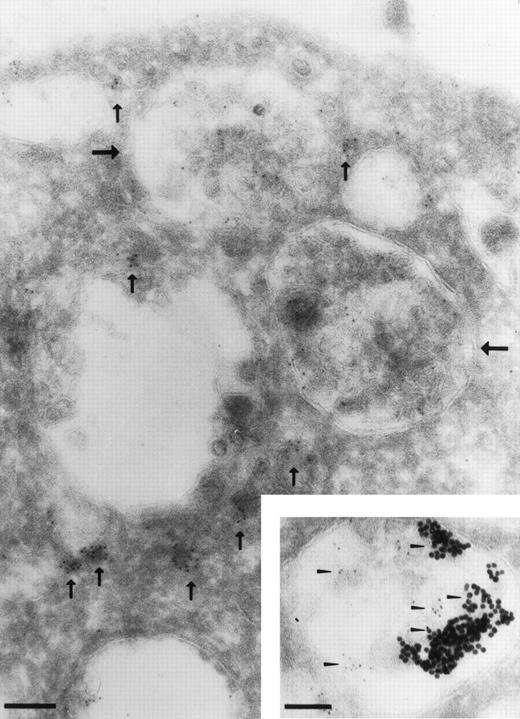
To better characterize the structures in which the intracellular CR1 is stored in RBL-CR1 cells, we prepared cryosections, which were immunogold-labeled for CR1 and analyzed in the electron microscope. Figure 7 shows that a small amount of CR1 is found on the cell surface, while much more is in small vesicles and in multivesicular bodies. The small vesicles (small arrows) are very similar to the small vesicles in which CR1 is present in resting PMN,6 while the large multivesicular bodies (large arrows) resemble the compartment into which CR1 is internalized in activated PMN.11
Immunoelectron microscopic localization of CR1 in resting RBL. Cryosections were stained with a mixture of two monoclonal antibodies directed against CR1 (3D9 and C543) followed by rabbit antimouse IgG conjugated to 5 nm gold. CR1 is shown on the cell surface, in small vesicles (small vertical arrows), and in multivesicular bodies (large horizontal arrows). The inset shows RBL-CR1 cells that were incubated with BSA conjugated to 20 nm gold for 30 minutes at 37°C before preparation for cryosectioning. CR1 was labeled with 5 nm gold as described above. Colocalization of the endocytic marker BSA-gold (large particles) and CR1 (small arrow heads) was observed in multivesicular bodies. Bar = 0.1 μm.
Immunoelectron microscopic localization of CR1 in resting RBL. Cryosections were stained with a mixture of two monoclonal antibodies directed against CR1 (3D9 and C543) followed by rabbit antimouse IgG conjugated to 5 nm gold. CR1 is shown on the cell surface, in small vesicles (small vertical arrows), and in multivesicular bodies (large horizontal arrows). The inset shows RBL-CR1 cells that were incubated with BSA conjugated to 20 nm gold for 30 minutes at 37°C before preparation for cryosectioning. CR1 was labeled with 5 nm gold as described above. Colocalization of the endocytic marker BSA-gold (large particles) and CR1 (small arrow heads) was observed in multivesicular bodies. Bar = 0.1 μm.
To investigate if the CR1 containing multivesicular bodies in RBL-CR1 are also formed by endocytosis, as in PMN,11 we incubated the cells in the presence of the endocytic tracer BSA-gold and then immunostained for CR1 as well. Figure 8shows that small vesicles and tubule-like structures at the periphery of RBL-CR1 cells, which contain the endocytic tracer (large gold particles) also are positive for CR1 (small gold particles, arrows), suggesting that small vesicles containing CR1 and the tracer are formed during this process, as we have previously shown in PMN.6 11 The inset to Fig 7 shows that CR1 and BSA-gold subsequently become colocalized in the multivesicular bodies. These data suggest that, like in PMN, the CR1 in the multivesicular bodies has been expressed on the cell surface and reinternalized by endocytosis.
Higher magnification immunoelectron micrograph of periphery of RBL-CR1 incubated with 10 nm BSA-gold endocytic tracer (large particles) and immunostained for CR1 (5 nm gold, arrows). Many vesicles contain both CR1 and endocytic tracer. Bar = 0.1 μm.
Higher magnification immunoelectron micrograph of periphery of RBL-CR1 incubated with 10 nm BSA-gold endocytic tracer (large particles) and immunostained for CR1 (5 nm gold, arrows). Many vesicles contain both CR1 and endocytic tracer. Bar = 0.1 μm.
DISCUSSION
Many cells respond to environmental stimuli by regulating the expression of functionally important proteins on their surface. Human PMN respond by translocating an intracellular pool of small, CR1-containing, secretory vesicles to the cell surface, thus increasing the plasma membrane expression of this receptor several fold. The mechanism by which CR1 and other proteins are sequestered within this compartment remains unclear. One possibility is that proteins to be stored in the secretory vesicles are all synthesized together, at a certain specific time during the development of the PMN, and thus are all packaged together as the vesicles are initially formed by budding off of the endoplasmic reticulum. This would be in agreement with the mechanism that is used to specifically store the respective constituents of the primary and secondary granules in PMN.14,35 Alternatively, specific protein sequences could govern the intracellular sorting of proteins, as has been reported for numerous other integral membrane proteins in a variety of cells.36 To determine whether differential gene expression or the sequence of the protein itself is responsible for the storage of CR1 within secretory vesicles, we used a vector in which CR1 gene expression is driven by a cytomegalovirus promoter so that the receptor would be synthesized continuously, rather than only during a particular phase of cell development.19
We initially transfected CR1 into COS-1 cells, a nonsecretory type of cell derived from monkey kidney fibroblasts. In these cells, CR1 is constitutively expressed on the plasma membrane with little or no intracellular storage pool and no upregulation in response to increased intracellular Ca++. These results may be due to the absence from COS cells of compartments similar to those in which CR1 is present in PMN. COS cells have been reported to be deficient in packaging markers of other types of rapidly translocatable vesicles as well. For example, synaptophysin, a component of synaptic vesicles in nerve cells, is produced after transfection, but is not targeted correctly in these cells, either.37 We subsequently expressed CR1 in RBL cells, as these cells, like PMN, are of granulocytic origin and have secretion as a major function. Other investigators have reported that granular proteins of PMN are correctly processed and targeted to granules in RBL, as well.38 We therefore hypothesized that these cells might use vesicle trafficking and membrane retrieval mechanisms similar to those found in PMN.
This cell line indeed proved more suitable for modeling the behavior of CR1 in PMN. Having found that only a minority of the human CR1 produced in the RBL transfectants was expressed on the plasma membrane, we subsequently investigated whether CR1 could be upregulated on activation of the RBL-CR1. With 1 μM A23187 and 1.2 mmol/L Ca++, which results in maximal expression of CR1 in PMN,9 the plasma membrane CR1 expression in RBL-CR1 increased 3-fold to 10-fold, while plasma membrane MHC class I expression decreased 3-fold to 6-fold. The fact that the upregulation of CR1 coincides with the downregulation of MHC class I suggests that the upregulation of CR1 is specific, not just the result of general membrane perturbation, and that it is accompanied by an increased level of endocytosis, as has been described for PMN. The rapidity with which the CR1 expression increases and the lack of effect of the protein synthesis inhibitors suggest that new protein synthesis is not involved in the increased cell surface expression and therefore that the upregulation must be due to the translocation of the intracellular storage compartments. These data thus show that the packaging of CR1 into intracellular compartments that are capable of translocating to the cell surface in response to stimulation does not require differential timing of gene expression.
Having established that the upregulation of CR1 on RBL-CR1 cells is due to the translocation of an intracellular pool of receptors, we investigated whether CR1 was present in the traditional granules or in smaller vesicles that do not contain the cells' major secretory products. Subcellular fractionation experiments showed that the bulk of the CR1 in RBL-CR1 was present in a light membrane fraction quite similar to the “γ-band” in which CR1 is found in PMN, and which is quite distinct from the traditional granules.1,31Indirect immunofluorescence of CR1 and serotonin, one of the main constituents of the RBL granules, showed that their distribution differed considerably. While the serotonin granules are relatively larger and primarily located close to the nucleus, the CR1 appeared to be in small punctate structures, which were widely distributed throughout the cytoplasm. Furthermore, upregulation of CR1 expression on the plasma membrane could be achieved without secretion of the granular enzyme β-hexosaminidase. This suggests that like in PMN, CR1 in RBL-CR1 is stored separately from the major secretory products and that RBL-CR1 cells are capable of secretory responses in which the mobilization requirements of the CR1-containing compartments are different from those of the β-hexosaminidase–containing granules. This is in agreement with the situation in PMN, where maximal upregulation of plasma membrane expression of CR1 does not necessarily coincide with the release of the constituents of the classical granules.1 10
In resting PMN, CR1 is found primarily in small electrolucent vesicles.6 When the PMN are activated, the receptors are rapidly translocated to the cell surface, after which they are reinternalized into multivesicular bodies.11 Our immunoelectron microscopy studies in unstimulated RBL-CR1 show that CR1 is already present in multivesicular bodies, as well as in small electrolucent vesicles. Using BSA-gold to define the endocytic pathway, we determined that small vesicles bearing CR1 and containing this tracer are formed during endocytosis, and that the multivesicular bodies in RBL, like those in PMN,11 also result from this process. These findings suggest that CR1 is not only correctly packaged in vesicles in RBL, but that subsequent trafficking of CR1 in RBL parallels that in activated PMN. Thus, the RBL cells, in their steady-state, resemble partially activated PMN, as multivesicular bodies are not found in resting PMN and only develop when the cells are activated.11 However, the presence of CR1 in small vesicles that morphologically resemble the secretory vesicles in resting PMN; and the fact that ionophore activation of the transfected RBL cells results in upregulation of CR1 on the plasma membrane, indicate that RBL-CR1 cells retain some qualities of resting PMN, as well. The “secretory vesicles” in which CR1 is found in resting PMN are believed to arise in a process involving endocytosis, as soluble plasma proteins are also found in these vesicles and are secreted when the vesicles fuse with the plasma membrane.1 30 Our observations that in RBL-CR1 as well, small CR1-bearing vesicles also contain endocytic tracers, suggests that the formation of these vesicles in the RBL-CR1 involve processes similar to those in PMN.
Because the CR1 gene in the RBL cells is driven by a CMV promoter, our data suggest that the sorting and regulated plasma membrane expression of CR1 does not rely on the timing of the gene expression, but is determined by the protein itself when it is expressed in cells that use the appropriate sorting and trafficking mechanisms. The small vesicles, which contain CR1 functionally and structurally, resemble the small vesicles in which functionally important membrane proteins are stored in many types of cells. Like the vesicles in which CR1 is stored in PMN, the glucose transporter GLUT-4 is stored in small “insulin responsive vesicles” in fat and brain cells.18,39 These vesicles can be rapidly mobilized, markedly increasing those cells' uptake of glucose in response to their specific stimulus, insulin. Unlike CR1, GLUT-4 is a complex protein with 12 transmembrane domains whose amino and carboxy termini are cytoplasmic. This molecule has been extensively studied and several sequences including a di-leucine motif and a specific phenylalanine residue have been found to determine its intracellular trafficking and storage.18,39 Only the C-terminal of CR1 is cytoplasmic, and its tail lacks both of these signals, indicating that other previously unrecognized sorting sequences32 or mechanisms are responsible for the storage and trafficking of CR1 in myeloid cells.
Thus, the characterization of a cell line such as RBL-CR1, in which CR1 is stored and translocated in much the same way as in PMN, will greatly facilitate studies of the mechanisms involved in the packaging and trafficking of CR1 in myeloid cells. In particular, the use of mutant CR1 genes will allow investigation of the sequences that determine the sorting of this important protein.
Supported by Grant No. AI 22687 from the National Institutes of Health, Bethesda, MD (to M.B.). L.K. is the recipient of an Investigator Award from the Arthritis Foundation, Atlanta, GA.
Address reprint requests to Melvin Berger, MD, PhD, Immunology Division, Rainbow Babies and Children's Hospital, 2101 Adelbert Rd, Cleveland, OH 44106.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal