The CD20 antigen is an attractive target for specific treatment of B-cell lymphoma. Antibody-directed enzyme prodrug therapy (ADEPT) aims at the specific activation of a nontoxic prodrug at the tumor site by an enzyme targeted by a tumor-specific antibody such as anti-CD20. We constructed a fusion protein of the single-chain Fv anti-CD20 mouse monoclonal antibody (MoAb) 1H4 and human β-glucuronidase for the activation of the nontoxic prodrug N-[4-doxorubicin-N-carbonyl(-oxymethyl) phenyl] O-β-glucuronyl carbamate to doxorubicin at the tumor site. The cDNAs encoding the light- and heavy-chain variable regions of 1H4 were cloned, joined by a synthetic sequence encoding a 15-amino acid linker and fused to human β-glucuronidase by a synthetic sequence encoding a 6-amino acid linker. An antibody-enzyme fusion protein-producing cell line was established by transfection of the construct into human embryonic kidney 293/EBNA cells. The yield of active fusion protein was 100 ng/mL transfectoma supernatant. Antibody affinity, antibody specificity, and enzyme activity were fully retained by the fusion protein. Immunoprecipitation and analysis by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) showed that the fusion protein has a relative molecular weight (Mw) of 100 kD under denaturing conditions. Gel filtration analysis indicated that the enzymatically active form of the fusion protein is a tetramer with an Mw of approximately 400 kD. The nontoxic prodrug N-[4-doxorubicin-N-carbonyl(-oxymethyl) phenyl] O-β-glucuronyl carbamate was hydrolyzed by the fusion protein at a hydrolysis rate similar to that of human β-glucuronidase. When the fusion protein was specifically bound to Daudi lymphoma cells, the prodrug induced similar antiproliferative effects as doxorubicin. Thus, it is feasible to construct a eukaryotic fusion protein consisting of a single-chain anti-CD20 antibody and human β-glucuronidase for future use in the activation of anticancer prodrugs in B-cell lymphoma.
MOST PATIENTS WITH non-Hodgkin's lymphoma receive chemotherapy, with or without radiotherapy.1 High-dose chemotherapy followed by stem cell rescue is now being evaluated for its effect on the clinical course of the disease and has shown improved event-free and overall survival.2 Despite high response rates, 50% of all patients will eventually relapse. Therefore, alternative treatment approaches, such as the use of monoclonal antibodies (MoAbs), are evaluated. MoAbs directed against tumor-associated antigens are being applied for both the detection and the treatment of cancer. MoAb-directed therapy includes the chemically coupled radioisotopes, cytostatic agents, or toxins to MoAbs.
The CD20 antigen is a suitable candidate for targeted therapy in patients with non-Hodgkin's lymphoma: it is uniquely and abundantly expressed on normal and malignant B cells, but not on hematopoietic stem cells. It is a 33- to 35-kD integral membrane phosphoprotein, which plays a role in B-cell proliferation and differentiation. The CD20 antigen is not subject to modulation. MoAbs directed against CD20 have been successfully used in radioimmunotherapy trials in patients with B-cell malignancies.3,4 In the majority of patients resistant to chemotherapy, durable responses have been observed. Recently, phase I and phase II trials have been performed with a human/mouse chimeric anti-CD20, which shows antibody-dependent cell-mediated cytotoxicity. The response rate in the phase II study was 42%.5
A novel and promising strategy for targeted therapy is the use of MoAbs to direct enzymes to tumor tissue, which then convert relatively nontoxic prodrugs into active cytostatic agents (ADEPT, antibody-directed enzyme prodrug therapy).6-8 This approach overcomes the problem of heterogeneity in MoAb distribution, as the released drug can diffuse throughout the tumor and destroy tumor cells irrespective of their antigen expression. The catalytic activity of the enzymes can be very high and, therefore, a high concentration of drug can be achieved within the tumor, whereas little or no toxic effects are expected in nontarget tissues. The higher tumor concentration of the drug is expected to result in a higher response rate and might overcome drug resistance.
For ADEPT, we have previously used chemically coupled antibody and enzyme.9 10 A major disadvantage of such conjugates is their intrinsic heterogeneity due to the lack of specificity of the reagents used for conjugation. This necessitates additional purification steps and often results in reduced enzymatic activity or diminished antibody binding of the conjugate. As an alternative, recombinant DNA technology can be used to prepare conjugates consisting of a uniform product with predictable properties. Single chain antibodies (scFv), consisting of the variable VH and VL chains offer the advantage of being approximately threefold smaller than intact IgG and can penetrate tumor lesions more readily. In addition, the lack of an Fc portion should greatly reduce its immunogenicity.
We constructed a fusion protein consisting of the genes encoding the variable regions of MoAb 1H4, an MoAb reactive with the CD20 antigen11,12 and human β-glucuronidase. The fusion protein was expressed in eukaryotic cells, because human β-glucuronidase needs to be glycosylated to form an enzymatically active tetramer.13 The antibody binding specificity and enzymatic activity of the fusion protein was analyzed and compared with that of 1H4 and human β-glucuronidase, respectively. Because treatment regimens for non-Hodgkin's lymphoma frequently include doxorubicin, we investigated the activating capacity of the fusion protein in the presence of a doxorubicin-glucuronide prodrug, N-[4-doxorubicin-N-carbonyl(-oxymethyl) phenyl] O-β-glucuronyl carbamate (Fig 1).14
Chemical structure of prodrug N-[4-doxorubicin-N-carbonyl (-oxymethyl) phenyl] O-β-glucuronyl carbamate (DOX-GA3).
Chemical structure of prodrug N-[4-doxorubicin-N-carbonyl (-oxymethyl) phenyl] O-β-glucuronyl carbamate (DOX-GA3).
MATERIALS AND METHODS
Reagents.
Restriction enzymes were purchased from New England Biolabs (Beverly, MA) and GIBCO-BRL (Breda, The Netherlands). T4 polynucleotide kinase, T4 DNA polymerase, T4 DNA ligase, Rnase H, Terminal Deoxynucleotidyl Transferase and DNA polymerase I (Klenow fragment) were from GIBCO-BRL. Pwo DNA polymerase (Boehringer, Almere, NL) was used for polymerase chain reaction (PCR) amplifications. All enzymes were used as recommended by the suppliers.
Oligonucleotides were synthesized by Pharmacia Biotech (Roosendaal, NL). Purification of DNA was done using spin columns (Qiagen, Hilden, Germany).
Cloning of variable regions.
cDNA clones encoding the variable region domains were obtained as described.15 Briefly, RNA was isolated from the anti-CD20 hybridoma 1H4 and cDNA was synthesized by reverse transcription using an oligo-dT primer. The RNA template was destroyed with RNAse H and an oligo-dC anchor sequence was added to the 3′ end of the cDNA using Terminal Deoxynucleotidyl Transferase and deoxycytidine triphosphate (dCTP). This material was then used for a PCR using primers specific for the constant regions of the heavy chain: HC1 tggaattcggggccagtggatagac or light chain: HL2 tgaagcttagatggatacagttggt16 and an oligo-dG primer: gtgaattcggggggggggggg. The PCR products were cloned in pUC 19 and sequenced by the dideoxy method using a T7 kit (Pharmacia).
Construction of pscFv-CD20–human β-glucuronidase.
Oligonucleotide primers were designed, encoding the linkers between the light chain and heavy chain, and the heavy chain and human β-glucuronidase. The oligonucleotide primer pair CD20VHfor 5′ gggaagcttcaccatg ggatggagttgtatc 3′ CD20VHback 5′ ccacacgaattcggatccgcctcctcctgaggagactgtgagagtgg 3′ amplified a fragment coding for the eukaryotic ribosome binding site, translation initiation codon (bold) and the N-terminal of the signal sequence of MoAb 1H4 and part of a synthetic 15-amino acid linker (gly4-ser)3 as described by Huston.17 The primers contained an HindIII site at the 5′ end and a BamHI and EcoRI site at the 3′ end indicated in italics.
The oligonucleotide pair CD20VLfor 5′ tgtaagcttggatccggcggaggcggaagcggtggcggaggctctcaaattgttctctcccagtc 3′ CD20VLback 5′ ccaagaattcctcgagtcatccggaacctttgatttccagcttggtgc 3′ amplified a fragment encoding part of the linker between VH and VL and the MoAb 1H4 light chain without its signal sequence. The primers contained anHindIII and BamHI site at the 5′ end and anXho I and EcoRI site at the 3′ end (italics). The 3′ terminus also contained a BspEI site followed by a stop codon (bold) to enable expression of scFv-CD20 without fusion to human β-glucuronidase. The BspEI site is preceded by part of a gly4ser2 linker for the joining to β-glucuronidase. The oligonucleotide pair GUSfor 5′ tgtgaagctttccggaggttctggtctgcagggcgggatgctgtacc 3′ GUSback 5′ ccaagaattcctcgagcagtagcgactttcatgccaactctttatttcc 3′ was used to amplify a DNA fragment encoding part of the gly4ser2 linker and human β-glucuronidase18 without its signal peptide. The PCR primers contained an HindIII and BspEI site at the 5′ end and an Xho I and EcoRI site at the 3′ end.
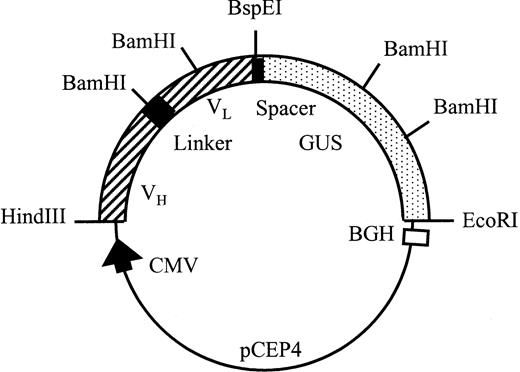
All PCR fragments were cloned into pUC19 after digestion withHindIII and EcoRI and the sequences were checked by the dideoxy method using T7 DNA polymerase (Pharmacia). pscFv-CD20 was constructed by inserting the HindIII/BamHI fragment of partially digested VL CD20 into the VH CD20 vector digested with the same enzymes. β-Glucuronidase cDNA was amplified with the indicated primers, cut with HindIII and Kpn I, and cloned into aKpn I and HindIII-digested pUC19 vector. TheKpn I-EcoRI fragment of the original cDNA encoding the enzyme was then ligated into this vector, cut with Kpn I andEcoRI, thus reconstructing the open reading frame encoding β-glucuronidase. The pscFv-CD20–β-glucuronidase was constructed by insertion of the BspEI/EcoRI fragment encoding β-glucuronidase into the pscFv-CD20 (Fig2).
Schematic representation of the pCEP4 scFv-CD20–β-glucuronidase expression vector. Transcription is driven by the human cytomegalovirus (CMV) enhancer-promotor sequence. The polyadenylation signal and transcription termination signal are from the bovine growth hormone gene.
Schematic representation of the pCEP4 scFv-CD20–β-glucuronidase expression vector. Transcription is driven by the human cytomegalovirus (CMV) enhancer-promotor sequence. The polyadenylation signal and transcription termination signal are from the bovine growth hormone gene.
Expression and purification of scFv-CD20–β-glucuronidase.
The insert containing scFv-CD20–β-glucuronidase was prepared byEcoRI digestion, blunt ends preparation by treatment with dNTPs (2′-deoxynucleoside 5′-triphosphates) and Klenow polymerase, followed by subsequent HindIII digestion. The isolated HindIII-EcoRI/blunt fragment of scFv-CD20–β-glucuronidase was ligated into Xba I (blunt)/HindIII digested vector pcDNA3 (InVitrogen, Leek, NL). An enzyme control vector was constructed using the human β-glucuronidase–encoding cDNA.18 The plasmids were transfected into COS-7 cells by diethyl aminoethyl (DEAE)-dextran for transient expression.19Cells were grown in Dulbecco's modified Eagle's medium (DMEM) containing 5% fetal calf serum (FCS) for 72 hours and the medium containing scFv-CD20–β-glucuronidase or β-glucuronidase was collected. To obtain stable transfectants, the fusion protein insert was isolated from the pcDNA3 vector by digestion with HindIII and Xho I and ligated into the pCEP4 vector (InVitrogen) digested with the same enzymes. The pCEP4 vector is an Epstein-Barr virus (EBV)-based vector, which is maintained and replicated extrachromosomally in primate and canine cells. This vector was used to transform 293/EBNA (InVitrogen) cells using DOSPER (Boehringer). Transfected cells were selected in medium containing 400 μg/mL hygromycin B (Boehringer). After 2 to 3 weeks resistant clones were isolated and recloned by limiting dilution. The scFv-CD20–β-glucuronidase fusion protein was purified from transfectoma supernatants by ion-exchange chromatography as described for human β-glucuronidase.20
Characterization.
The molecular weight of the fusion protein or β-glucuronidase was determined by immunoprecipitation and gel filtration. For immunoprecipitation, COS-7 cells were metabolically labeled 48 hours after transfection. First the cells were starved for 1 hour in DMEM plus 5% dialyzed fetal bovine serum, which lacked methionine and cysteine. For labeling, the same medium was used containing 100 μCi of Trans35S-label (ICN, Zoetermeer, The Netherlands) for 4 hours. Cell extracts and supernatants were then immunoprecipitated with rabbit antihuman β-glucuronidase as described18 and analyzed by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) as described by Laemmli.21
Gel filtration was performed with an SX200 column (1.6 × 60 cm; Pharmacia, Roosendaal, NL) in phosphate-buffered saline (PBS) to estimate the molecular mass of the native fusion protein. The column was run at 0.5 mL/min and fractions (0.5 mL) were assayed for β-glucuronidase activity. The column was calibrated with bovine serum albumin (67 kD), IgG (150 kD), and β-galactosidase (480 kD).
β-Glucuronidase activity was measured fluorometrically. To 50 μL of transfection supernatant or purified enzyme 100 μL substrate (4-methylumbelliferyl–β-glucuronide, 1 mmol/L in 100 mmol/L sodium acetate buffer pH 4.5) was added and incubated for 30 minutes at 37°C. The reaction was stopped by the addition of 1 mL 0.1 mol/L Glycine-NaOH pH 10.5. Fluorescence was measured using an excitation wavelength of 370 nm and an emission wavelength of 450 nm.
The binding of the fusion protein was determined with Daudi cells.22 Purified fusion protein was added to Daudi cells (105) at various concentrations for 1 hour at 4°C in 100 μL. Cells were washed three times with PBS and resuspended in 100 μL 4-methylumbelliferyl–β-glucuronide in buffer. After 2 hours at 37°C, 1 mL 0.1 mol/L glycine-NaOH was added and the fluorescence was measured as described above. Binding was determined by subtracting the values obtained with cells preincubated with an excess of anti-CD20 MoAb BCA-B206 from the fluorescence of the samples.
Prodrug activation.
The kinetics of the hydrolysis by human β-glucuronidase or fusion protein was determined with the prodrug N-[4-doxorubicin-N-carbonyl(-oxymethyl) phenyl] O-β-glucuronyl carbamate14 at a concentration of 100 μmol/L in PBS containing 0.1% BSA at pH 6.8 and at 37°C. Hydrolysis was followed by high-performance liquid chromatography (HPLC) analysis using a silica-C18 column (4.6 × 100 mm, 3 μm CP; MicroSpher, Chrompack, Middelburg, NL) and an isocratic eluent, which consisted of 2 mmol/L triethylamine in 20 mmol/L NaH2PO4 (pH 4.0)-acetonitrile (2:1, vol/vol) at a flow rate of 1 mL/min. Before each analysis, samples were diluted in ice-cold methanol to precipitate proteins. The eluate was analyzed with a fluorescence detector using an excitation wavelength of 480 nm and an emission wavelength of 580 nm. Peak areas were used to calculate the concentrations of drug and prodrug.
In vitro antiproliferative effects.
Daudi lymphoma cells22 were incubated with fusion protein (10 μg/mL) or PBS for 1 hour at 4°C, washed three times and seeded into 96-well plates at 2 × 104 cells/well. To determine the specificity of the fusion protein, a separate set of Daudi cells was pretreated (30 minutes, 4°C) with an excess of anti-CD20 antibody BCA-B20,6 washed, and then incubated with fusion protein. The cells were then exposed to the prodrug N-[4-doxorubicin-N-carbonyl(-oxymethyl) phenyl] O-β-glucuronyl carbamate at concentrations ranging from 0.01 to 50 μmol/L. In separate wells an excess of β-glucuronidase was present to determine the antiproliferative effects of prodrug completely hydrolyzed by the enzyme. After 24 hours, 200-μL medium was added and the cells were allowed to proliferate for another 72 hours. Cells were fixed with trichloroacetic acid and stained with Sulforhodamine B as described.12 The antiproliferative effects were determined and expressed as IC50 values, the concentrations that give 50% growth inhibition compared with control growth.
RESULTS
Cloning of the 1H4 variable cDNAs and construction of scFv-CD20–β-glucuronidase fusion protein.
The cDNAs encoding the 1H4 variable domains were cloned by reverse transcriptase PCR from total RNA isolated from the 1H4 hybridoma. The nucleotide sequence of the heavy and light variable chain cDNA was determined. At least three independent clones were sequenced to ensure that the DNA polymerase used in the PCR induced no mutations. The nucleotide and deduced amino acid sequence of the cDNA encoding the scFv-CD20 is shown in Fig 3. The sequences were used to design primers for the construction of the fusion protein.
Nucleotide and deduced amino acid sequence of the VH and VL of anti-CD20 MoAb 1H4. The primers used for cloning are underlined. The cleavage site of the leader peptide (bold) is indicated by the arrow.
Nucleotide and deduced amino acid sequence of the VH and VL of anti-CD20 MoAb 1H4. The primers used for cloning are underlined. The cleavage site of the leader peptide (bold) is indicated by the arrow.
The plasmid pCD20-β–glucuronidase was constructed as shown in Fig 2. A 15-amino acid linker containing (gly4ser)3, as described previously, separated the variable VH and VL antibody domains.17 The cDNA encoding human β-glucuronidase18 was inserted in frame of the scFv separated by a 6-amino acid linker segment. The signal peptides of VL and human β-glucuronidase were removed. The open reading frame encoded a recombinant protein, which after cleavage of the secretory signal sequence and C-terminal processing, was composed of 878 amino acids with a predicted molecular weight (Mw) of 100 kD.
Expression and characterization of fusion protein.
The fusion protein was expressed in eukaryotic cells because human β-glucuronidase needs to be glycosylated to form an enzymatically active tetramer.13 The supernatants of the transfected COS-7 cells contained approximately 10 ng/mL of enzymatically active fusion protein, which was approximately 10-fold less than that of COS-7 cells transfected with vector encoding human β-glucuronidase. For continuous production of fusion protein, 293/EBNA cells were transfected with pCEP4 vector encoding the fusion protein. Clones were selected with hygromycin B and subcloned by limiting dilution. Culture supernatants from these cells produced approximately 100 ng/mL fusion protein.
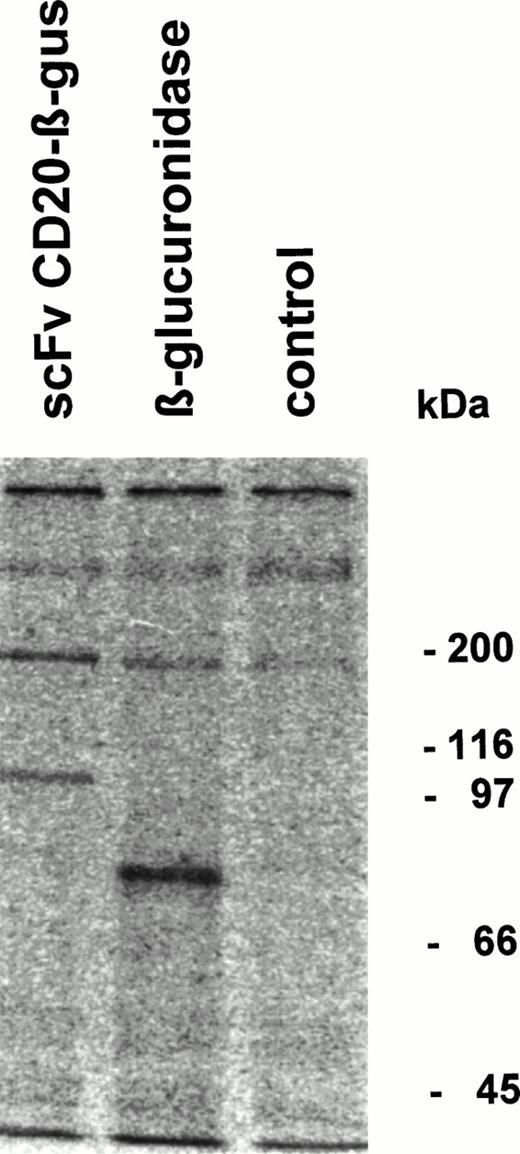
Fusion protein or β-glucuronidase was analyzed by immunoprecipitation followed by SDS-PAGE. The fusion protein migrated with an apparent Mw of 100 kD, whereas β-glucuronidase monomers showed an Mw of approximately 80 kD (Fig 4). Gel filtration analysis of the fusion protein showed that it migrates as a tetrameric protein with the expected Mw of approximately 400 kD (data not shown).
Immunoprecipitation of scFv-CD20–β-glucuronidase. COS-7 cells were transfected with scFvCD20-β–glucuronidase or β-glucuronidase cDNA. The cells were labeled 48 hours after transfection with 35S-methionine/cysteine for 4 hours, the supernatant was immunoprecipitated with rabbit anti-β–glucuronidase antibody and analyzed by SDS-PAGE.
Immunoprecipitation of scFv-CD20–β-glucuronidase. COS-7 cells were transfected with scFvCD20-β–glucuronidase or β-glucuronidase cDNA. The cells were labeled 48 hours after transfection with 35S-methionine/cysteine for 4 hours, the supernatant was immunoprecipitated with rabbit anti-β–glucuronidase antibody and analyzed by SDS-PAGE.
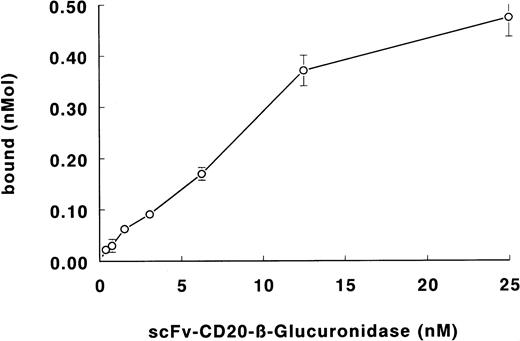
The binding of the fusion protein to Daudi lymphoma cells is shown in Fig 5. The specificity of binding was determined with the blocking anti-CD20 antibody BCA-B20. Binding of the fusion protein was less than 5% in the presence of excess antibody. From the binding data, the apparent affinity of the fusion protein was calculated to be 3 × 109 L/mol.
Binding of scFv-CD20–β-glucuronidase to Daudi lymphoma cells. Cells were incubated with fusion protein. Cells were washed and the binding was determined by measuring the β-glucuronidase activity.
Binding of scFv-CD20–β-glucuronidase to Daudi lymphoma cells. Cells were incubated with fusion protein. Cells were washed and the binding was determined by measuring the β-glucuronidase activity.
Prodrug activation and antiproliferative effects.
The enzymatic activity of the fusion protein was compared with that of native recombinant β-glucuronidase using the prodrug N-[4-doxorubicin-N-carbonyl(-oxymethyl) phenyl] O-β-glucuronyl carbamate as a substrate. At 100 μmol/L prodrug concentration, the activation rates for the conversion of prodrug to drug were similar (T1/2 = 130 minutes) for both the fusion protein and the enzyme (Fig 6), showing that the fusion protein possessed the full enzymatic activity of human β-glucuronidase.
Hydrolysis of the prodrug N-[4-doxorubicin-N-carbonyl (-oxymethyl) phenyl] O-β-glucuronyl carbamate (100 μmol/L, closed symbols) to the cytotoxic drug doxorubicin (open symbols) by human β-glucuronidase (▵ and ▴, 1 μg/mL) or scFv-CD20–β-glucuronidase (○ and •, 1 μg/mL) as determined by reversed-phase HPLC and fluorescence detection.
Hydrolysis of the prodrug N-[4-doxorubicin-N-carbonyl (-oxymethyl) phenyl] O-β-glucuronyl carbamate (100 μmol/L, closed symbols) to the cytotoxic drug doxorubicin (open symbols) by human β-glucuronidase (▵ and ▴, 1 μg/mL) or scFv-CD20–β-glucuronidase (○ and •, 1 μg/mL) as determined by reversed-phase HPLC and fluorescence detection.
Daudi lymphoma cells were used to determine the specific activation of the prodrug N-[4-doxorubicin-N-carbonyl (-oxymethyl) phenyl] O-β-glucuronyl carbamate by scFv-CD20–β-glucuronidase fusion protein. In the presence of an excess of β-glucuronidase, the prodrug was activated to doxorubicin. Similarly, preincubation of Daudi cells with the fusion protein increased the antiproliferative effects of the prodrug as a result from activation (Fig7). Specificity was evident, because cells preincubated with an excess of BCA-B20 blocking antibody and fusion protein showed no increase in growth inhibition when compared with incubation with the prodrug alone.
Antiproliferative effects of doxorubicin, prodrug N-[4-doxorubicin-N-carbonyl(-oxymethyl) phenyl] O-β-glucuronyl carbamate (DOX-GA3), and prodrug combined with scFv-CD20–β-glucuronidase. Cells were incubated with fusion protein, blocking antibody, and fusion protein, or PBS. Cells were then seeded in 96-wells plates and exposed to drug or prodrug for 24 hours. After 72 hours, cell growth was measured with a protein dye, Sulforhodamine B.
Antiproliferative effects of doxorubicin, prodrug N-[4-doxorubicin-N-carbonyl(-oxymethyl) phenyl] O-β-glucuronyl carbamate (DOX-GA3), and prodrug combined with scFv-CD20–β-glucuronidase. Cells were incubated with fusion protein, blocking antibody, and fusion protein, or PBS. Cells were then seeded in 96-wells plates and exposed to drug or prodrug for 24 hours. After 72 hours, cell growth was measured with a protein dye, Sulforhodamine B.
DISCUSSION
We have constructed an expression plasmid for the production of a single-chain antibody-enzyme fusion protein composed of the anti-CD20 antibody 1H4 and the human lysosomal enzyme β-glucuronidase. The scFv-CD20–β-glucuronidase fusion protein was expressed in eukaryotic cells and was shown to exist in a tetrameric form. The fusion protein bound to CD20-expressing Daudi lymphoma cells; the antigen specificity was confirmed by competition experiments. The enzymatic activation of the prodrug N-[4-daunorubicin-N-carbonyl (-oxymethyl) phenyl] O-β-glucuronyl carbamate by the fusion protein was at the same rate as that for β-glucuronidase. The fusion protein, when specifically bound to Daudi lymphoma cells, completely activated the nontoxic prodrug and growth inhibition was similar to that obtained with doxorubicin.
Several chemically prepared β-glucuronidase immunoconjugates have been described for activation of anticancer prodrugs. We have used bothEscherichia coli (E coli)-derived β-glucuronidase and human β-glucuronidase conjugated to MoAb 323/A3 for the activation of anthracycline prodrugs such as epirubicin-glucuronide and daunorubicin-glucuronide.11,12,14 Roffler et al23 have used E coli β-glucuronidase for the activation of an aniline mustard prodrug. Antibody-enzyme conjugates prepared with chemical cross-linkers have the disadvantage that the conjugates differ in the amount and position of the cross-links resulting in inconsistent fractions of enzyme and antibody activity. Recombinant DNA techniques provide the means of directly producing a fusion protein with defined characteristics. Furthermore, when scFv fragments are used, the antibody size is reduced from 150 kD to approximately 25 kD. The smaller size of a fusion protein prepared with scFv fragments instead of IgG will result in better tumor penetration properties.24 In addition, scFv are expected to be less immunogenic than whole IgG antibodies.25 26
The yield of enzymatically active fusion protein was low: 10 ng/mL in COS-7 transfectoma supernatants. This was approximately 10-fold lower than the expression level of β-glucuronidase in the same vector. Southern blot analysis of transcripts indicated that RNA levels were reduced in cells transfected with the fusion protein when compared with those of the enzyme (data not shown). We could increase the expression level 10-fold in 293/EBNA cells, which were transfected with the pCEP4 vector. This vector is EBV-based and is maintained and replicated extrachromosomally in primate and canine cells.
Several antibody-enzyme fusion proteins have been described. Goshorn et al27 and Rodrigues et al28 have developed Fv-β–lactamase fusions. These fusion proteins, prepared with a bacterial enzyme, showed selective activation in vitro of cephalosporin prodrugs of a nitrogen mustard and doxorubicin, respectively. Bosslet et al29,30 have developed an antibody-human β-glucuronidase fusion protein. This fusion protein was tested in mice bearing human tumor xenografts and found to be rapidly cleared from the blood resulting in tumor:blood ratios of >100:1 at 7 days after injection. The combination of this fusion protein with a doxorubicin prodrug showed superior growth inhibition when compared with doxorubicin alone. The fusion protein of Bosslet et al29 differs from our scFv-CD20–β-glucuronidase fusion protein in that the enzyme was fused to a humanized VH containing an IgG3 hinge region, which was joined to the VL of the antibody by disulfide bond formation. The antibody moiety was a F(ab)2fragment and not a scFv. The apparent native Mw of this fusion protein was 250 kD, which is conflicting with the observation that β-glucuronidase needs a tetrameric configuration with an apparent Mw of 400 kD to be enzymatically active.13 Apparently, processing of the c-terminal end of β-glucuronidase in the baby hamster kidney cells used by Bosslet et al differs from that of other cells, which leads to dimeric enzyme molecules.31
The observed Mw of 400 kD of the scFv-CD20–β-glucuronidase fusion protein is similar to the size of the chemically linked conjugate with Mw 400 kD.20 Unfortunately, the large size of the tetrameric protein will impede tumor penetration. We have constructed a similar fusion protein between human β-galactosidase, which is a monomeric lysosomal enzyme, and scFv-CD20. The scFv-CD20–β-galactosidase fusion protein had an apparent Mw of 100 kD, both on SDS-PAGE, as well as by gel filtration (data not shown) and should have improved in vivo tumor penetration properties.
Of importance, the design of the expression cassette used for scFv-CD20–β-glucuronidase enables the rapid exchange of both the scFv and the enzyme. Thus, fusion proteins may be constructed that target different tumor types and use prodrugs activated by other enzymes.
Human dendritic cell derived from peripheral blood progenitors with recombinant interleukin-4 and granulocyte-macrophage colony-stimulating factor (GM-CSF). The cells have long microvillous surface processes and numerous cytoplasmic lysosomes in addition to a well-formed golgi apparatus (uranyl acetate, lead citrate, original magnification ×2,500). (Courtesy of Jonathan W. Said, Kai Chien, Walter Verbeek, Jeffrey Weber, H. Phillip Koeffler, UCLA Center for the Health Sciences, University of Southern California, and Cedars Sinai Medical Center, Los Angeles, CA.) {/ANNT;80256n;;center;0n}
Human dendritic cell derived from peripheral blood progenitors with recombinant interleukin-4 and granulocyte-macrophage colony-stimulating factor (GM-CSF). The cells have long microvillous surface processes and numerous cytoplasmic lysosomes in addition to a well-formed golgi apparatus (uranyl acetate, lead citrate, original magnification ×2,500). (Courtesy of Jonathan W. Said, Kai Chien, Walter Verbeek, Jeffrey Weber, H. Phillip Koeffler, UCLA Center for the Health Sciences, University of Southern California, and Cedars Sinai Medical Center, Los Angeles, CA.) {/ANNT;80256n;;center;0n}
ACKNOWLEDGMENT
We gratefully acknowledge the gift of the cDNA encoding human β-glucuronidase and the rabbit antiserum against human β-glucuronidase from Prof. W. Sly.
Address reprint requests to Hidde J. Haisma, PhD, Department of Medical Oncology, Academic Hospital Vrije Universiteit, PO Box 7057, 1007 MB Amsterdam, The Netherlands; e-mail:hj.haisma.oncol@med.vu.nl.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

![Fig. 1. Chemical structure of prodrug N-[4-doxorubicin-N-carbonyl (-oxymethyl) phenyl] O-β-glucuronyl carbamate (DOX-GA3).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/1/10.1182_blood.v92.1.184.413k26_184_190/5/m_blod41326001x.jpeg?Expires=1769120093&Signature=5QKI9FVK9TnA5W-MQCydtNeaCT21xpQay7Hjy0HtNLj7zTYL2kzdQaZc0Z4J0Tqr-0besG7NJHq3V98P0hcPs2WnUmR9dHr8MqMwW5YdtUBF0PumgyByrjOOJpl~ohj1AH1S7MmMBTROsqBOM6IYI~pPZj15Ry1V-T5YuNX53J5VtjzvTetTlFt6WFm7Q3ZbvlHpSsiKVKHtPxmh9kCn918sxjzvBK9kNtIO0KM3vdnsq6b17uS06rLy4j06hunBZCdTlvBG3fEss14VpmxCZONFMCFCboaaETi5dm3Fqn2kCZQXfvfgfWepI~py-jSvGx3KCWLwAVH~eenF~KwARg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 6. Hydrolysis of the prodrug N-[4-doxorubicin-N-carbonyl (-oxymethyl) phenyl] O-β-glucuronyl carbamate (100 μmol/L, closed symbols) to the cytotoxic drug doxorubicin (open symbols) by human β-glucuronidase (▵ and ▴, 1 μg/mL) or scFv-CD20–β-glucuronidase (○ and •, 1 μg/mL) as determined by reversed-phase HPLC and fluorescence detection.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/1/10.1182_blood.v92.1.184.413k26_184_190/5/m_blod41326006x.jpeg?Expires=1769120093&Signature=ANlr98wNHt1eBbg~C8qh9axJmY0vqRqrouklZiKWnOZyarQwQwdRMkcyv6sdjuaZOXBcl7tNnlwsjhRJsPu3-jFKSZJvbKrqYYa-KsdiBXy6ZaT-wXni8aZSIwb7OaUjFw9xAGT8SponL1Hm6G1NlyqOkuR5IIgUVOddNaKU6nB5FR--FSt62dpeF-~x-YYA0Kd04lOkjAbd6RM8FhZKDj6thGoCYMiR2TA3ARxX6~WtFOWXvs-n5IRxSJ77wx9qRbeE0k7NXI1mdI47HRObPJKeMI-m72vayJPrFckd6Cx-VLyFc3C5wka4Z8M1G6kE8gP30QgW9PXG8uL4AVI9ng__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 7. Antiproliferative effects of doxorubicin, prodrug N-[4-doxorubicin-N-carbonyl(-oxymethyl) phenyl] O-β-glucuronyl carbamate (DOX-GA3), and prodrug combined with scFv-CD20–β-glucuronidase. Cells were incubated with fusion protein, blocking antibody, and fusion protein, or PBS. Cells were then seeded in 96-wells plates and exposed to drug or prodrug for 24 hours. After 72 hours, cell growth was measured with a protein dye, Sulforhodamine B.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/1/10.1182_blood.v92.1.184.413k26_184_190/5/m_blod41326007x.jpeg?Expires=1769120093&Signature=I8SdBb1-lT5qGTL3MMCF2g6VFVObNg3kl8an3qACYeLeBAG6pLZ9QwR9HGxc7z170MMx5kHpzF0vyZOZKvMpyWjBJcgDt4AWFfUS~zhh-cimiB7DDtKEfFzH0nO1smktp6~~KcDZ9atVyrlq-8gwJCwbKfMQhh-JX8ZihjHpM6PJykzA652LSOqnJ4SXs-6VzvYc7MD~jwG2J6WQZ9hAcmX4ezJTbEGOXVv7mInZcL~WXyHIYBBPBcq9rHVXfbhfyyYFI3BqgcJrRPqQH5nYwntjZhZJKan8emR43njiw3t5SyPoXT73oY5~Wfu6VRFQdlSu0ZFl2FJ4bSwYSQFcow__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal