The role of neutrophils during Epstein-Barr virus (EBV) infection is not known. Disruption of the initial and nonspecific immune response may favor the spread of EBV infection. We have previously shown that EBV interacts with human neutrophils and modulates protein expression. In this study we have investigated the ability of EBV to infect neutrophils. Electron microscopy studies showed penetration of virus and its subsequent localization to the nucleus. The presence of viral genomes in isolated nuclei from neutrophils was also shown by polymerase chain reaction (PCR). Expression of viral transcripts like EBNA-2 (Epstein-Barr nuclear antigen-2) and ZEBRA (BamHI Z EBV replication activator) was not detected by reverse transcriptase (RT)-PCR, suggesting that EBV does not seem to establish a latent or a lytic infection in neutrophils. However, at 20 hours post-EBV infection, 77% of cells were apoptotic as compared to 22% in uninfected cell cultures, as evaluated by flow cytometry. This EBV-induced apoptosis was prevented by the addition of granulocyte-macrophage colony-stimulating factor to the cell cultures. Apoptotic cell death seems to implicate the Fas/Fas ligand (L) pathway, as reflected by an increase of Fas/Fas L expression on neutrophils treated with EBV and an increase of soluble Fas L, which may function in an autocrine/paracrine pathway to mediate cell death. Lastly, EBV genome was detected from neutrophils of infectious mononucleosis (IM) patients in contrast to neutrophils obtained from healthy EBV-seropositive donors. Our findings on the interactions of EBV with neutrophils will then provide new insights on the immunosuppressive effects associated with EBV infection.
IMMUNE RESPONSE GENERATED against viral infections involves both nonspecific and antigen-specific mechanisms. Nonspecific defense mechanisms, delivered by macrophages and neutrophils, are rapidly inducible and play a crucial role during the early phase of viral infections. In fact, the number and distribution of neutrophils throughout the body ensures that they are often the first leukocytes to encounter invading organisms. The immune functions of neutrophils in the control of infectious agents are mainly associated with phagocytosis and the production of degradative enzymes and oxygen-free radicals. Neutrophils can also synthesize and release several immunoregulatory proteins after stimulation with different agonists.1,2 Therefore, impairment of these functions may partially suppress the immune response. The role of neutrophils in the control of viral infection is not well documented. It was previously reported that human immunodeficiency virus, cytomegalovirus, and influenza virus can interact with phagocytes, resulting in a decrease in natural immunity.3-7 However, the outcome of an interaction between Epstein-Barr virus (EBV) and neutrophils has yet to be studied.
EBV, which belongs to the family of “Herpesviridae,” is known to infect human B lymphocytes and epithelial cells of the oropharynx through the CD21 receptor.8 However, growing evidence suggests that EBV may interact with a wider range of cells than previously believed. For example, several reports have described patients with EBV-genome–positive T-cell lymphoma.8 EBV was also found to bind and infect human thymocytes and T-cell lines HSB-2, Jurkat, and HPB-ALL.9-12 Furthermore, it was observed that EBV can bind to and activate CD8+ peripheral T lymphocytes and monocytes without penetrating the cells.13-15 These studies showed that EBV binding involved a ligand distinct from CD21, suggesting the existence of an additional EBV receptor as previously proposed by others.11 16
We have recently reported that EBV can bind to neutrophils and cause cellular aggregation and protein synthesis.17 18 Here we report the penetration of EBV into neutrophils and its subsequent localization to the nucleus. Although no evidence is supportive of the establishment of a latent or a lytic infection, we observed that EBV induces apoptosis in human neutrophils. This effect of EBV on neutrophils may represent an alternative mechanism by which the virus suppresses the immune response.
MATERIALS AND METHODS
Neutrophil isolation.
Neutrophils were isolated from venous blood obtained from normal healthy volunteers using Ficoll-Hypaque density centrifugation (Pharmacia-Biotech Inc, Baie d'Urfé, Canada) as previously described.18 All neutrophil preparations contained fewer than 1% monocytes as determined by monoesterase staining and less than 0.3% of B and T lymphocytes, as evaluated by cytometry using anti-CD2, anti-CD3, and anti-CD19 monoclonal antibodies (MoAbs) (Becton Dickinson, San Jose, CA). Viability, estimated by the trypan blue-dye exclusion procedure, was greater than 99% in all preparations.
General incubation conditions.
Neutrophils were incubated in Hank's balanced saline solution (HBSS) (GIBCO-BRL, Burlington, Ontario, Canada) supplemented with 1% of heat-inactivated autologous plasma and 10 mmol/L of HEPES buffer for all experiments, except for studies of apoptotic cells which were performed in RPMI-1640 supplemented with 10% of heat-inactivated fetal bovine serum (FBS). Culture medium tested for the presence of endotoxin by the limulus amebocyte assays (Sigma, St Louis, MO) contained less than 10 pg/mL of contaminating endotoxins. Neutrophils were incubated at specified cells densities with either infectious EBV or 3 nmol/L recombinant human granulocyte-macrophage colony-stimulating factor (GM-CSF; provided by Genetics Institute, Boston, MA) for the indicated times.
Virus preparations.
Viral preparations of EBV strain B95-8 were produced as previously described.18 Briefly, B95-8 cells (which were mycoplasma-free tested) were grown in RPMI-1640 medium supplemented with 10% heat-inactivated FBS. When the viability of cell cultures reached 20% or less, as determined by trypan blue-dye exclusion, cell-free culture supernatants were obtained and filtered through a 0.45-μm pore size filter, and viral particles were purified by differential ultracentrifugation. Virus stocks were resuspended in RPMI-1640, aliquoted, and stored at −80°C until use. Viral titers were measured and evaluated at 107 transforming units (TFU/mL).
Electron microscopy.
Neutrophils were resuspended in cold HBSS and incubated with EBV at 4°C for 5 to 15 minutes to allow binding to the cell surface. Virus-bound cells were then cultured at 37°C for varying time periods and processed for electron microscopy examination as described.19 20 Briefly, cells were fixed for 1 hour in 1.5% glutaraldehyde in 0.1 mol/L cacodylate buffer pH 7.3. After fixation, cells were pelleted and pre-embedded in 20% bovine serum albumin (BSA), polymerized with 25% glutaraldehyde, and cut in one millimeter3. The blocks were rinsed with buffer, postfixed in 1% buffered osmium tetroxyde, treated with 0.1% buffered tannic acid, and stained in bloc with 2% ethanolic uranyl acetate. The neutrophils were dehydrated through a graded series of ethanol and propylene oxide, and embedded in Epon 812 (JBem Services Inc, Quebec, Canada). Ultrathin sections were cut with a diamond knife on an Ultracut S ultramicrotome (Leica Canada Inc, Montreal, Canada) and mounted on 200 mesh copper grids. The sections were stained with 2% uranyl acetate and 0.5% lead citrate, and examined with a Jeol 1010 electron microscope (Jeol Canada Inc, St Hubert, Canada).
DNA isolation and Southern blot analysis.
Neutrophils were incubated for various periods of time in the presence or absence of EBV and then washed three times with HBSS to remove residual virus. For Southern blotting analysis, DNA was extracted as described.21 RNA was removed by RNASE ONE (Promega, Madison, WI) treatment and DNA was digested with BamHI. Ten micrograms of DNA cells were loaded onto a 0.6% agarose gel and size-fractionated by electrophoresis. Transfer onto HYBOND-N membrane (Amersham Canada Limited, Oakville, Ontario, Canada) was performed by capillary diffusion in 10× sodium saline citrate (SSC) overnight. After prehybridization the membranes were hybridized with random-primed 32P-labeled probes in 50% formamide overnight at 42°C. The membranes were then washed and exposed to Kodak X-OMAT films (Eastman Kodak, Rochester, NY) with an intensifying screen at −70°C. The BamHI W probe was a 400-bp polymerase chain reaction (PCR) amplicon located in the BamHI W region of the EBV genome (primer 1: 5′ GCAGTAACAGGTAATCTCTG 3′, positions 20124-20143 and primer 2: 5′ ACCAGAAATAGCTGCAGGAC 3′, positions 20523-20504.22 The glyceraldehyde 3-phosphate dehydrogenase (GAPDH) cDNA probe was used as control to demonstrate equal loading of DNA in each lane. For each sample the amount of viral DNA was quantitated relatively to their respective levels of GAPDH. The given ratio was compared with that obtained in unstimulated cells, using this formula: (optical density [OD] of DNA of the sample/OD of GAPDH of the sample)/(OD of DNA of unstimulated cells/OD of GAPDH of unstimulated cells).
Preparation of nuclei.
Neutrophils (10 × 106 cells/mL) were pretreated with cytochalasin B (an inhibitor of phagocytosis) (Sigma, Oakville, Ontario, Canada) at 10 μmol/L and infected with EBV. After 10 hours of culture, cells were washed and resuspended in ice-cold buffer containing sucrose 0.25 mol/L, HEPES 10 mmol/L, EGTA 1 mmol/L, and protease inhibitors, phenylmethylsulfonyl fluoride (PMSF) 1 mmol/L, aprotinin and leupeptin 100 μg/mL. Nuclei were then extracted as previously described.23 Briefly, neutrophils were sonicated on ice (3 × 20 seconds, at a power setting of 2 and 60% duty cycle) in a Branson Ultrasonic Processor (VWR/Canlab, Montreal, Quebec, Canada). Sonicates were centrifuged at 12,000g, 10 minutes, 4°C. The corresponding pellets referred to nuclei of neutrophils and the supernatants referred to cellular membranes and cytosols. Nuclei were resuspended in HBSS in DNA was extracted as described above. The presence of EBV genome was evaluated by PCR using theBamHI W primers detailed in the previous section.
Detection of EBV genome in neutrophils from infectious mononucleosis (IM) patients.
Neutrophils from IM patients were isolated and DNA was extracted as described above. Neutrophils obtained from healthy EBV-seropositive donors were also used as negative controls. The presence of EBV genome was evaluated by PCR analysis using the BamHI-W primers detailed in the previous section. PCR was performed in Perkin Elmer Gene Amp 9600 (Cetus, Emeryville, CA) for 35 cycles (denaturation: 60 seconds at 95°C, ramp 60 seconds; annealing: 30 seconds at 55°C , ramp 30 seconds; and extension: 30 seconds at 72°C, ramp 60 seconds). Amplified product was detected by hybridization to an32P-end-labeled oligonucleotide probe: 5′-TATCTTTAGAGGGG AAAAGAGGAATAAG-3, positions 20313-20340.
RNA isolation and RT-PCR amplification.
Unstimulated cells and EBV-treated neutrophils (50 × 106 cells/mL) were cultured for various time periods before RNA extraction. Total RNA was extracted by the TRIzol Reagent (GIBCO-BRL) method according to the manufacturer. First-strand cDNA was made using Moloney-murine leukemia virus (M-MLV) reverse transcriptase (RT) using downstream primer. For EBNA-2 detection, the downstream primer was 5′ TGACGGGTTTCCAAGACTATCC 3′, exon Y3/P, positions 48583 to 48562 and the upstream primer was 5′ AGAGGAGGTGGTAAGCGGTTC 3′, exon W2, positions 14802 to 14822. The PCR fragments were separated on a 2% agarose gel and transferred to a nylon membrane (Hybond N; Amersham) using 10× SSC. Amplified EBNA-2 products were detected by hybridization to an end-labeled oligonucleotide probe (5′ GAGAGTGGCTGCTACGCATT 3′, Y2 exon, positions 47885-47904). cDNA from Raji cell lines was used as positive controls. For BZLF-1 detection, the downstream primer used was 5′-GGCAGCAGCCA CCTCACGGT-3′, exon 2/3 splice, positions 102330-102341/102426-102433, and the upstream primer was 5′-TTCCACAGCCTGCACCAGTG-3′, exon 1, positions 102719-102700. Amplified BZLF-1 products were detected by hybridization to an end-labeled oligonucleotide probe (5′-CTTAAACTTGGCCCGGCATT-3′, exon 2, positions 102450-102469). cDNA from B95-8 cell lines were used as positive controls. The sequences of EBV PCR primers and probes used to detect BZLF-1 and EBNA-2 transcript have been validated in another study.24 The β-actin cDNA was used as internal control to demonstrate equal concentration of RNA in each sample. Sequences of primers used have been previously reported.25
Analysis of apoptotic cells by flow cytometry.
After EBV treatment, we identified living, apoptotic, and necrotic cells based on the differences in their stainability with propidium iodide (PI) and Hoechst (HO) 33342. Analyses were done by flow cytometry based on previously described procedures.26 27Briefly, human neutrophils (2 × 106/mL) were cultured in RPMI-1640 containing 10% FBS in the presence or absence of EBV and obtained at specified times. Cells were washed in phosphate-buffered saline (PBS) (pH 7.4), and 50 μL of PI (20 μg/mL) was added to the pellet. Tubes were mixed and kept on ice for 30 minutes. After this incubation time, 950 μL of 25% ethanol and 25 μL of HO 33342 (112 μg/mL) were added to each tube. Samples were vortexed and kept at 4°C in the dark for 4 hours. Cells were analyzed by cytofluorometry (EPICS ELITE ESP; Coulter, Hialeah, FL) with the following settings: the fluorescence emission was first filtered through a 488-nm dichroic filter with the <488-nm fluorescence filtered through a 450-nm long-pass filter (HO33342 fluorescence). The >488-nm fluorescence was filtered through a 515-nm long-pass filter and a 560-nm dichroic filter with the >560-nm fluorescence filtered through 610-nm long-pass filters (PI fluorescence). Analyses were performed from the samples of 10,000 cells. When indicated, cells were pretreated with GM-CSF (3 nmol/L) for 3 hours before EBV infection.
Detection of Fas and Fas L expression.
Neutrophils (3 × 106 cells/mL) were cultured in the presence or absence of EBV during 20 hours and obtained for immunofluorescence staining. Cell-surface expression of Fas and Fas L was assayed by flow cytometry using murine MoAbs anti-human Fas UB2 (Immunotech, Burlington, Ontario, Canada) and anti-human Fas L NOK-1 (PharMingen, Mississauga, Ontario, Canada) for primary straining and revealed with fluorescein isothiocyanate (FITC)-conjugated purified F(ab′)2 goat anti-mouse IgG (Cappel, Durham, NC) for secondary staining. Negative control staining was performed with irrelevant murine IgG. Cells were stained with specific antibodies for 30 minutes at 4°C, washed with PBS, and fixed with 0.5% paraformaldehyde in PBS before cytometric analysis.
Detection of soluble Fas L.
Cell-free supernatants from unstimulated and EBV-treated neutrophils (10 × 106 cells/mL) were obtained at indicated times and tested for the release of soluble Fas L using an enzyme-linked immunosorbent assay (ELISA) kit (Medicorp, Montreal, Canada).
Statistical analysis.
Statistical analyses were performed using Student's paired (two-tailed) t-test, and significance was attained atP < .05.
RESULTS
EBV penetration in neutrophils.
We have previously reported that EBV binds to human neutrophils (approximately 30%) and recognizes a receptor distinct from the CD21 antigen.17 Such interactions result in cellular aggregation and in de novo protein synthesis by neutrophils. Here we have examined the association between neutrophils and EBV by electron microscopy. Viral particles were incubated with neutrophils at 4°C to allow binding and then at 37°C for various periods of time. EBV adsorption to the outer cell membrane of neutrophils could be observed (Fig 1A and insert). Fully mature virions, with electron dense core and bilayer membrane, were found associated to the cells. In Fig 1B, a virion is engaged in internalization, as seen by the fusion of the viral envelope with the cellular membrane. Nucleocapsids of EBV were observed later in the cytoplasma and in the nuclei of neutrophils (Fig 1C and D). Chromatin was condensed following EBV localization to the nucleus and this process was observed between 5 to 15 minutes of incubation. Some EBV particles were also observed in cellular vacuoles, showing that viral particles were also phagocytized by neutrophils (data not shown). Virions within the neutrophils were identical to those seen in the cytoplasma of B95-8 cell line. The percentage of EBV-infected neutrophils evaluated by electron microscopy was similar to the one observed in binding assay.17
EBV infection of human neutrophils. Neutrophils were incubated with EBV for 5 to 15 minutes to allow binding to the cell surface. Virus-bound cells were then cultured at 37°C for varying time periods. (A) Virus was in contact with the cell membrane (original magnification [OM] × 76,000); (inset) at higher magnification, characteristic EBV virion in contact with cell membrane, showing its electron dense core representing viral DNA (OM × 90,000). (B) Fusion of viral and cellular membranes was found (OM × 80,000). (C and D) Internalization and presence of viral capsids were observed within the cytoplasm and the nucleus of neutrophils (OM × 39,000). N, nucleus. These results are represenative of six other experiments. Approximately one third of the cells were found to be infected by EBV.
EBV infection of human neutrophils. Neutrophils were incubated with EBV for 5 to 15 minutes to allow binding to the cell surface. Virus-bound cells were then cultured at 37°C for varying time periods. (A) Virus was in contact with the cell membrane (original magnification [OM] × 76,000); (inset) at higher magnification, characteristic EBV virion in contact with cell membrane, showing its electron dense core representing viral DNA (OM × 90,000). (B) Fusion of viral and cellular membranes was found (OM × 80,000). (C and D) Internalization and presence of viral capsids were observed within the cytoplasm and the nucleus of neutrophils (OM × 39,000). N, nucleus. These results are represenative of six other experiments. Approximately one third of the cells were found to be infected by EBV.
Detection of EBV genome in isolated nuclei obtained from neutrophils.
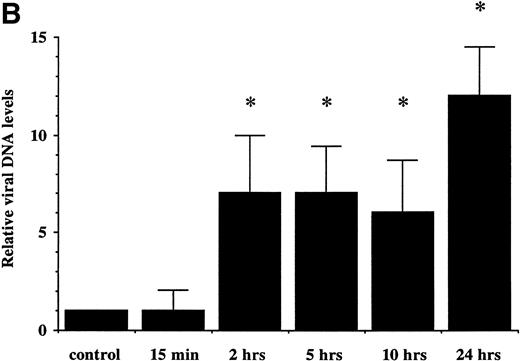
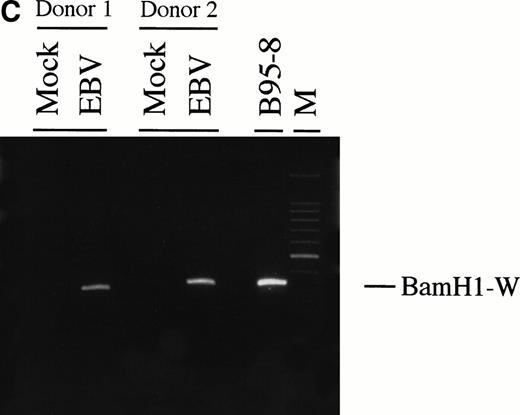
To further confirm the results obtained by electron microscopy, we first examined the presence of EBV genome in neutrophils by Southern blotting analysis (Fig 2A). We used aBamHI W fragment from the internal repeats of EBV genome. Neutrophils were treated with EBV for different periods of time (from 15 minutes to 24 hours). Expression of EBV fragments was detectable at 2 hours postinfection and increased at 24 hours postinfection, suggesting that the number of copies of viral genome or the number of infected neutrophils is increasing with time. Because viral internalization can occur by phagocytosis, we performed an additional experiment to confirm the presence of EBV in the nuclei of neutrophils. Cells were first pretreated with cytochalasin B, an inhibitor of phagocytosis, then treated with EBV. At 10 hours postinfection, DNA was extracted from isolated nuclei and tested for EBV genomic DNA by PCR. As shown in Fig 2C, EBV genome was detected in isolated nuclei from neutrophils treated with EBV, suggesting that EBV can penetrate neutrophils independently of phagocytosis. As negative control, when P815 cells (murine mastocytoma exerting phagocytosis) were pretreated with cytochalasin B before EBV treatment, no specific amplification of EBV DNA was detected in isolated nuclei under the same experimental conditions (data not shown).
(A) Detection of EBV genome in human neutrophils. Cells were incubated for increasing time periods before DNA isolation and Southern blot analysis. EBV DNA was detected by hybridization with aBamHI W probe. Raji cell line was used as positive control. Neutrophils were either cultured in absence (control) or in presence of EBV (15 minutes and 2, 5, 10, and 24 hours). The experiment is representative of two other experiments. (B) Densitometric analysis of viral DNA levels in EBV-infected neutrophils. Results are the mean of three experiments. (C) Detection of EBV genome in isolated nuclei. Neutrophils from two healthy donors were preincubated with the phagocytosis inhibitor cytochalasin B (10 μmol/L) for 15 minutes and then treated with EBV or culture medium (mock) for 10 hours. Cells were obtained and genomic DNA was extracted from purified nuclei. EBV genomic DNA was detected by PCR as described in Materials and Methods. B95-8 cells were used as positive control. M represents a 100-bp molecular weight marker.
(A) Detection of EBV genome in human neutrophils. Cells were incubated for increasing time periods before DNA isolation and Southern blot analysis. EBV DNA was detected by hybridization with aBamHI W probe. Raji cell line was used as positive control. Neutrophils were either cultured in absence (control) or in presence of EBV (15 minutes and 2, 5, 10, and 24 hours). The experiment is representative of two other experiments. (B) Densitometric analysis of viral DNA levels in EBV-infected neutrophils. Results are the mean of three experiments. (C) Detection of EBV genome in isolated nuclei. Neutrophils from two healthy donors were preincubated with the phagocytosis inhibitor cytochalasin B (10 μmol/L) for 15 minutes and then treated with EBV or culture medium (mock) for 10 hours. Cells were obtained and genomic DNA was extracted from purified nuclei. EBV genomic DNA was detected by PCR as described in Materials and Methods. B95-8 cells were used as positive control. M represents a 100-bp molecular weight marker.
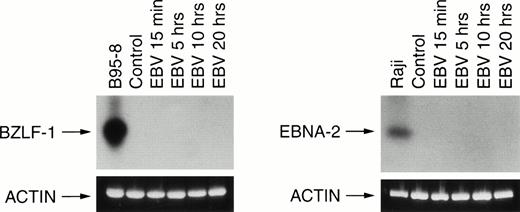
Transcription of EBNA-2 or BZLF-1 was not detected in EBV-infected neutrophils.
We next investigated the expression of two viral transcripts in neutrophils. First, we amplified by PCR the early lytic BZLF-1 gene that encodes the Zebra protein and second, the EBNA-2 transcript that is associated with EBV immortalization. In both cases, no transcripts were detected in EBV-infected neutrophils in contrast to the positive controls B95-8 and Raji cells (Fig 3).
RT-PCR analysis of viral BZLF-1 and EBNA-2 mRNA expression. Neutrophils were incubated in absence (control) or in presence of EBV for various times (15 minutes and 5, 10, and 20 hours). Total RNA was reverse transcribed and amplified using specific BZLF-1 and EBNA-2 primers as described in Materials and Methods. Control cells were the EBV+ B95-8 cells line for BZLF-1 analysis and the EBV+ Raji cells line for EBNA-2 analysis. Amplification were Southern blotted and probed as described in Materials and Methods. The size of PCR products are 182 bp for BZLF-1 and 381 bp for EBNA-2. Results are representative of three different donors.
RT-PCR analysis of viral BZLF-1 and EBNA-2 mRNA expression. Neutrophils were incubated in absence (control) or in presence of EBV for various times (15 minutes and 5, 10, and 20 hours). Total RNA was reverse transcribed and amplified using specific BZLF-1 and EBNA-2 primers as described in Materials and Methods. Control cells were the EBV+ B95-8 cells line for BZLF-1 analysis and the EBV+ Raji cells line for EBNA-2 analysis. Amplification were Southern blotted and probed as described in Materials and Methods. The size of PCR products are 182 bp for BZLF-1 and 381 bp for EBNA-2. Results are representative of three different donors.
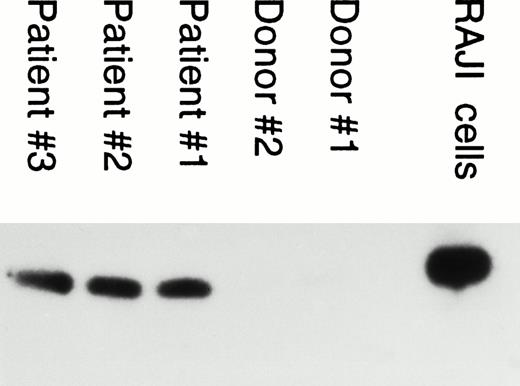
Neutrophils from IM patients are infected by EBV.
Because EBV was found to infect human neutrophils in vitro, we tested for the presence of EBV genome in neutrophils from IM patients. This was performed by PCR amplification of a specific region of the EBV genome using DNA isolated from neutrophils of EBV patients. Interestingly, EBV genome was detected in neutrophils from EBV patients in contrast to healthy seropositive donors (Fig 4).
PCR analysis of EBV genome in neutrophils from IM patients. PCR amplification was performed with DNA isolated from neutrophils obtained from EBV patients and from healthy EBV-seropositive donors (negative control). Amplification was performed using BamHI W primers as described in Materials and Methods. DNA extracted from Raji cells was used as positive control.
PCR analysis of EBV genome in neutrophils from IM patients. PCR amplification was performed with DNA isolated from neutrophils obtained from EBV patients and from healthy EBV-seropositive donors (negative control). Amplification was performed using BamHI W primers as described in Materials and Methods. DNA extracted from Raji cells was used as positive control.
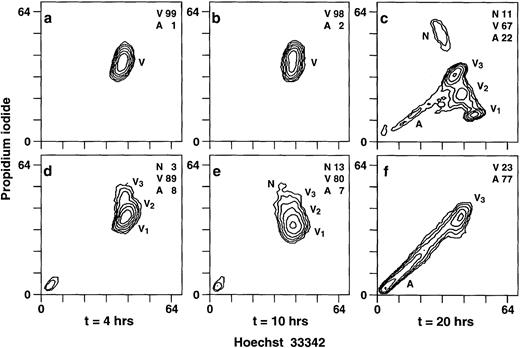
EBV induces apoptosis in neutrophils.
Because EBV can induce or inhibit apoptosis in mononuclear cells28 29 and the fact that neutropenia is observed in the majority of IM patients, we next investigated the effects of EBV on neutrophil viability. This was performed by flow cytometric analysis using the dye HO33342 and DNA intercalating dye propidium iodine. This technique allows the discrimination between necrotic, apoptotic, and viable cells. Viable cells can be divided into three subpopulations, V1, V2, and V3, based on their degree of permeability to PI. Cells in V1 are less permeable to PI than cells in V2, and cells in V2 are less permeable to PI than cells in V3. Permeability is inversely proportional to cellular viability. The more permeable cells, found in the V3 area, are thus closer to death than cells located in the V1 or V2regions. In one representative experiment, after 20 hours of culture, neutrophils treated with EBV showed 77% apoptotic cells as compared to 22% for unstimulated cells (Fig 5). Necrotic neutrophils were not detected at 20 hours postinfection, suggesting that EBV causes neutrophil death by apoptosis rather than necrosis. In addition, cells in V3 area were only detected in EBV-treated cultures, indicating a decrease in cellular viability. These results were consistently observed throughout several experiments. The apoptotic process induced by EBV in neutrophils was confirmed by two other techniques, eg, condensation of chromatin (percent of apoptotic cells: control, 31% ± 6%; EBV-treated cells, 59% ± 10%; P < .05; n = 3) and DNA fragmentation studies (data not shown).
EBV induces apoptosis in human neutrophils. Cells were cultured in the presence or absence of EBV and were obtained at 4, 10, and 20 hours posttreatment for DNA staining as described in Materials and Methods. Uptake of dyes was evaluated by flow cytometry. (a through c) Unstimulated cells cultured for 4, 10, and 20 hours, respectively. (d through f) EBV-treated neutrophils cultured for 4, 10, and 20 hours, respectively. N, necrotic cells; V, viable cells; V1, V2, V3, three smaller groups of viable cells, from the more viable to the less viable cells, respectively; A, apoptotic cells. Numbers indicate the percentage of positive cells. The results displayed in each histogram are representative of seven experiments. After 20 hours of culture, the percentages of apoptotic cells in controls and in EBV-treated cells are 26% ± 8% and 68% ± 14% (n = 7), respectively. Values are significantly different atP < .05.
EBV induces apoptosis in human neutrophils. Cells were cultured in the presence or absence of EBV and were obtained at 4, 10, and 20 hours posttreatment for DNA staining as described in Materials and Methods. Uptake of dyes was evaluated by flow cytometry. (a through c) Unstimulated cells cultured for 4, 10, and 20 hours, respectively. (d through f) EBV-treated neutrophils cultured for 4, 10, and 20 hours, respectively. N, necrotic cells; V, viable cells; V1, V2, V3, three smaller groups of viable cells, from the more viable to the less viable cells, respectively; A, apoptotic cells. Numbers indicate the percentage of positive cells. The results displayed in each histogram are representative of seven experiments. After 20 hours of culture, the percentages of apoptotic cells in controls and in EBV-treated cells are 26% ± 8% and 68% ± 14% (n = 7), respectively. Values are significantly different atP < .05.
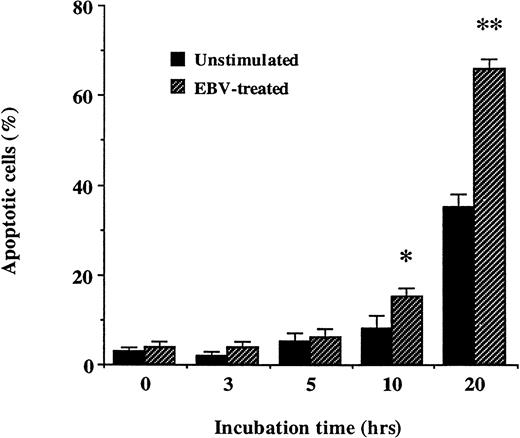
Kinetics of neutrophil cell death induced by EBV.
Human neutrophils were incubated with or without EBV for increasing period of time and apoptosis was assessed by flow cytometric method (Fig 6). The number of apoptotic cells significantly increased after 20 hours in EBV-infected cell cultures as compared with unstimulated neutrophils.
Kinetic of EBV-induced apoptosis in human neutrophils. Neutrophils were incubated with or without EBV for increasing periods of time at 37°C. Cells were obtained and DNA was stained as described in Materials and Methods. The results presented in this figure represent the mean ± SD of experiments performed in triplicate on neutrophils from four individuals. *Significantly different from unstimulated control value at P < .05;**significantly different from unstimulated control value at P < .01.
Kinetic of EBV-induced apoptosis in human neutrophils. Neutrophils were incubated with or without EBV for increasing periods of time at 37°C. Cells were obtained and DNA was stained as described in Materials and Methods. The results presented in this figure represent the mean ± SD of experiments performed in triplicate on neutrophils from four individuals. *Significantly different from unstimulated control value at P < .05;**significantly different from unstimulated control value at P < .01.
GM-CSF protects neutrophils against apoptosis induced by EBV.
GM-CSF is known to prolong survival of human neutrophils in vitro.30 To determine if this effect was also protective on EBV-treated neutrophils, we incubated unstimulated and EBV-infected neutrophils with or without GM-CSF. After 20 hours of incubation, the percentage of apoptotic cells was evaluated by flow cytometry. As shown in Table 1, treatment with GM-CSF strongly suppressed the ability of EBV to induce neutrophil apoptosis. In fact, the number of apoptotic cells in EBV-treated cultures was similar to that from unstimulated cell cultures.
Influence of GM-CSF on EBV-Induced Apoptosis in Neutrophils
| . | Treatments . | |||
|---|---|---|---|---|
| Control . | GM-CSF . | EBV . | EBV + GM-CSF . | |
| Donor 1 | 26 | 15 | 65 | 25 |
| Donor 2 | 28 | 17 | 57 | 31 |
| Donor 3 | 23 | 14 | 43 | 9 |
| Donor 4 | 38 | 21 | 68 | 42 |
| Mean of apoptotic cells ± SD | 29 ± 7 | 17 ± 3-150 | 58 ± 11 | 27 ± 14-150 |
| . | Treatments . | |||
|---|---|---|---|---|
| Control . | GM-CSF . | EBV . | EBV + GM-CSF . | |
| Donor 1 | 26 | 15 | 65 | 25 |
| Donor 2 | 28 | 17 | 57 | 31 |
| Donor 3 | 23 | 14 | 43 | 9 |
| Donor 4 | 38 | 21 | 68 | 42 |
| Mean of apoptotic cells ± SD | 29 ± 7 | 17 ± 3-150 | 58 ± 11 | 27 ± 14-150 |
PMN (2 × 106/mL) were incubated with EBV, GM-CSF (3 nmol/L), or with both agonists for 20 hours. Cells were then stained and the number of apoptotic cells was evaluated by flow cytometry, as described in Materials and Methods. Results are expressed in percentage (%) of apoptotic cells for each culture performed in duplicate. The mean of apoptotic cells from GM-CSF–treated cells was compared with that obtained from control cells, and the mean of apoptotic cells from EBV + GM-CSF–treated cells was compared with that obtained from EBV-treated cells.
Significantly different at P < .01.
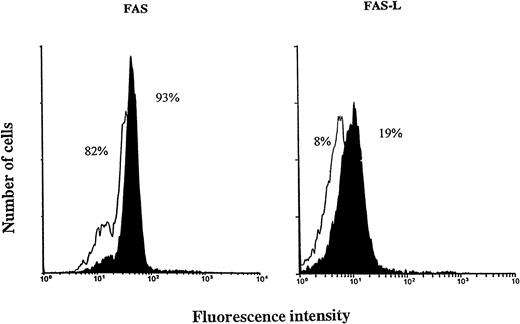
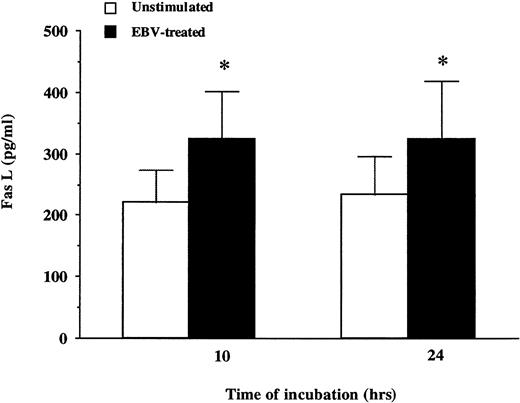
EBV increases the expression of Fas and Fas L on neutrophils.
The Fas/Fas L system is an important cellular pathway mediating apoptosis in neutrophils.31 We therefore evaluated the effects of EBV on the expression of Fas/Fas L antigens on neutrophils. As shown in Fig 7, the expression of Fas and Fas L was significantly increased on neutrophils infected by EBV (93% and 19%, respectively). The time course of appearance of Fas and Fas L antigens was similar to the kinetics of neutrophil death induced by EBV. Furthermore, the release of soluble Fas L was also increased in culture supernatants of EBV-treated neutrophils (Fig 8). These results suggest that EBV may induce apoptosis in neutrophils via the Fas/Fas L system and that soluble Fas L may function in an autocrine/paracrine pathway to mediate cell death.
Cell-surface expression of Fas/Fas L on EBV-infected neutrophils. Immunofluorescence flow cytometry was performed on unstimulated neutrophils (white surface) and on EBV-infected neutrophils (black surface) after 20 hours of culture. Analyses were performed on 10,000 cells per sample and data are representative of four independent expriments. The averages for the controls are: Fas, 84 ± 8; Fas L, 6 ± 3; and for EBV-treated cells: Fas, 93 ± 4; Fas L, 15 ± 5.
Cell-surface expression of Fas/Fas L on EBV-infected neutrophils. Immunofluorescence flow cytometry was performed on unstimulated neutrophils (white surface) and on EBV-infected neutrophils (black surface) after 20 hours of culture. Analyses were performed on 10,000 cells per sample and data are representative of four independent expriments. The averages for the controls are: Fas, 84 ± 8; Fas L, 6 ± 3; and for EBV-treated cells: Fas, 93 ± 4; Fas L, 15 ± 5.
EBV induced release of soluble Fas L by neutrophils in vitro. Neutrophils (10 × 106 cells/mL) were incubated with or without EBV during 24 hours. At indicated times, cell-free supernatants were tested for the presence of Fas L. Results (pg/mL) presented in this figure represent the mean ± SD of experiments performed in triplicate on neutrophils from five different individuals. Values significantly different from unstimulated controls are indicated by asterisks (P < .05).
EBV induced release of soluble Fas L by neutrophils in vitro. Neutrophils (10 × 106 cells/mL) were incubated with or without EBV during 24 hours. At indicated times, cell-free supernatants were tested for the presence of Fas L. Results (pg/mL) presented in this figure represent the mean ± SD of experiments performed in triplicate on neutrophils from five different individuals. Values significantly different from unstimulated controls are indicated by asterisks (P < .05).
DISCUSSION
As a key element in the nonspecific defense mechanism, neutrophils are likely to encounter invading agents such as viruses. Two of the major functions of neutrophils are phagocytosis and the release of inflammatory mediators. Growing evidence shows the ability of neutrophils to induce inflammatory response by releasing several immunoregulatory cytokines upon stimulation.1,2 In this study we show that EBV infects and induces apoptosis of human neutrophils, which could disrupt the initial defense against EBV infection. Electron microscopy studies show contact between EBV and neutrophils, as well as fusion between cellular and viral membranes. These results support a previous study17 showing that EBV binds to a surface membrane antigen on neutrophil via a receptor different from CD21. This is also in agreement with other studies using T-cell lines.11,16 The increased expression of EBV fragments in neutrophils over time either may suggest that EBV can replicate in these cells or that more neutrophils get infected over time. However, we cannot overlook that a number of viral particles can also enter into neutrophils by phagocytosis. In this regard, the results obtained with isolated nuclei from EBV-infected neutrophils are of particular interest. In fact, the presence of EBV genome in these nuclei indicates that EBV can penetrate into neutrophils without being phagocytosed because all cell cultures were treated with cytochalasin B, an inhibitor of phagocytosis. Taken together, the results indicate that although some EBV particles are phagocytosed, others penetrate into neutrophils via an alternate route. Since EBV can penetrate neutrophils, we looked for the synthesis of viral transcripts EBNA-2 and ZEBRA. EBNA-2, readily detectable in the first 24 hours of infection,32 is required for the initiation of lymphocyte immortalization and is an essential transactivator of viral gene expression.33,34 ZEBRA is a key immediate-early protein essential for lytic cell induction and is synthesized during the first hours postinfection.35 36 None of these two genes were found to be expressed in EBV-infected neutrophils, suggesting an abortive type of infection. However, because EBV can encode several other genes, further studies are required to identify viral genes expressed in EBV-infected neutrophils.
Death of neutrophils may represent a strategy of host cells to abort the infectious process. Indeed, after 20 hours of culture, neutrophils treated with EBV showed more than 70% of apoptotic cells which may significantly affect the establishment of productive infection. Such a cellular defense, named apoptosis or programmed cell death, has been reported with Sinbis virus37 and with influenza A and B virus.38-40 It was previously shown that the expression of the EBV latent membrane protein 1 (LMP-1) protects infected B cells from apoptosis and that this effect is mediated, at least in part, by the upregulation of the bcl-2 proto-oncogene.28,41EBNA-2, another EBV latent protein, can increase the effect of LMP-1 onbcl-2 expression, thus improving the protection against apoptosis. This mechanism does not seem to be used by EBV with regard to neutrophils, especially as EBNA-2 protein was not detected in neutrophils; moreover, bcl-2 and bcl-x expression is likely to be absent in this cell type.42 43 However, we evaluated the regulation of the human bfl-1 gene, abcl-2 homologue, in EBV-treated neutrophils. Unfortunately, no significant difference was observed between unstimulated and EBV-infected neutrophils after 20 hours of culture.
It was recently reported that apoptosis in neutrophils may be regulated, at least in part, by the coexpression of cell-surface Fas and Fas L. In the present study our results suggest that the Fas/Fas L system may be involved in neutrophil death induced by EBV. The presence of EBV in cell cultures significantly increased the expression of Fas and Fas L on the membrane surface of neutrophils, as compared with unstimulated cells. Similarly, EBV was found to induce the release of soluble Fas L by neutrophils. However, treatment of freshly isolated neutrophils with supernatants obtained from EBV-treated neutrophils did not significantly modify the rate of spontaneous apoptosis observed in unstimulated neutrophils. At present we cannot exclude the involvement of the Fas/Fas L system in the apoptotic process induced by EBV. Other soluble mediators induced by EBV in addition to virally encoded proteins (still unidentified) may act in synergy with the Fas/Fas L to induce apoptosis of neutrophils. EBV-induced apoptosis of neutrophils is a complex phenomenon necessitating multiple events. In fact, viral entry is necessary but not sufficient to induce apoptosis. This statement is supported by the fact that UV-irradiated particles do not cause apoptosis of neutrophils even though viruses enter the cells (data not shown). The fact that EBV infects and induces apoptosis in neutrophils in vitro and that we detect EBV genome in neutrophils from IM patients are in accordance with some clinical observations made in IM and immunocompromised patients. In 60% to 90% of IM patients, an absolute neutropenia has been detected between the third and fourth week of illness.44-48 This was also observed in severe chronic EBV infection where the number of circulating neutrophils was found to decrease. Moreover, antineutrophil antibodies have been detected in sera of a high proportion of patients with active IM,49 50 a process possibly associated with death of neutrophils.
In conclusion, we showed that EBV penetrates and causes apoptosis of neutrophils. Interactions of EBV with phagocytes may alter the primary immune response and favor the spread of EBV infection. Further studies on the mechanisms of EBV-induced apoptosis in neutrophils should give us new insights on the interactions between EBV and this cell type.
ACKNOWLEDGMENT
We thank Pierrette Côté for her excellent secretarial assistance. We also thank Dr Robert Delage, who provided blood samples from IM patients.
J.G. is the recipient of a Scholarship from the Medical Research Council of Canada. L.F. currently holds a Scholarship from the Fonds de la Recherche en Santé du Québec.
Address reprint requests to Jean Gosselin, PhD, Laboratory of Viral Immunology, Centre de recherche en Rhumatologie et Immunologie, CHUQ, Pavillon CHUL, Room T 1-49, 2705 boul. Laurier, Sainte-Foy, Québec, G1V 4G2, Canada.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

![Fig. 1. EBV infection of human neutrophils. Neutrophils were incubated with EBV for 5 to 15 minutes to allow binding to the cell surface. Virus-bound cells were then cultured at 37°C for varying time periods. (A) Virus was in contact with the cell membrane (original magnification [OM] × 76,000); (inset) at higher magnification, characteristic EBV virion in contact with cell membrane, showing its electron dense core representing viral DNA (OM × 90,000). (B) Fusion of viral and cellular membranes was found (OM × 80,000). (C and D) Internalization and presence of viral capsids were observed within the cytoplasm and the nucleus of neutrophils (OM × 39,000). N, nucleus. These results are represenative of six other experiments. Approximately one third of the cells were found to be infected by EBV.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/1/10.1182_blood.v92.1.291.413k34_291_299/5/m_blod41334001y.jpeg?Expires=1765881920&Signature=pMbJMiDsY8Hv3KJAseM-aSPbYeXt98hq~7kwj~osaRzhlf0kS1x52Y7ba37KczJsiibLhODmrzgi6~DSV1nooRSQaflzqNAsa0uQyMUbhfOb-LC-dBuRqzJEzRapvuoGJpc-owZN6KFQB3xeMbKv3fIgE3xAanGTi4N1lkxPilynBcO3HkjtEW6pCF2qV3ovm-1wtWsFLGE3U5HNtKCeVWOwDt6lG~tP~ymV6zWHjgPgX4uuyHr5Ip6qJ5hv96Ko3EVODpKDEAY-ekJn4k9n0W8s6BV4qOQ-mcECob0ryB-0cwP9BZQoJWzQJLiN6YyV5o4JXCWjdZrphu4xfA1~uQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal