Dendritic cells (DC) are migratory cells which exhibit complex trafficking properties in vivo, involving interaction with vascular and lymphatic endothelium and extracellular matrix (ECM). The underlying mechanisms involved in these processes are still ill defined. In the present study we have investigated the ability of DC to interact in vitro with human vascular endothelial cells (EC) and ECM. DC were differentiated from monocytes by in vitro exposure to granulocyte-macrophage colony-stimulating factor and interleukin-13 for 7 days. In adhesion assays a considerable proportion of DC bound to resting EC monolayers: (17% ± 4%, mean ± SE of eight experiments). Adhesion to tumor necrosis factor (TNF)-activated EC was increased to 29% ± 5% (n = 8). Binding to resting EC was strongly inhibited by anti-CD11a and CD11b, but not by CD11c monoclonal antibodies (MoAbs); on TNF-activated EC, anti–VLA-4 in concert with anti-CD18 inhibited adhesion by more than 70%. Binding to a natural ECM, derived from cultured EC, or to purified fibronectin was high: 52% ± 6% (n = 8) involved VLA-4 and VLA-5 integrins. In a transmigration assay, 10% ± 2% (n = 6) of input cells were able to cross the EC monolayer. Unlike adhesion, transendothelial migration was significantly reduced by anti-CD31 MoAb. The amount of DC transmigrated through a monolayer of EC was increased twofold to threefold by a defined set of C-C chemokines including RANTES, MIP1α, MIP5, and, to a lesser extent, by MIP1β and MCP-3. Most importantly, in view of the trafficking pattern of these cells, a significant proportion of DC (13% ± 4% of input cells seeded) was able to migrate across the endothelial basement membrane and, subsequently, across the endothelial barrier (reverse transmigration). The adhesion molecules and chemoattractants characterized herein are likely to underlie the complex trafficking of DC in vivo.
DENDRITIC CELLS (DC) are bone marrow (BM)-derived leukocytes specialized in antigen capture, processing, and presentation to T lymphocytes. DC are most potent among antigen-presenting cells (APC) and are believed to be indispensable to initiate a primary immune response.1-5 DC progenitors from the BM enter the blood and seed nonlymphoid tissues. Immature DC are localized in epithelia, such as skin epidermis (Langherans cells), gastrointestinal and genito-urinary tract, airways, and the interstitial space of many solid organs (heart, liver, kidney).1 6
Upon the encounter of an antigen, DC migrate from the site of residence to the T-cell areas of regional lymph nodes. These migratory cells also undergo maturation from a “processing” to a “presenting” stage, characterized by the loss of antigen uptake and by an increased capacity to stimulate T cells.1-5 Locally produced inflammatory cytokines (eg, tumor necrosis factor [TNF] and interleukin-1 [IL-1]) and bacterial products can stimulate the maturation and migration of DC from resident tissues to lymph nodes.6-11
Some pathways of DC migration in vivo and recruitment of DC precursors from blood to tissues have been characterized. TNF and possibly other lipopolysaccharide (LPS)-derived cytokines quickly recruited DC in the airway epithelia in a model of respiratory infection.8Intradermal administration of granulocyte-macrophage colony-stimulating factor (GM-CSF) lead to an increase in the number of DC within the human dermis.12 After intravenous injection of inert particles, phagocytic cells are recruited to the hepatic sinusoid and these cells then translocate to the hepatic lymph.13 After intratracheal injection of an antigen, antigen-loaded DC are found in the draining lymph nodes.14 Systemic administration of LPS also induced a profound loss of major histocompatibility complex (MHC) class II+ cells from mouse heart and kidney, probably because of migration.10 11
Large quantities of cells with the typical features of DC can be easily differentiated in vitro from CD34+ stem cells upon culture with GM-CSF and TNF,15,16 or from monocytes with GM-CSF and IL-4.17-19 We recently showed that IL-4 can be fully substituted by IL-13.20
Although some pathways of DC migration have been identified, the molecular mechanisms regulating their tissue trafficking are not fully elucidated. We and others have characterized the capacity of in vitro cultured DC to respond to chemotactic signals, including classical peptides (eg, C5a) and lipid (eg, platelet-activating factor [PAF]) agonists as well as chemokines.21-25 DC respond to a unique spectrum of chemokines which overlaps but is distinct from that active on other leukocytes, which includes the CC molecules MCP-3, Rantes, MIP1α, MIP1β, MCP-4, HCC2/MIP5, and the C-X-C chemokine SDF-1.22,26 Moreover, the recently identified chemokine macrophage-derived chemokine (MDC) is 100 times more active on DC than on monocytes.27 There is heterogeneity among DC in chemokine responsiveness as illustrated by the observation that CD34-derived, but not monocyte-derived, DC express CCR6 and, accordingly, respond to MIP3α.28 29 The chemotactic response to chemokines suggest that these molecules may have a role in regulating the trafficking of DC in vivo.
During migration from the tissue of residence to afferent lymphatics, DC interact with extracellular matrix (ECM) and with the endothelial lining of lymphatic vessels. DC interact also with blood endothelial cells (EC) during recruitment of precursors from the blood into tissues and during migration to spleen.6
No information is available on the ability of DC to adhere and to transmigrate through vascular EC. In the present study we have addressed the question of how DC interact with EC and ECM in vitro, which adhesion molecules are engaged, and which chemotactic signals direct their transendothelial migration. Moreover, we devised a simple methodological approach to investigate the capacity of DC to enter vessels from tissues, which we named reverse transmigration.
MATERIALS AND METHODS
Culture media and reagents.
The following reagents were used for separation of effector cells, cell culture, and experimental assays: pyrogen-free saline for clinical use and distilled water (Bieffe, Sondrio, Italy); medium RPMI 1640 (10× concentrated; Biochrom KG, Berlin, Germany); medium 199 (GIBCO, Paisley, UK); glutamine (GIBCO); penicillin and streptomycin (GIBCO); aseptically collected fetal calf serum (FCS; Hyclone, Logan, UT). The routinely used tissue culture medium was RPMI 1640 with 2 mmol/L glutamine, 50 μg/mL of gentamicin, 10% FCS.
Cytokines.
Human recombinant GM-CSF was from Sandoz (Basel, Switzerland). Human recombinant (r) TNF-α was from BASF (Knoll, Germany). Human rIL-13 and MCP-3 were from Sanofi Elf Bio Recherches (Labège, France). Human rMIP-1β was from PeproTech Inc (Rocky Hill, NJ). Human rMIP-1α was from Dr L. Czaplewski (British Biotechnology Limited, Cowley, UK). RANTES and MIP-5/HCC2 were chemically synthesized.30 MIP-5/HCC2 is a novel human CC chemokine that shows high sequence identity to MIP-3 (76.7%), MIP-4 (63.2%), MIP-1α (75.4%), and MIP-1β (66.7%).26 Cytokines were endotoxin free as assessed by Limulus Amebocyte assay (BioWhittaker, Walkersville, MD).
DC culture.
DC were differentiated in vitro as previously described.20,22,26 31 Highly enriched blood monocytes (>95% CD14+) were obtained from buffy coats (through the courtesy of Centro Trasfusionale, Ospedale Sacco, Milan, Italy) by Ficoll and Percoll gradients and purified by panning on CD6-coated plastic dishes. Monocytes were cultured for 7 days at 1 × 106/mL in 6-well Multiwell tissue culture plates (Falcon, Becton Dickinson, Lincoln Park, NJ) in RPMI 1640 + 10% FCS supplemented with 50 ng/mL GM-CSF and 10 ng/mL IL-13.
In a limited number of experiments, DC were differentiated from CD34+ cells purified from human cord blood and cultured for 14 days in the presence of stem cell factor (50 ng/mL), GM-CSF (50 ng/mL), and TNF (10 ng/mL).
Mixed leukocyte reaction.
Cultured DC were irradiated (3,000 rad) and added in graded doses to 1 × 105 purified allogeneic T cell in 96-well round-bottomed microtest plates. Responder cells were depleted of autologous APC by passage with CD14- and CD19-coated Dynabeads (Unypath, Milan, Italy). Each group was performed in triplicate.3H-thymidine incorporation was measured on day 5 by 16-hour pulse (5 Ci/μmol; Amersham, Amersham, UK).
Monoclonal antibodies (MoAbs).
The following MoAbs were used: L243 (IgG2a, anti–MHC class II) purchased from American Type Culture Collection (ATCC; Rockville, MD); NA1/34 (IgG2a, anti-CD1a; Dako, Glostrup, Denmark); UCHM1 (IgG2a, anti-CD14; a kind gift from Dr P. Beverly, London, UK); TS1/22 (IgG1, anti-CD11a) and TS1/18 (IgG1, anti-CD18) from ATCC; 44a (IgG2a, anti-CD11b; a kind gift from Dr R. Todd, Ann Arbor, MI); L29 (IgG1 anti-CD11c; a kind gift from Dr L. Lanier, DNAX Palo Alto, CA); HP2/1 (IgG1 anti-VLA4; a kind gift from Dr. F. Sanchez-Madrid, University of Madrid, Madrid, Spain); M89D3 [an F(ab′)2 fragment of anti CD31; a kind gift from Dr R. Zocchi, Ist. S. Raffaele, Milan, Italy]; SK11 (IgG2a anti-CD62L) and RUU-PL (IgG1 anti-CD61) were from Becton Dickinson (Mountain View, CA).
In adhesion or transmigration assays, optimally diluted MoAbs were preincubated with the cells for 20 minutes before plating; medium control contained an isotype-matched irrelevant MoAb. For fluorescence-activated cell sorter (FACS) analysis, cell staining with primary MoAb was followed by fluorescein isothiocyanate (FITC)-conjugated affinity purified, isotype-specific goat anti-mouse antibodies (Valter Occhiena, Torino, Italy). Results are expressed as percent of positive cells and as relative fluorescence intensity (RFI), calculated according to the formula: RFI = Mean Fluorescence (sample) − Mean Fluorescence (control)/Mean Fluorescence (control).
Preparation of EC.
Human EC were obtained from umbilical vein (HUVEC) and cultured as previously described.32 Routinely we used confluent cells (105/2-cm2 culture well) between the first and fourth passage maintained in 199 medium with 20% bovine serum (Hyclone) supplemented with endothelial cell growth supplement (50 μg/mL; Collaborative Research Inc, Lexington, MA) and heparin (100 μg/mL; Sigma Chemical Co, St Louis, MO). The purity of EC cultures was checked by expression of von Willebrand factor and found to be greater than 99% positive.
Adhesion assay.
Adhesion of DC to EC was studied as described previously.33EC were grown to confluence in flat-bottomed 48-well trays.51Cr-labeled DC (Amersham, UK) were coincubated with EC monolayers at 37°C for 1 hour. At the end nonadherent cells were washed away and adherent cells were solubilized with 0.1 mL of 0.1% sodium dodecyl sulfate and radioactivity was counted in a gamma counter. Results represent the percent of adherent cells ± SD of three replicates/group.
Transmigration and reverse transmigration assay.
This assay was performed as previously described.34 In brief, EC were grown to confluence on polyvinylpyrrolidone (PVP)-free polycarbonate filters (5-μm pore) and mounted on modified Boyden chambers over a nitrocellulose filter.51Cr-labeled DC were seeded in the upper compartment and coincubated with EC monolayers for 1 hour at 37°C. Nonadherent cells were gently washed away, the radioactivity in the double filter and in the lower compartment referred to transmigrated cells. The adherent cells were considered to comprise both cells bound to EC as well as those that had transmigrated.
In the reverse transmigration assay an upper polycarbonate filter was coated with ECM, and the lower polycarbonate filter was coated by a monolayer of EC, placed upside down, and mounted in the Boyden chamber. The ECM was prepared by growing a monolayer of EC on filters for at least 5 days; EC were then stripped away by a short treatment with a 20 mmol/L NH4OH solution + 0.5% Triton X-100 (Sigma, St Louis, MO) for 30 seconds. In this assay the radioactivity present in the lower compartment only accounted for the transmigrated cells.
In some experiments polycarbonate transwell inserts (5-μm pore; Corning, Costar, Cambridge, MA) were used. Inserts were coated with EC monolayers; chemoattractants, usually 100 ng/mL, were seeded in the lower compartment.
RESULTS
In vitro adhesion of DC to EC monolayers and ECM.
DC were differentiated in vitro from highly purified human monocytes in the presence of GM-CSF + IL-13 for 7 days. As previously reported these cultured DC expressed high levels of CD1a and MHC class II and had low levels of CD14. As APC, they were strong stimulators of allogeneic T-lymphocyte proliferation in MLR.20 26
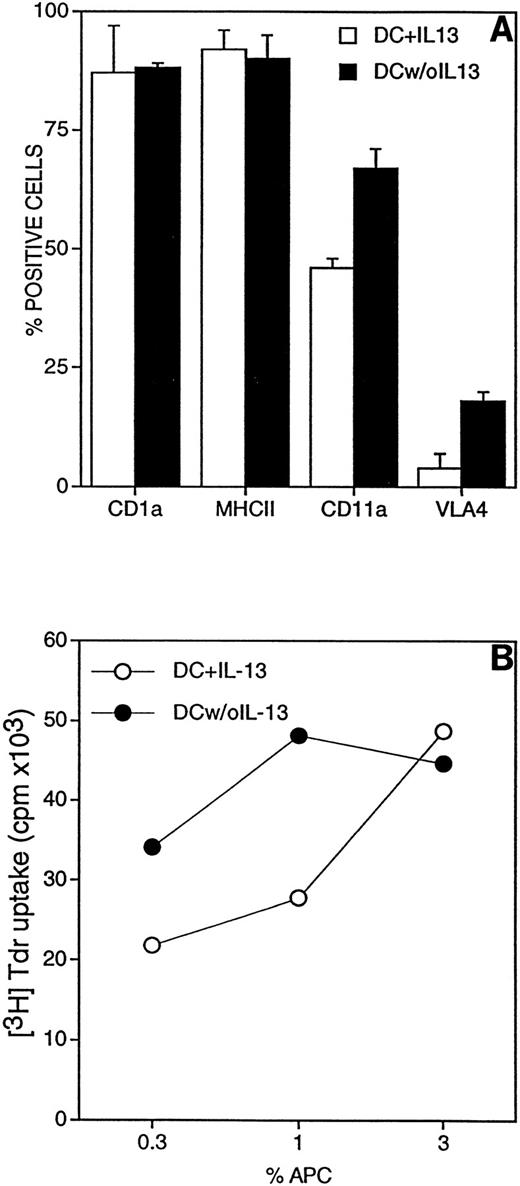
In preliminary experiments we evaluated the role of IL-13 on the adhesive ability of cultured DC. There was a concern in performing adhesion assays with cells cultured in the presence of IL-13, as we reported that IL-4, a functionally related cytokine, strongly inhibits leukocyte adhesion to vascular endothelium.35 To verify this possibility, DC were washed at day 5 of culture to remove IL-13 and then incubated for 2 additional days in the presence of GM-CSF only. These cells (termed DC w/o IL-13) were compared with conventional DC cultures exposed to IL-13 for 7 days (DC + IL-13). In vitro adhesion to monolayers of resting or TNF-activated EC indeed showed that adhesion of DC w/o IL-13 was higher compared with DC + IL-13 (Fig1).
Adhesion of cultured DC to monolayers of resting or TNF-activated EC (10 ng/mL for 24 hours). DC were differentiated from blood monocytes by 5-day culture with 50 ng/mL GM-CSF and 10 ng/mL IL-13. (□) DC were cultured for 2 additional days in the presence of GM-CSF and IL-13. (▪) Cells were washed and cultured for 2 additional days without IL-13. 51Cr-labeled DC were coincubated for 1 hour at 37°C with EC monolayers. Results are expressed as percent of adherent cells, mean ± SEM of three replicates. Three distinct experiments are shown.
Adhesion of cultured DC to monolayers of resting or TNF-activated EC (10 ng/mL for 24 hours). DC were differentiated from blood monocytes by 5-day culture with 50 ng/mL GM-CSF and 10 ng/mL IL-13. (□) DC were cultured for 2 additional days in the presence of GM-CSF and IL-13. (▪) Cells were washed and cultured for 2 additional days without IL-13. 51Cr-labeled DC were coincubated for 1 hour at 37°C with EC monolayers. Results are expressed as percent of adherent cells, mean ± SEM of three replicates. Three distinct experiments are shown.
Analysis of adhesion molecules, relevant for interaction with the vascular endothelium, showed that DC + IL-13 expressed lower levels of CD11a and VLA-4 than DC w/o IL-13 (Fig 2A).It was important to verify that removal of IL-13 in the last 2 days of culture did not prevent DC differentiation. Fig 2A shows that CD1a and MHC class II were expressed to the same extent in both populations. Figure 2B shows that DC w/o IL-13 were more potent APC for allogeneic T cells, compared with DC + IL-13 for the whole period of culture. At 1% APC stimulation index (SI) was 28 and 48 for DC + IL-13 and DC w/o IL-13, respectively. Based on these results all subsequent experiments were performed with DC cultured in the absence of IL-13 for the last 48 hours.
(A) Characterization of adhesion molecules expressed by DC. After 5 days in vitro with GM-CSF and IL-13, DC were cultured for 2 additional days in the presence (□) or absence (▪) of IL-13. Cells were labeled with the designed MoAb and then with FITC-labeled goat anti-mouse Ig. (B) Mixed leukocyte reaction (MLR). Responder cells were allogeneic T lymphocytes depleted of autologous monocytes and B lymphocytes; they were plated at 1 × 105cells/well. DC cultured for last 2 days with (○) or without (•) of IL-13 were mixed at the indicated concentrations. 3H-Tdr was added in the last 18 hours of a 5-day experiment.
(A) Characterization of adhesion molecules expressed by DC. After 5 days in vitro with GM-CSF and IL-13, DC were cultured for 2 additional days in the presence (□) or absence (▪) of IL-13. Cells were labeled with the designed MoAb and then with FITC-labeled goat anti-mouse Ig. (B) Mixed leukocyte reaction (MLR). Responder cells were allogeneic T lymphocytes depleted of autologous monocytes and B lymphocytes; they were plated at 1 × 105cells/well. DC cultured for last 2 days with (○) or without (•) of IL-13 were mixed at the indicated concentrations. 3H-Tdr was added in the last 18 hours of a 5-day experiment.
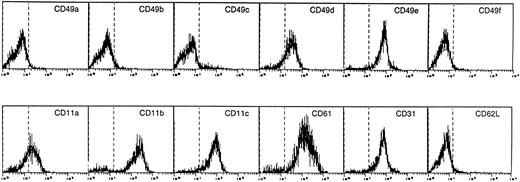
Analysis of expression of β1 and β2 integrins is reported in Fig3. DC expressed high levels of CD11b and CD11c and intermediate levels of CD11a. Among β1 integrins, CD49d and CD49e (VLA-4 and VLA-5) were expressed at high levels. VLA-4 levels were variable among donors (26.3% ± 13%; range, 3.4 to 77.3; mean of five experiments). All the cells stained positive for CD61 (β3) and CD31 (PECAM-1), and were negative for CD62L (L-selectin).
Flow cytometric analysis of adhesion molecules expressed by DC. Cells were labeled with the designed MoAb and then with FITC-labeled goat anti-mouse Ig.
Flow cytometric analysis of adhesion molecules expressed by DC. Cells were labeled with the designed MoAb and then with FITC-labeled goat anti-mouse Ig.
The identification of adhesion molecules involved in the binding to resting EC was performed by using functional blocking MoAb. Anti-CD11a, anti-CD11b, and anti-CD18 MoAbs partially inhibited adhesion (52%, 64%, and 68%, respectively, in the representative experiments shown in Fig 4A). It is of interest that CD11c, although expressed to a very high extent on DC, is not involved in binding to endothelium. These results were confirmed in a larger series of experiments (mean inhibition with anti-CD18, 61% ± 4%; mean ± SE of seven experiments).
Molecules involved in the adhesion of DC to resting (A) and TNF-activated EC (B). 51Cr-labeled DC were preincubated for 20 minutes at room temperature with the indicated MoAb; thereafter DC were coincubated with the EC monolayers for 1 hour at 37°C. Results are expressed as percent of adherent cells, mean ± SEM of three replicates. **Statistically significant at P < .01 versus medium control.
Molecules involved in the adhesion of DC to resting (A) and TNF-activated EC (B). 51Cr-labeled DC were preincubated for 20 minutes at room temperature with the indicated MoAb; thereafter DC were coincubated with the EC monolayers for 1 hour at 37°C. Results are expressed as percent of adherent cells, mean ± SEM of three replicates. **Statistically significant at P < .01 versus medium control.
On TNF-activated EC expressing high levels of ICAM-1 and VCAM-1, basal adhesion of DC was increased from 17.3% ± 4.4% to 28.7% ± 5.6% (mean ± SE of eight experiments). Anti–VLA-4 alone did not inhibit adhesion (not shown) but the combination of anti-CD18 + anti–VLA-4 was more inhibitory compared with anti-CD18 alone (Fig 4B), a phenomenon already observed with other leukocytes.33 These results show the important role of VLA-4 in leukocyte adhesion, although it is secondary to β2 integrins.
Binding of DC to a natural ECM, derived from cultured EC, or to purified fibronectin, was higher than to EC monolayers: 52% ± 6% (mean ± SE of eight experiments) (not shown).
Direct and reverse transendothelial migration.
We previously described an in vitro method to measure transmigration through monolayers of EC cultured on porous polycarbonate filters.34 In this assay, freshly isolated monocytes showed to have high adhesive (40% ± 13%) and transmigrating ability (16.8 ± 2; mean ± SE of three experiments) compared with other leukocytes.34 In vitro cultured DC, differentiated from monocytes, effectively transmigrated through resting EC monolayers. The proportion of adhesive and transmigrated cells compared with input cells in a 1-hour assay was 35.8% ± 2% and 10.6% ± 1.6%, respectively (mean ± SE; n = 6). Because migration is preceded by adhesion, anti-CD18 inhibited both adhesion (79%) and transmigration (75%) in the representative experiment shown in Fig5. Treatment of EC with anti-CD31 MoAb did not affect adhesion, but did inhibit transmigration by 38% (Fig5).
Molecules involved in the transmigration of DC across resting EC. A porous polycarbonate filter was coated with a monolayer of EC and placed in modified Boyden chamber. 51Cr-labeled DC were preincubated for 20 minutes at room temperature with the indicated MoAb and then seeded in the upper compartment and coincubated with EC for 1 hour at 37°C. Results are expressed as percent of adherent and transmigrated cells (see Materials and Methods), mean ± SEM of three replicates. **Statistically significant at P < .01 versus medium control.
Molecules involved in the transmigration of DC across resting EC. A porous polycarbonate filter was coated with a monolayer of EC and placed in modified Boyden chamber. 51Cr-labeled DC were preincubated for 20 minutes at room temperature with the indicated MoAb and then seeded in the upper compartment and coincubated with EC for 1 hour at 37°C. Results are expressed as percent of adherent and transmigrated cells (see Materials and Methods), mean ± SEM of three replicates. **Statistically significant at P < .01 versus medium control.
Adhesion to ECM and reverse transmigration of DC. (A) Molecules involved in the adhesion of DC to EC-derived matrix (ECM). ECM were prepared by culturing a monolayer of EC on polycarbonate filters for at least 5 days; EC were then stripped by an NH4OH + Triton solution for 30 seconds. Results are expressed as percent of adherent cells, mean ± SEM of three replicates. **Statistically significant at P < .01 versus medium control. (B) Reverse transmigration of DC. A double-filter system was used. The upper filter was coated with an ECM and the lower filter was coated with a monolayer of EC placed upside down. 51Cr-labeled DC were seeded in the upper compartment of the chamber and coincubated with EC monolayer for 3 hours at 37°C. Results are expressed as percent of adherent and transmigrated cells (see Materials and Methods), mean ± SEM of three replicates. Two independent experiments are shown.
Adhesion to ECM and reverse transmigration of DC. (A) Molecules involved in the adhesion of DC to EC-derived matrix (ECM). ECM were prepared by culturing a monolayer of EC on polycarbonate filters for at least 5 days; EC were then stripped by an NH4OH + Triton solution for 30 seconds. Results are expressed as percent of adherent cells, mean ± SEM of three replicates. **Statistically significant at P < .01 versus medium control. (B) Reverse transmigration of DC. A double-filter system was used. The upper filter was coated with an ECM and the lower filter was coated with a monolayer of EC placed upside down. 51Cr-labeled DC were seeded in the upper compartment of the chamber and coincubated with EC monolayer for 3 hours at 37°C. Results are expressed as percent of adherent and transmigrated cells (see Materials and Methods), mean ± SEM of three replicates. Two independent experiments are shown.
GM-CSF augments the adhesive properties of monocytes.36Therefore, experiments were performed with DC washed away of GM-CSF and rested for 24 hours. Adhesion and transmigration values were not different from conventional cultures: eg, 28% ± 1% (adhesion) and 7.5% ± 1% (transmigration) for DC with GM-CSF; 27% ± 4% and 8.6% ± 1% for DC rested without GM-CSF (mean ± SE of two experiments).
Circulating DC precursors migrate from the blood compartment into tissues. From there, DC reach lymphoid organs via lymph or blood.6 Therefore, we established an assay to mimic the tissue-to-lymph/blood part of the natural history of DC (reverse transmigration). In this assay the upper filter (of the two-filter system) is coated with ECM, and the lower filter is coated by a monolayer of EC, placed upside down. As shown in Fig6A, this natural matrix was highly adhesive for DC. Anti–VLA-4 + VLA-5 MoAbs or anti-CD29 inhibited most of the binding (>75% inhibition) while (as expected) anti-CD18 had no effect. Figure 6B shows the results of two experiments of reverse transmigration. The mean amount of transmigrated cells relative to input was 13% ± 4%, which corresponded to 20.8% of adherent cells.
Effect of chemokines on DC transendothelial migration. Polycarbonate Transwell inserts were coated with a monolayer of EC and chemoattractants (100 ng/mL) were seeded in the lower compartment.51Cr labeled DC were placed in the upper compartment and incubated for 1 hour at 37°C. **Statistically significant atP < .01 and *P < .05 versus medium control.
Effect of chemokines on DC transendothelial migration. Polycarbonate Transwell inserts were coated with a monolayer of EC and chemoattractants (100 ng/mL) were seeded in the lower compartment.51Cr labeled DC were placed in the upper compartment and incubated for 1 hour at 37°C. **Statistically significant atP < .01 and *P < .05 versus medium control.
Response of DC to chemokines.
We and others have previously reported that cultured DC migrate in vitro in response to chemoattractants including formyl peptides and C5a and a distinct set of CC and CXC chemokines.22-26Therefore, it was important to assess whether CC chemokines affected the capacity of DC to interact with and migrate across EC monolayers. Polycarbonate Transwell inserts were coated with a monolayer of EC and chemoattractants were seeded in the lower compartment. As shown in Fig7, increased migration was induced in response to MIP-1α, RANTES, HCC2/MIP-5, and to a lesser extent to MIP-1β and MCP-3. These results are in line with our previous findings of chemotactic response in a classical chemotaxis assay.22 26
DISCUSSION
The present study is the first analysis of the interaction of DC with EC and their ECM. DC obtained from monocytes by culture with GM-CSF and IL-13 expressed CD31, the β2 integrins LFA-1, Mac-1, and p150,95, the β1 integrins VLA-4 and VLA-5, but were negative for other β1 integrins and for L-selectin. They bound resting and activated EC via LFA-1, Mac-1, and VLA-4, and EC-derived ECM via VLA-4 and VLA-5. DC were able to migrate across EC monolayers; unlike adhesion, transendothelial migration involved engagement of CD31. Most importantly, in view of the trafficking of these cells, DC were able to perform reverse transmigration.
We and others have reported that DC express receptors for chemokines and respond to chemotactic signals.22-26,37 Active chemoattractants included formyl peptides and C5a and a distinct set of CC and CXC chemokines, which overlaps with but is distinct from those active on other leukocytes.22-26,37 In the present study we assessed the response of DC to CC chemokines in a transendothelial migration assay. RANTES, MIP-1α, and HCC2/MIP-5 augmented the transmigration of DC. Intriguingly MCP-3, a strong attractant in a conventional assay,22 26 had little effect in this assay. The reason for the dissociation of the capacity of this chemokine to attract DC in the presence and absence of an EC monolayer is elusive. One could speculate that at least the EC population used here does not efficiently present MCP-3 to migrating DC.
The interaction of leukocytes with the luminal surface of vascular endothelium and their passage into tissues has been extensively investigated in vitro in adhesion and transmigration assays. Among leukocytes, lymphocytes and DC have the ability to undergo the reverse process of migrating from tissue into the lumen of blood or lymphatic vessels. In an effort to model this tissue-to-lymph/blood component of the natural history of certain leukocytes we have set up a simple reverse transmigration assay. As expected, DC were able to migrate across the basal membrane and then through the endothelial monolayer to reach the luminal surface. The reverse transmigration assay may represent a new useful tool to investigate this aspect of leukocyte trafficking.
DC are heterogeneous populations of BM-derived elements. One pathway of DC differentiation is closely related to the monocyte-macrophage lineage.17-19,38-41 The population used in the present study was obtained by culturing monocytes with GM-CSF and IL-13 and therefore differentiated from monocytic precursors. It is well established that CD34+ precursors cultured with stem cell factor, GM-CSF, and TNF give rise to a population containing different types of DC. At early time points of culture two subsets have been phenotypically identified: one CD1a+CD14−, the other CD1a−CD14+. Both subsets eventually mature into typical DC: the CD1a precursors generate cells with the typical features of epidermal Langerhans cells, while the CD14+ precursors, related to the monocytic lineage, express markers of dermal DC.41 In a limited series of experiments (n = 4) we tested DC obtained from CD34+ cord blood cells, cultured as described above. The CD34+-derived DC behaved similarly to monocyte-derived DC in terms of expression of adhesion molecules and binding ability. Adhesion to activated EC was 22% ± 3% of input cells and anti-CD18 used in concert with anti–VLA-4 inhibited up to 80%. Hence, at least some of the results reported here with monocyte-derived DC may also apply to CD34+-derived DC.
EC are heterogeneous in terms of morphology and function, both within the same vascular bed and among different organs.42Lymphatic EC in particular differ considerably from blood-vessel EC.43 In the present study we used conventional HUVEC as a model EC population. Further work and the development of appropriate reliable culture technique will be required to analyze the interaction of DC with more relevant EC populations such as those that line lymphatics.
DC undergo complex trafficking patterns.6 Circulating precursors localize in tissues and this process can be increased by local inflammation. At epithelial lining surfaces, after antigen capture, DC traffic to lymph nodes via lymphatics. The same route is followed by DC present in the interstitium of organs such as heart and kidney; however, these cells can also reach the spleen via the blood stream. A subset of DC present in the liver can capture circulating antigen and travel along the lymph/lymph nodes as well as the blood/spleen route. The molecular pathways of interaction with EC and ECM proteins described herein are likely to be important in at least some of the trafficking patterns of DC.
ACKNOWLEDGMENT
We are indebted to Drs A. Minty (Sanofi, Labège, France) and G. Guidi (Novartis, Milan, Italy) for their generous gifts of IL-13 and GM-CSF, respectively.
G.D. and G.B. have contributed equally to this work.
Supported in part by the National Research Council (Finalized Project Biotec) and by MURST 40% fund (Project Tumori). The Italian Association for Cancer Research (AIRC) is gratefully acknowledged.
Address reprint requests to Paola Allavena, MD, Istituto di Ricerche Farmacologiche “Mario Negri,” Via Eritrea 62, 20157 Milan, Italy; e-mail: Allavena@irfmn.mnegri.it.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal