Recent studies have shown that transfusions of HLA-compatible donor lymphocytes may induce complete remission in marrow-grafted patients with relapses of acute myeloblastic leukemia (AML). We investigated the in vitro generation of antileukemia T-cell clones obtained from the peripheral blood mononuclear cells of a partially HLA-compatible donor (HLA-A2 and B7 molecules in common with the leukemic blasts) after stimulation with a pool of naturally processed peptides extracted from leukemic blast cells collected at diagnosis from a patient with hyperleucocytosis AML. We recovered a significant quantity of peptides that bound to the HLA-A2 or HLA-B7 molecules that were able to induce cytolytic T-lymphocyte (CTL) lines and clones specific for the eluted AML peptides and restricted to the HLA-A2 or B7 molecules. Such CTL line did not recognize the patient's nonleukemic cells, and one clone was able to interact with the leukemic blasts from which the naturally processed peptides had been eluted. Such T-cell clones might provide a rationale for the development of adoptive immunotherapy and could be used to improve the efficiency of HLA-compatible T-lymphocyte transfusions and the graft-versus-leukemia response in patients with AML.
IN RECENT YEARS, several immunological strategies have been suggested for restoring or enhancing antitumor immunity in cancer patients. It may be possible to develop an optimal immunological therapy that induces a memory response against malignant cells using stimulation with tumor-specific antigens in association with potent costimulation signals. This would first require identification of the tumor-associated antigens. Such antigens have been identified using tumor-specific lymphocytes isolated from cancer patients and genetic approaches.1 In a few cases, total acid extraction, immunoaffinity purification of major histocompatibility complex (MHC) molecules, repeated fractionations, and tandem mass spectrometry have been used to characterize and sequence naturally processed tumor epitopes.2 3 However, powerful immunotherapeutic strategies should include different tumor-associated epitopes to avoid the escape of tumor cells and to limit the risk of selecting antigen-loss tumor variants. This would require the definition of the multiple tumor antigens associated with different cancers. This limits the development of immunological therapies, because tumor antigens have been identified in only a small number of malignant diseases.
The pool of naturally processed peptides extracted from tumor cells by acid elution represents a promising strategy for generating antitumor T lymphocytes for adoptive cell therapy since tumor-eluted peptides have been shown to stimulate the cytolytic lymphocyte response.4,5 Several studies in animals have shown that immunization with acid-eluted peptides prevents the growth of preestablished tumor in vivo.6-8
Patients with acute myeloid leukemia (AML) are currently treated with allogeneic bone marrow transplantation (BMT). Allogeneic BMT has an associated immune-mediated antileukemia effect, the graft-versus-leukemia (GVL) effect. Clinical observations in patients given marrow transplants have provided evidence of a T-cell–mediated antileukemia effect.9 Complete remissions were induced in about 30% of AML patients who relapsed after BMT by transfusion of allogeneic HLA-compatible donor lymphocytes.10 However, about half of these patients in remission developed graft-versus-host-disease (GVHD). These effects are sometimes lethal. In regard, the infusion of HLA-compatible antileukemia T-cell clones generated in vitro against naturally processed peptides provided by the tumor cells might be useful in improving the efficiency of HLA-compatible T-lymphocyte transfusion and the GVL response in BM-grafted patients with AML relapse. This approach may also limit detrimental GVHD.
Therefore, we investigated the induction of a leukemia-specific immune response, using a pool of naturally processed peptides extracted from leukemic blasts collected at diagnosis from a patient with de novo AML without known chromosome abnormalities and predetermined candidate antigen. We investigated whether cytolytic T-lymphocyte (CTL) lines with different specificities and restricted by different HLA class I molecules could be generated from the peripheral blood mononuclear cells (PBMC) of a partially HLA-compatible donor. We also isolated clones derived from these CTL lines to investigate recognition of the AML blasts from which the naturally processed peptides were eluted. This work suggests that in AML patients who relapse after BMT, antileukemia T lymphocytes could be generated from the PBMC of the allogeneic HLA-compatible donor providing the BM.
MATERIALS AND METHODS
AML patients and healthy donor.
Leukapheresis samples were drawn from patients with hyperleucocytosis AML after obtaining their informed consent to provide leukemic blasts. PBMC of these patients were mostly leukemic blasts (>95%). Patient HAR had an AML of M4 French-American-British (FAB) subtype and HLA-A-2/28; B-7/44 phenotype. Patient LED had an AML of M1 FAB subtype and HLA-A-24/28; B-8/44 phenotype. No cytogenetic abnormalities are found in classical karyotypic examination. PBMC were also obtained from patient HAR after complete remission was achieved using mitoxantrone (7 mg/m2/J; J1, J3, J5), cytarabine (100 mg/m2/J; J1-7), etoposide (100 mg/m2/J; J1-5). More than 80% of the leukemic blasts from the patients HAR and LED were HLA class I+. Leukapheresis samples from a healthy subject whose HLA class I typing was A-2/11; B-7/27 were provided by the Cochin Hospital blood bank.
Isolation of peptides from leukemic cells.
The leukemic blasts were washed three times with NaCL 0.9% and the tumor-associated peptides isolated using a previously described procedure.11 Aliquots of 5 × 109 leukemic blasts were pelleted, frozen, and stored at −80°C. Pellets were thawed, resuspended in 10 mL citrate-phosphate buffer (0.131 mol/L citric acid/0.066 mol/L Na2HPO4, pH 3.3) containing protease inhibitors (Boehringer Mannheim, GmbH, Mannheim, Germany), and immediately centrifuged at 15,000g. The supernatants were loaded onto C18 SepPak (Walters, Milford, MA). Peptides were eluted from these columns using 3 mL 60% acetonitrile in water. The eluate was collected in five vials (Minisorp; Nunc, Roskilde, Denmark), each containing peptides extracted from approximately 109 leukemic blasts, and lyophilized.
Peptide fractionation and detection of HLA assembly.
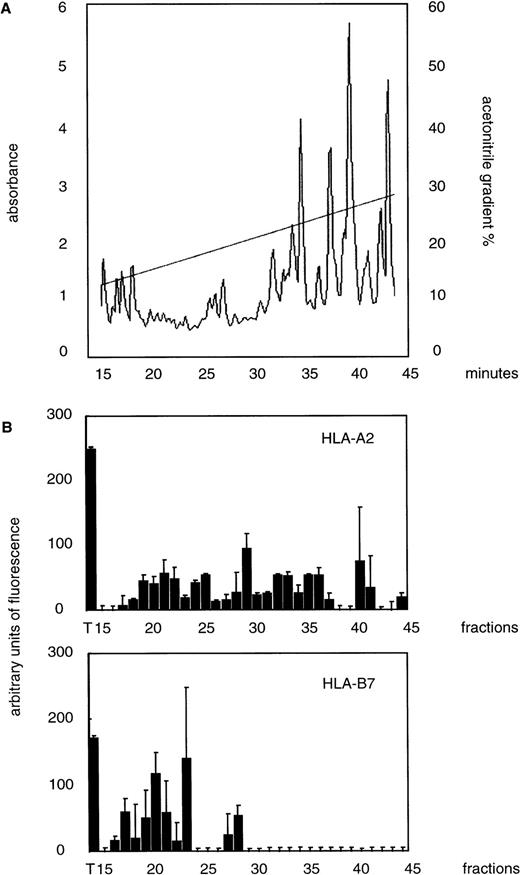
The peptides extracted from 3 × 109 leukemic blasts were reconstituted after lyophilization in 200 μL of 0.1% trifluoroacetic acid in water and fractionated by reverse-phase high-performance liquid chromatography (HPLC) as previously described.11 Elution with increasing concentrations of acetonitrile was monitored by measuring absorption of UV light at 214 nm (Fig1A). Individual HPLC fractions were lyophilized and reconstituted in 150 μL of buffer (0.05% Tween, 3 mmol/L sodium azide, 1 mmol/L phenylmethylsulfonyl fluoride [PMSF], and 10 mg/mL albumin in phosphate-buffered saline [PBS]).
Binding of fractionated eluted leukemia-associated peptides to HLA-A2 and -B7 molecules. (A) Acid-eluted peptides were fractionated by HPLC and detected by absorbance at 214 nm. (B) Individual fractions were assayed for their ability to promote HLA-A2 and -B7 assembly. The influenza virus matrix peptide, M58-66, and an endogenous peptide, (APRTVALTAL), are HLA-A2 and -B7 ligands and were used as positive controls (T). The values obtained with the negative control peptide NP 383-391 (nonspecific binding) have been substracted from the results shown. Means of duplicates and standard deviations are given.
Binding of fractionated eluted leukemia-associated peptides to HLA-A2 and -B7 molecules. (A) Acid-eluted peptides were fractionated by HPLC and detected by absorbance at 214 nm. (B) Individual fractions were assayed for their ability to promote HLA-A2 and -B7 assembly. The influenza virus matrix peptide, M58-66, and an endogenous peptide, (APRTVALTAL), are HLA-A2 and -B7 ligands and were used as positive controls (T). The values obtained with the negative control peptide NP 383-391 (nonspecific binding) have been substracted from the results shown. Means of duplicates and standard deviations are given.
The ability of eluted peptides from patient HAR to promote HLA-A2, -B7, and A-29 assembly using purified molecules was tested as previously described.12 Nonspecific binding was analyzed with the HLA-A29 molecule, not present on the HAR leukemic blasts. The synthetic reference peptides used were the M 58-66 epitope from the Influenza A virus matrix (GILGFVFTL) for HLA-A2, the endogenous peptide (APRTVALTAL) for HLA-B7, and a poly-Gly peptide (GEFGGGGY) for HLA-A29. The peptide NP 383-391 from the influenza A virus nucleoprotein which binds to HLA-B27 was used as a negative control. Purified HLA-A2.1, -B7, or -A29 molecules were allowed to refold in the presence of exogenous β2-microglobulin and reconstituted eluted peptides for 24 hours at 4°C. The reassembled HLA-A2, -B7, -A29 molecules were then incubated overnight at 4°C in the wells of microtiter plates coated with an anti–HLA-A2 monoclonal antibody (BB7.2.), an anti–HLA-B antibody (B1.23.2), or an anti-HLA total antibody (B9.12.1). Correctly folded HLA complexes were detected using anti–β2-microglobulin Ig M28 coupled to alkaline phosphatase. Phosphatase activity was detected using 4-methyl-umbelliferyl phosphate as the substrate and the resulting fluorescence measured at 360/460 nm.
Induction of primary cytotoxic T lymphocytes using acid-eluted unfractionated leukemia-associated peptides from human naive precursors.
A previously described protocol was used to induce CTL from the PBMC of a healthy donor.13 The PBMC from this healthy subject, whose HLA class I typing was A-2/11; B-7/27 were shown to proliferate with tetanus toxoid protein (data not shown). CTL lines were generated in complete medium containing RPMI 1640, 50 IU/mL penicillin, 50 μg/mL streptomycin, nonessential amino acids (1×, 10 mmol/L HEPES, and glutamine (all these regents from Life Technology, Paisley, Scotland), 1 mmol/L pyruvate (ICN, Costa Mesa, CA), and 10% human AB serum.
Unfractionated PBMC (106/mL) from the healthy donor were pulsed with peptides extracted from 109 leukemic blasts (patient HAR) in RPMI medium. The PBMC were diluted to 2 × 106/mL with tetanus toxoid protein (1 μg/mL) after 4 hours and seeded in 96-well plates (4 × 105 cells/200 μL/well). Interleukin-7 (IL-7) (20 IU/mL; Pepro Tech, London, UK) was added on day 3 or 4. Replicates for each culture were treated independently and restimulated with irradiated peptide-pulsed PBMC on day 8. The PBMC (10 × 106/mL) were pulsed with peptides eluted from 109 leukemic blasts. The pulsed PBMC were then diluted to 106/mL. Half the volume of each replicate supernatant was removed and replaced with the same volume of complete medium containing 105 PBMC. One day later, half the volume of each replicate was again removed and replaced with an equal volume of complete medium containing IL-2 (10 IU/mL) and IL-7 (20 IU/mL). This was repeated 4 days later, then every week. The concentration of IL-2 was finally adjusted to 50 IU/mL after five restimulations, when there was significant cytolytic activity. Some cytolytic T-cell lines were then cloned (0.3 cells/well) with irradiated allogeneic PBMC (105/well) in 100 μL complete medium containing 0.5 μg/mL phytohemagglutinin (PHA) and 50 IU/mL IL-2.
Cytolytic activity assay.
Cytolytic activity was tested by a standard 4-hour [51Cr] assay on day 5 after the fifth stimulation. The target cells were Epstein-Barr virus (EBV)-transformed cell lines (HLA class I typing A2/3; -B7/8 or A1/3; B7/8), HLA-A2 T2 TAP-deficient cell line, or nonleukemic PHA blasts and macrophages prepared from the PBMC of patient HAR collected at complete remission. PHA blasts were obtained from PBMC stimulated with 0.5 μg/mL PHA for 24 hours and the macrophages obtained from PBMC cultured for 5 days with 800 IU/mL granulocyte-macrophage colony-stimulating factor (GM-CSF) in 5% fetal calf serum (FCS) complete medium. The target cells (106) were incubated overnight with peptides extracted from 108leukemic blasts, then labeled with 100 μCi of sodium chromate ([51Cr], 10 mCi/mL; Dupont-NEN Research Prods, Boston, MA) for 1 hour, washed twice with 0.9% NaCl medium containing 5% FCS, and dispensed at 5,000 cells per well. Chromium release was measured after 4 hours. Spontaneous releases never exceeded 25% of the maximum [51Cr] uptake. The percent lysis was determined as follows:
Tumor necrosis factor-α (TNF-α) release assay.
CTL or clones (5 × 104) were incubated with stimulator cells (105) in 250 μL complete medium. The stimulator cells were AML blasts from patient HAR and patient LED, PBMC of the healthy donor pulsed with eluted peptides from AML HAR or AML LED blasts. PBMC (10 × 106/mL) were pulsed for 4 hours with peptides eluted from 109 leukemic blasts. The supernatants (100 μL) were collected after 24 hours and mixed with 2 × 104 TNF-α–sensitive WEHI-16/173 cells in 100 μL of medium. The amount of TNF-α released was estimated by comparing the effect of known concentrations of recombinant (r)TNF-α (Pepro Tech, London, UK) on WEHI-16/173 survival.
RESULTS
Binding of fractionated peptides eluted from leukemia blasts to purified HLA-A2 and -B7 molecules.
Peptides from the HAR leukemia blasts (HLA-A-2/28; -B-7/44) were subjected to mild acid extraction, eluted, and separated by acetonitrile gradient on an HPLC column. Individual fractions were assayed for their ability to promote HLA-A2 and -B7 assembly. The assembly of these eluted fractions with the irrelevant HLA-A29 was also assayed to analyze the specificity of the interaction for a pool of peptides. Positive control [M58-66 for HLA-A2, the endogenous peptide (APRTVALTAL) for HLA-B7, and a poly-Gly peptide (GEFGGGGY) for HLA-A29] as well as negative controls with an unrelated peptide (NP 383-391 which binds to HLA-B27) were used. We observed that some of the fractions between fractions 15 and 45 contained peptides that caused a significant assembly of HLA-A2 and HLA-B7 molecules compared to the control peptides (Fig 1B) whereas no assembly of the HLA-A29 molecule was detected (data not shown). The fractions containing eluted peptides that promote HLA-A2 or -B7 molecule assembly were not those containing the greatest quantity of peptides. Peptides promoting the assembly of HLA-A2 molecules were broadly distributed from the fractions 17 and 44, whereas the fractionation of peptides binding to the HLA-B7 molecules was more restricted (between fractions 16 and 28). HLA-B7 assembly was more efficient than HLA-A2 assembly with some fractions (eg, 20 and 23). This suggests that fractions 20 and 23 contained more peptides that bound to the HLA-B7 molecule than the fractions containing HLA-A2–binding peptides. This could also suggest that fractions 20 and 23 contain peptides that bound more strongly than those of other fractions.
Our results show that the peptides that bound to a particular HLA molecule, HLA-B7, could be isolated in only a small number of fractions, whereas the peptides that bind to another HLA molecule, HLA-A2, were present in a large number of fractions. This is consistent with the results of preliminary experiments in which we fractionated using HPLC synthetic and well-studied HLA-binding peptides such as peptides from the matrix of the influenza virus or the p53 protein. The HLA-A2–binding peptides were eluted in a large number of fractions from the beginning to the end of the acetonitrile gradient, whereas HLA-B7–binding peptides were present only in a limited number of fractions toward the beginning of the gradient (data not shown). These variations were probably caused by the structural constraints on the HLA anchor residues and to the different chemical properties of the amino acids involved in the binding of the peptide to the various HLA molecules.
Our results showed that acid-treatment of leukemia blasts led to the recovery of a significant quantity of peptides that bound to the HLA-A2 and -B7 molecules. They also suggest that HPLC fractionation could be used to enrich the pool of peptides eluted from the tumor for peptides that bound to a given HLA molecule.
Generation of CTL lines from naive precursors of a partially HLA-compatible donor with unfractionated peptides eluted from leukemia blasts.
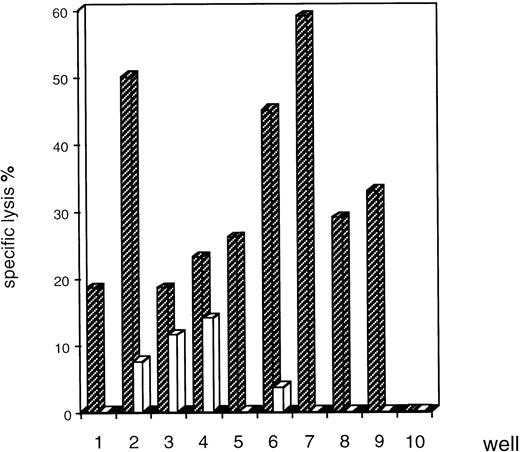
Peptides naturally processed by leukemic cells were isolated by mild acid-extraction at pH 3.3. Primary CTL lines were generated from the PBMC of a partially HLA-compatible donor who had HLA-A2 and B7 molecules in common with the patient. We used a simple, highly efficient protocol involving activated CD4+ lymphocytes. The activated helper lymphocytes had to provide certain cytokines in order to allow generation of CD8+ CTL from naive precursors.13 PBMC (110 replicates) were stimulated each week with the unfractionated eluted peptides. CTL were selected according to their ability to lyse an allogeneic EBV-transformed cell line expressing the HLA-A2 and -B7 molecules, which had previously been pulsed with eluted AML peptides or which was unpulsed. Two different inductions of T-cell lines with separate preparations of eluted leukemia peptides gave similar results. Only a limited number of replicates (9 of 110) contained T-cell lines with significant lytic activities and most of the cytotoxic T-cell lines lysed target cells pulsed with the eluted AML peptides, but not unpulsed target cells (Fig2). The lytic activity of some of these lines was relatively high (for example, CTL 2, 6, 7).
Screening of eluted AML peptide-specific CTL lines in a cytolytic assay. CTL lines were generated from the PBMC of a partially HLA-compatible donor (HLA class I typing A2/11; B7/27) seeded in 110 wells in microplates and stimulated with the pool of eluted peptides (HLA class I typing A2/28; B7/44). Cytolytic activity was analyzed after five stimulations on HLA-A-2/3; -B7/8 EBV-transformed target cells unpulsed (□) or pulsed () with AML peptides eluted from HAR blasts. There was cytolytic activity in 9 of 110 wells (CTL 1-9). The other wells had no such activity, as shown for well 10. We found that these results were consistent with two different inductions. The results shown are the means of duplicates and are representative of three cytolytic assay, for an effector:target (E:T) ratio of 40:1.
Screening of eluted AML peptide-specific CTL lines in a cytolytic assay. CTL lines were generated from the PBMC of a partially HLA-compatible donor (HLA class I typing A2/11; B7/27) seeded in 110 wells in microplates and stimulated with the pool of eluted peptides (HLA class I typing A2/28; B7/44). Cytolytic activity was analyzed after five stimulations on HLA-A-2/3; -B7/8 EBV-transformed target cells unpulsed (□) or pulsed () with AML peptides eluted from HAR blasts. There was cytolytic activity in 9 of 110 wells (CTL 1-9). The other wells had no such activity, as shown for well 10. We found that these results were consistent with two different inductions. The results shown are the means of duplicates and are representative of three cytolytic assay, for an effector:target (E:T) ratio of 40:1.
Recognition specificities and HLA restriction.
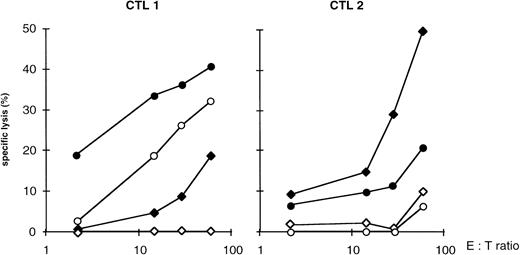
We investigate the recognition of nonleukemic mononuclear cells from patient HAR by the CTL. CTL 1 lysed both PHA blasts from HAR patient previously pulsed with AML peptides and unpulsed PHA blasts as though there were less lysis of unpulsed than of pulsed PHA blasts (Fig3). More interestingly, CTL 2 did not lyse unpulsed PHA blasts (Fig 3). We also observe that CTL 2 did not recognize unpulsed macrophages whereas it recognized pulsed macrophages (data not shown).
Recognition of the PHA blasts of patient HAR. Cytolysis of nonleukemic PHA blasts obtained from patient HAR and HLA-A-2/3; -B7/8 EBV-transformed target cells unpulsed (○) or pulsed (•) with peptides eluted from the blasts of patient HAR were assayed. PHA blasts were prepared from the PBMC of patient HAR. collected upon complete remission. Representative results and means of duplicates are shown for CTL 1 and 2. (⧫), EBV HLA-A2/3; B7/8 + AML HAR peptides; (◊), EBV HLA-A2/3; B7/8.
Recognition of the PHA blasts of patient HAR. Cytolysis of nonleukemic PHA blasts obtained from patient HAR and HLA-A-2/3; -B7/8 EBV-transformed target cells unpulsed (○) or pulsed (•) with peptides eluted from the blasts of patient HAR were assayed. PHA blasts were prepared from the PBMC of patient HAR. collected upon complete remission. Representative results and means of duplicates are shown for CTL 1 and 2. (⧫), EBV HLA-A2/3; B7/8 + AML HAR peptides; (◊), EBV HLA-A2/3; B7/8.
We investigated the HLA-restriction of the CTL by studying cytolytic activity after presentation of the peptides eluted by target cells with different HLA class I phenotypes. We found that some CTL lines were restricted by HLA-A2 molecules in the same way as CTL 1 or by HLA-B7 molecules in the same way as the CTL 7 (Fig4).
HLA restriction of the CTL lines. Cytolysis of the T2 HLA-A2–deficient line and HLA-A-1/3; -B7/8 EBV-transformed target cells unpulsed or pulsed with AML peptides eluted from the blasts of patient HAR was assayed. Representative results are shown for CTL 1 and 7 for an E:T ratio of 4:1. (▩), T2 HLA-A2 + AML HAR peptides; (□), T2 HLA-A2; (), EBV HLA-A1/3; B7/8 + AML HAR peptides; (▧), EBV HLA-A1/3; -B7/8.
HLA restriction of the CTL lines. Cytolysis of the T2 HLA-A2–deficient line and HLA-A-1/3; -B7/8 EBV-transformed target cells unpulsed or pulsed with AML peptides eluted from the blasts of patient HAR was assayed. Representative results are shown for CTL 1 and 7 for an E:T ratio of 4:1. (▩), T2 HLA-A2 + AML HAR peptides; (□), T2 HLA-A2; (), EBV HLA-A1/3; B7/8 + AML HAR peptides; (▧), EBV HLA-A1/3; -B7/8.
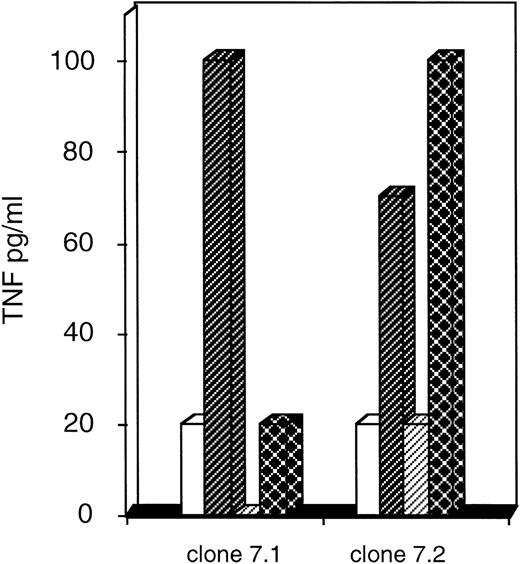
We then wanted to know whether the AML blasts from patient HAR would be recognized by clones 7.1 and 7.2. These clones were derived from the CTL 7 for which the greatest peptide-specific cytotoxic activity was detected (Fig 2). Because fresh AML blasts HAR were not available to be used for targets in a cytolytic assay, we quantified the TNF-α released by clones stimulated by thawed leukemic blasts or PBMC of the partially HLA-compatible donor pulsed with eluted AML peptides (Fig5). The AML blasts used in this experiment were from patient HAR (AML M4, HLA class I typing A-2/28; B-7/44) and from patient LED (AML M1, HLA class I typing A-24/28; B-8/44). We found that clone 7.2 recognized the HAR blasts because it released a significant quantity of TNF-α after stimulation with these leukemic cells. Furthermore, the recognition of HAR blasts was specific because this 7.2 clone did not release any TNF-α after stimulation with the LED blasts. These results showed that the recognized peptides were well associated with HLA-A2 or B7 molecules and not with HLA-A28 or -B44, since the LED blasts did not have the HLA-A2 and B7 molecules but did have the HLA-A28 and B44 molecules in common with those of HAR. On the contrary, another peptide-specific clone (7.1) poorly recognized the HAR blasts (Fig 5) compared with the clone 7.2. We also observed that CTL 2 (shown in Fig 3, did not recognize nonleukemic cells of patient HAR) specifically recognizes HAR blasts, although the quantity of TNF-α secreted was much lower than that released by the 7.2 clone (data not shown).
Recognition of leukemic blasts of patient HAR. Secretion of TNF by clones was quantified (as described in Materials and Methods) after stimulation by the PBMC of the donor pulsed with AML peptides eluted from patient HAR () or patient LED (□) and after stimulation by leukemic blasts from patient HAR (▩) or patient LED (▨). Representative results are shown for the 7.1 and 7.2 clones.
Recognition of leukemic blasts of patient HAR. Secretion of TNF by clones was quantified (as described in Materials and Methods) after stimulation by the PBMC of the donor pulsed with AML peptides eluted from patient HAR () or patient LED (□) and after stimulation by leukemic blasts from patient HAR (▩) or patient LED (▨). Representative results are shown for the 7.1 and 7.2 clones.
DISCUSSION
In this work, we investigated the feasability of generating T lymphocytes from the PBMC of a partially HLA-compatible donor that are stimulated in vitro with eluted leukemia-associated peptides. We showed that the use of naturally processed peptides is a promising approach for generating anti-leukemic T lymphocytes from the PBMC of the allogeneic HLA-compatible donors for adoptive immunotherapies in AML patients who relapse after BMT.
We assume that the use of eluted peptides to generate antileukemia T-cell clones would be more efficient than using entire leukemic cells because it would limit the induction of allogeneic clones, increase the concentration of potential leukemia-specific and immunogenic peptides, and would avoid difficulties (relevant to coculture with tumor cells) such as very low expression of the B7.1 costimulation molecule, secretion of inhibitory proteases or the presence of suppressive cytokines (such as IL-10), or the possible production of FasL or TNF-α by the leukemic cells.
We assumed that most of the self peptides isolated from the leukemic blasts that bound to the HLA-A2 and -B7 molecules (these two HLA molecules were present on the tumor cells and on the donor PBMC) would not induce T-cell lines from the donor PBMC presuming that the pool of HLA-A2 and -B7 molecules restricted self peptides from the donor and the patients were likely to overlap, and thus generation of T cells would be limited by tolerance mechanisms.
We use a protocol based on culture in replicate microplates, which makes it possible to isolate T-cell lines of various specificities. Our approach allowed the isolation of CTL lines, although the percentage of microwells containing CTL was low (about 8%) as expected because generation of T-cell lines was performed using naive lymphocyte precursors. The CTL lines specifically recognize the eluted AML HAR peptides associated with the HLA-A2 or B7 molecules while (unpulsed) PHA blasts or macrophages HAR were not recognized. Interestingly, some clones recognized the HAR leukemic blasts (HLA-A-2/28; -B-7/44) from which the naturally processed peptides had been eluted. We confirmed that the clones are specific for peptides associated with HLA-A2 or -B7 molecules and are not specific to allogeneic HLA molecules or peptides because the LED leukemic blasts (HLA-A-24/28; -B-8/44) that had the HLA-A28 and -B44 molecules in common with HAR were not recognized by these clones derived from the HLA A-2/11; -B-7/27 donor.
However, the recognized peptides may be minor histocompatibility antigen (mH) present on myeloid precursors from patient HAR. Several mH antigens were identified by T-cell clones isolated from the donors after HLA-identical BMT. These antigens are recognized in the context of HLA molecules. The mH antigens identified so far were presented by the HLA-A2 molecule, and are either ubiquitous or restricted to the hematopoietic-cell lineage.14 They were identified using clones isolated from T-cell lines first stimulated in vivo by the host cells (nonleukemic) at the time of the transplantation, and then in vitro by the leukemic blasts.15,16 However, CD8+ cytolytic T-cell clones that recognized leukemic cells and that did not recognize nonleukemic host lymphocytes were isolated from T cells lines obtained after in vitro stimulation of donor mononuclear cells by the leukemic cells from the host.17 Some of these clones could recognize a normal myeloid progenitor, but one would imagine that the GVL effect may also involve a specific antileukemia effect. With respect to this, a B-cell leukemia-associated mH antigen has recently been described that is not present on normal cells of B lineage. This antigen is recognized on leukemic and EBV-transformed B cells but not on untransformed B cells.18
Moreover, eluted leukemia peptides may also be used to generate lymphocyte effectors from the autologous PBMC of patient. These lymphocytes may be transfered to the patient to avoid leukemia relapse, or used to purge in vitro autologous marrow and eliminate the leukemic blasts before autologous BMT. Using such an approach, we obtained some T-cell lines that were specific for eluted leukemia peptides in two patients with AML or chronic myeloid leukemia (data not shown).
Our results show that it is possible to generate antitumor T-cell lines or clones in vitro from the PBMC of a partially HLA-compatible donor when donor and patient have HLA class I molecules in common. Transfusion of HLA-compatible antileukemia T-cell clones generated in vitro is a promising approach for adoptive immunological therapy in BM-grafted patients in cases of leukemia relapse. This transfer may enhance the GVL response and limit detrimental GVHD.
Supported by grants from the Institut National de la Santé et de la Recherche Médicale, Ligue contre le cancer, Agence Française du Sang, and Fondation contre la Leucémie.
Address reprint requests to Marina Ostankovitch, PhD, ICGM, INSERM U445, Université René Descartes, Hôpital Cochin, 27 rue du faubourg Saint Jacques, 75 014 Paris, France; e-mail:ostankovitch@cochin.inserm.fr.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal