The platelet GPIb-V-IX complex is the receptor for the initial binding of von Willebrand factor (vWF) mediating platelet adhesion. The complex is composed of four membrane-spanning glycoproteins (GP): GPIbα, GPIbβ, GPIX, and GPV. Bernard-Soulier syndrome results from a qualitative or quantitative defect in one or more components of the platelet membrane GPIb-V-IX complex. We describe the molecular basis of a novel Bernard-Soulier syndrome variant in two siblings in whom GPIbα was not detected on the platelet surface but that was present in a soluble form in plasma. DNA sequence analysis showed that the affected individuals were compound heterozygotes for two mutations. One, inherited from a maternal allele, a T777 → C point mutation in GPIbα converting Cys65 → Arg within the second leucine rich repeat, the other, a single nucleotide substitution (G2078 → A) for the tryptophan codon (TGG) causing a nonsense codon (TGA) at residue 498 within the transmembrane region of GPIbα, inherited from a mutant paternal allele. The Bernard-Soulier phenotype was observed in siblings who were compound heterozygotes for these two mutations. Although GPIbα was not detected on the surface of the patient's platelets, soluble GPIbα could be immunoprecipitated from plasma. When plasmids encoding GPIbα containing the Cys65 → Arg mutation were transiently transfected into Chinese hamster ovary (CHO) cells stably expressing the GPβ-IX complex (CHOβIX), the expression of GPIbα was similar to the wild-type (WT) GPIbα, but did not bind vWF. When plasmids encoding GPIbα containing the Trp498 → stop were transiently transfected into CHOβIX, the surface expression of GPIbα was barely detectable compared with the WT GPIbα. Thus, this newly described compound heterozygous defect produces Bernard-Soulier syndrome by a combination of synthesis of a nonfunctional protein and of a truncated protein that fails to insert into the platelet membrane and is found circulating in plasma.
THE PLATELET glycoprotein Ib-V-IX complex consists of four type I membrane-spanning polypeptides that belong to the leucine-rich motif (LRM) family.1 These proteins have in common a structural motif containing one or more repeats of periodically spaced leucines. In addition, these polypeptides have regions flanking the LRM that are very similar and contain disulfide loops.2 GPIb consists of two subunits, α (140 kD) and β (27 kD), which are disulfide linked.2,3 GPIX is noncovalently associated with GPIbα.4 GPV and GPIb-IX also form a noncovalent complex in the platelet membrane.5
The α-subunit of GPIb consists of four major structural domains. The N-terminus contains seven leucine-rich repeats.6Immediately outside the platelet membrane is a threonine-, proline-, and serine-rich region that is highly O-glycosylated; this region is termed the macroglycopeptide domain. Following the macroglycopeptide domain is a transmembrane domain of 29 amino acids and a cytoplasmic domain of approximately 100 amino acids.6 The external portion of the α chain, termed glycocalicin (135 kD),7 can be cleaved from the platelet surface. It is found circulating in normal plasma8 at a concentration of 2.04 ± 0.46 μg/mL.9 The binding site for vWF has been localized to the N-terminus of the α-subunit.10 11
A qualitative or quantitative defect in the GPIb-V-IX complex results in a rare congenital disorder of platelets initially described by Bernard and Soulier in 1948.12 This syndrome, which is inherited in an autosomal recessive manner, is characterized by decreased numbers of platelets that are large and morphologically abnormal, and a prolonged cutaneous bleeding time.13Although Bernard-Soulier platelets are characterized by normal aggregation in response to agonists such as adenosine diphosphate (ADP) and epinephrine, they do not aggregate or agglutinate in the presence of ristocetin, a process that is dependent on the interaction between vWF and the platelet glycoprotein Ib-V-IX complex.14 A critical functional in the binding of vWF to the glycoprotein Ib-V-IX complex was initially suggested by studies of Bernard-Soulier platelets, in which there was absent surface expression of GPIb, GPIX, and GPV.15
The expression of the GPIb-V-IX complex is dependent on the coordinated assembly of at least three gene products, the α- and β-subunits of GPIb, and GPIX.16 The molecular basis of Bernard-Soulier syndrome has been characterized in several published cases to date providing further evidence that each of these subunits has a critical role in the coordinate assembly of the functional complex. Whereas GPV has also been shown to be absent in Bernard-Soulier syndrome, it does not seem to be necessary for the surface expression of the complex.17
Given that each polypeptide is encoded by its own gene and that the coordinate assembly of the complex is required, it is surprising that Bernard-Soulier syndrome is so rare. Most of the mutations characterized that result in Bernard-Soulier syndrome are within the GPIbα gene. These mutations have been caused by nonsense mutations producing a truncated GPIbα protein18-22 or mutations that have been localized to the leucine-rich motif (LRM) of GPIbα.23-26 A single case having a mutation in GPIbα that changed a cysteine residue involved in disulfide bonding has also been described.27 We and other investigators have recently characterized a mutation within the transmembrane region of GPIbα that affected anchoring of the GPIbα polypeptide in platelets and results in a circulating soluble GPIbα.28,29 A number of mutations have been identified within GPIX, two of which resulted from an amino acid change in the LRM or the region flanking the LRM in GPIX,30,31 and two other point mutations that changed a cysteine to a tyrosine in GPIX32 and another that caused a nonsense codon.22 There has been a single published report of a mutation within the promoter for GPIbβ that resulted in Bernard-Soulier syndrome.33
This report describes the molecular genetic basis for a novel variant mutation within the gene coding for GPIbα that is responsible for Bernard-Soulier syndrome.
MATERIALS AND METHODS
Case History
A 5-year-old girl and her 20-year-old brother from eastern Iceland were diagnosed as having Bernard-Soulier syndrome. The boy presented at 15 months of age with easy bruising and a platelet count of 29,000 to 67,000/mm3. He was treated initially with steroids. At 5 years of age, he underwent a splenectomy, as he was believed to have idiopathic thrombocytopenic purpura. At 8 years of age, an episode of epistaxis required hospital admission, and large platelets were noted on a blood smear. Platelet aggregation studies showed normal response to adenosine diphosphate (ADP), epinephrine, collagen, and arachidonic acid, with no response to ristocetin. The platelet count was 120,000/mm3, the bone marrow aspirate was normal, and the platelet count has remained at approximately 120,000/mm3since the splenectomy. The younger sister was first admitted to hospital at 16 months of age because of episodes of bleeding and easy bruising. Her bleeding time was prolonged (>13 minutes) and platelet count was 75,000/mm3. She has had several episodes of bleeding requiring transfusion. There are six other siblings, none of whom has any bleeding symptoms. The mother and father are unrelated and unaffected clinically with normal platelet counts and morphology.
Monoclonal antibodies and reagents.
The anti-GPIbα antibody AP-1 blocks vWF binding to GPIbα.14 MBC 142.2, 142.6, and 142.11 are monoclonal antibodies (MoAbs) raised against purified GPIbα that do not inhibit the binding of vWF to GPIbα. An anti-GPIX MoAb (FMC 25) was purchased from Harlan Bioproducts (Indianapolis, IN). AP2 is a MoAb against the GPIIb-IIIa complex.34 An affinity-purified platelet GPIbβ-specific rabbit polyclonal antibody4 was a generous gift of Dr Sandor S. Shapiro (Cardeza Foundation for Hematologic Research, Philadelphia, PA).
Blood.
Blood samples from the patients and the patient's parents were collected into acid-citrate-dextrose (National Institutes of Health formula A) and shipped by courier to Milwaukee. The samples arrived within 72 hours and were processed immediately. Because proteolysis of the extracellular portion of GPIbα could result in the decrease of detectable GPIbα on the platelet surface, blood samples were obtained simultaneously from three normal volunteers in Iceland and shipped with the patient samples. Platelet-rich plasma (PRP) from the parents and normal volunteers was prepared by centrifugation at 800g for 2 minutes. Platelets were isolated and washed three times by differential centrifugation and resuspended in a buffer containing 96.5 mmol NaCl, 85.7 mmol glucose, 1.1 mmol EDTA, 8.5 mmol Tris with 50 ng/mL prostaglandin E1 (PgE1) (Sigma Chemical Co, St Louis, MO). Because Bernard-Soulier platelets are typically large and morphologically abnormal, a previously described sedimentation technique was used to isolate platelets from the patients.29
Platelet lysates were prepared by resuspending the platelet pellet in 500 μL of lysis buffer (96.5 mmol NaCl, 85.7 mmol glucose, 1.1 mmol EDTA, 8.5 mmol Tris, 5 mmol N-ethylmaleimide, 100 μg/mL leupeptin, 1 mmol phenylmethylsulfonyl fluoride [PMSF], and 1% Triton X-100 [Pierce, Rockford, IL]). The lysate was vortexed for 3 minutes and then centrifuged at 4°C for 10 minutes at 16,000g. Aliquots of platelet lysate and platelet-poor plasma (PPP) were frozen at −80°C until analyzed.
Immunoblotting.
Platelet lysates were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on an 8% gel according to Laemmli.37 The separated proteins were electroblotted onto a polyvinylidine difluoride (PVDF) membrane (Novex, San Diego, CA) as described by Towbin et al,38 blocked in phosphate-buffered saline (PBS) containing 5% powdered milk, and then incubated overnight with the anti-GPIbα MoAbs MBC 142.6 or MBC 142.11 or the affinity-purified anti-GPIbβ polyclonal antibody. The membrane was washed three times with PBS containing 5% powdered milk, incubated with a goat anti-mouse or donkey anti-rabbit IgG conjugated with horseradish peroxidase (HRP), washed again three times, and treated with SuperSignal Substrate (Pierce).
Immunoprecipitation.
GPIbα MoAb 142.2 was coupled to cyanogen bromide-activated Sepharose 4B beads (Sigma). PPP was precleared by incubating with uncoupled Sepharose CL-4B beads for 1 hour at room temperature. The beads were centrifuged at 1,000g and the plasma added to the antibody-coupled beads and incubated overnight at 4°C. The beads were washed and the immunoprecipitated complexes from plasma eluted in 2% SDS. All samples were boiled at 100°C for 3 minutes. The samples were then analyzed by SDS-PAGE on an 8% to 16% exponential gradient in the presence of 20 mmol dithiothreitol. Immunoblotting was performed in the same manner as described above, using the antibody MBC 142.11.
Flow cytometry analysis of platelets in whole blood.
The platelet GPIb-IX complex and GPIIbIIIa were analyzed by flow cytometry, using a two-color, two-antibody technique.39 A total of 100 μL of whole blood was diluted 1:10 in PBS and divided into six 50-μL aliquots. Samples were then either unstained, incubated with 2 μg/mL of mouse IgG as a negative control, 2 μg/mL of fluorescein isothiocyanate (FITC)-conjugated MoAb-AP1, 2 μg/mL of FITC-conjugated-AP2, a combination of a biotin-conjugated AP2 plus FITC-AP1, or a combination of a biotin-conjugated AP1 plus FITC-conjugated AP2. The samples were incubated in the dark for 20 minutes at room temperature. Subsequently, streptavidin-conjugated phycoerythrin (PE) was then added to each sample except the unstained control. The samples were incubated for an additional 10 minutes in the dark at room temperature and analyzed immediately in a Becton Dickinson (San Jose, CA) FACScan flow cytometer. Fluorescence was used to identify the platelet population with AP2; the samples were then analyzed for AP1 fluorescence. This technique demonstrated that the anti-GPIbα MoAb AP1 failed to bind platelets that were recognized by the anti-GPIIbIIIa antibody. Further experiments were then performed to evaluate the surface expression of GPIbα and of GPIX on platelets using the anti-GPIbα antibodies 142.2 and 142.11 and the anti-GPIX antibody FMC-25. In these experiments, platelets were identified and gated, both by their PE-AP2 fluorescence intensity and by their physical properties on a forward versus side-scatter plot. Data for these platelets were collected through this gate and were analyzed for fluorescence with the antibodies 142.2 and 142.11 and FMC-25.
PCR amplification of genomic DNA.
Genomic DNA was isolated from peripheral blood lymphocytes (PBLs) as described.40 DNA was amplified by the polymerase chain reaction (PCR), using primer pairs based on the published genomic sequence of GPIbα.41 For DNA sequence analysis, the full-length coding region for mature GPIbα was amplified with primers 162-181 (GGCCTGCATTTCCTCCTCACC) and 2653-2634 (AAGCTCCCGATGCTGCATGGG). The target sequences were amplified in a 50-μL reaction volume containing 500 to 1,000 ng of genomic DNA, 30 pmol of each primer, and 0.2 mmol of each dNTP in a reaction buffer consisting of 60 mmol/Tris-HCl pH 9.0, 15 mmol (NH4)2SO4, 2 mmol/L MgCl2 and 1 U of Taq polymerase (Perkin Elmer, Foster City, CA) and 4% (vol/vol) DMSO. PCR amplification was performed in a programmable thermal cycler (model 9600, Perkin Elmer) for 35 cycles of 45 seconds denaturation at 96°C, annealing for 1 minute at 60°C, and extension for 1 minute at 72°C. PCR products containing the entire coding region for GPIbα were cloned into the pCRII.1 cloning vector using the TA cloning kit (Invitrogen, San Diego, CA).
DNA sequencing.
Direct sequence analysis of the entire coding region of PCR-amplified GPIbα from each subject and a minimum of three clones each from the two patients, the mother, and father was performed using the Prism Ready Reaction DyeDeoxy terminator cycle sequencing kit and an Applied Biosystems (Foster City, CA) model 373A DNA Sequencer. Sequencing primers were synthesized on an Applied Biosystems model 394/DNA synthesizer.
Transient expression.
A Bsg I and BsrGI restriction fragment (nucleotides 218–847) of the subcloned GPIbα PCR product amplified from genomic DNA containing the T → C substitution at nucleotide 777 in GPIbα (Cys65 → Arg) was subcloned into pGEM-7 containing the WT GPIbα cDNA, from which a BsgI and BsrGI restriction fragment had been excised. An XhoI/MluI restriction fragment, containing the entire coding region for GPIbα, was then excised from this pGEM-7GPIbαC65 → R and inserted into the mammalian expression vector pCI-NEO (Promega, Madison, WI), which carries the human cytomegalovirus (CMV) promoter and the SV40 origin of replication. This expression vector (pCI-NEOGPIbαC65 → R) now contained the entire coding region for GPIbα with the point mutation at codon 65. In a similar manner, a Pst I restriction fragment (nucleotides 1246-2206 and containing the G → A substitution at nucleotide 2078 resulting in W498 → stop) of the subcloned GPIbα PCR product was subcloned into pGEM-7 containing the WT GPIbα cDNA, from which aPst I restriction fragment had been excised. Again, anXho I/Mlu I restriction fragment, containing the entire coding region for GPIbα, was excised from pGEM-7GPIbαW498 → stop and inserted into the expression vector pCI-NEO. This expression vector (pCI-NEOGPIbαW498 → stop) now contained the entire coding region for GPIbα with the point mutation at codon 498. Constructs containing both the WT and mutant GPIbα were sequenced to ensure that no additional mutations had been introduced.
For transient expression studies, CHO βIX cells (kindly provided by Dr José A. López, Baylor College of Medicine, Houston, TX) were used. CHO βIX cells are CHO cells that stably surface express human GPIbβ and GP IX at high levels.42 These cells were additionally transiently transfected with either the WT GPIbα, the construct containing the pCI-NEOGPIbαC65 → R, the construct containing pCI-NEOGPIbαW498 → stop or mock-transfected with the plasmid pCI-NEO alone. Expression plasmids were introduced into CHO βIX cells in the presence of lipofectamine (GIBCO-BRL), following the protocol of Felgner et al.43 In brief, 1.5 × 106 cells were plated in 100-mm dishes and grown overnight. 6.6 mL of OPTI-MEM–reduced serum media (GIBCO-BRL) containing 72 μg of lipofectamine and 6 μg of the appropriate plasmid DNA was added, and the cells were incubated for 5 hours. The transfection media was removed and 8 mL of culture media was added; incubation was continued at 37°C for 48 hours.
Transfected cells were detached from tissue culture plates with 3 mmol EDTA, centrifuged at 250g and resuspended in HBSS with 1% bovine serum albumin (BSA) and 1% normal donkey serum. 3 × 105 cells were transferred to each well of a 96-well V-bottom plate (Dynatech, Chantilly, VA) and incubated for 30 minutes simultaneously with 5 μg/mL of vWF, 1 μg/mL of botrocetin the anti-GPIbα antibody MBC 142.2, and a rabbit polyclonal antibody to vWF (5 μg/mL and 3 μg/mL, respectively). A concentration of 1 μg/mL of botrocetin was used, as previous investigations from our laboratory have shown no significant difference in vWF binding with 1 μg/mL of botrocetin as compared with 5 μg/mL.44 The cells were then washed twice and incubated for an additional 30 minutes in a darkened room with a 1:100 dilution of PE-conjugated affinity-purified F(ab′)2 donkey anti-mouse IgG and a 1:320 dilution DTAF-conjugated affinity-purified F(ab′)2 donkey anti-rabbit IgG (Jackson Immunoresearch Laboratories, West Grove, PA) to detect vWF binding in cells expressing GPIbα. The cells were then washed twice, resuspended in 2% paraformaldehyde, and analyzed in a Becton Dickinson FACScan flow cytometer.
RESULTS
GPIbα Is Undetectable on the Patient's Platelet Surface
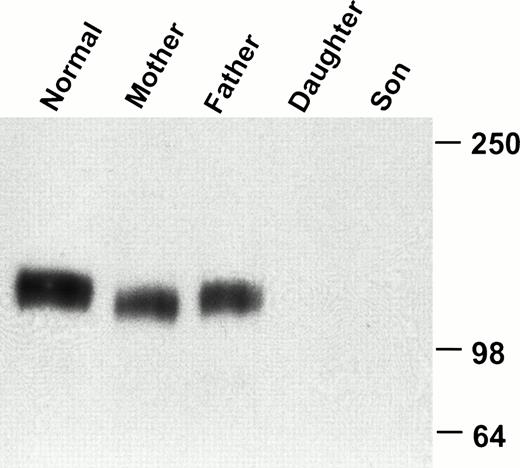
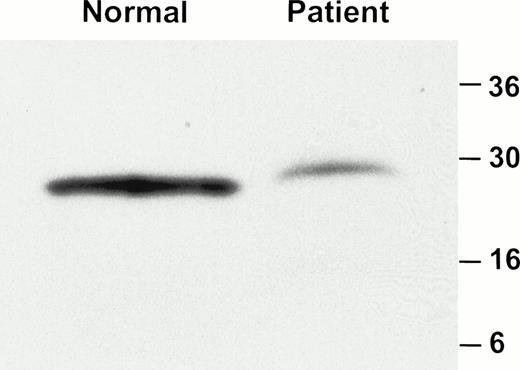
FACS analysis of whole blood showed that the MoAb AP1 failed to recognize GPIbα on the platelet surface of these two patients (Fig1). Although there was no detectable binding of AP1 to platelets from either of the affected siblings, the binding of AP1 was similar in both parents, as compared with three normal controls. By contrast, binding of AP2 was normal in both patients and their parents (data not shown). Further analysis of platelets from the eldest patient showed that neither of the anti-GPIbα antibodies, 142.2 and 142.11, bound to platelets (Fig2). By contrast, the expression of GPIX was normal in the patients platelets. Immunoblot analysis of platelet lysate with the monoclonal antibodies MBC 142.6 confirmed the absence of GPIbα in either of the patient's platelets (Fig3). Immunoblot analysis of platelet lysate under reduced conditions with the anti-GPIbβ polyclonal antibodies demonstrated that GPIbβ was present in the patients platelets, albeit in markedly reduced amounts (Fig 4).
Flow cytometric analysis of patients' and parents' platelets. Analysis was performed on whole blood with monoclonal antibodies against GPIbα (AP1) and GPIIbIIIa (AP2). Platelets were then analyzed for their binding to AP1. Filled area, mother and father; clear area, affected children. The results from analysis of an additional three normal volunteers are shown by thedashed lines overlapping the normal sample in the shaded area. There is no detectable GPIbα on the platelets of the two children, whereas the samples from the two parents are similar to those of the normal controls.
Flow cytometric analysis of patients' and parents' platelets. Analysis was performed on whole blood with monoclonal antibodies against GPIbα (AP1) and GPIIbIIIa (AP2). Platelets were then analyzed for their binding to AP1. Filled area, mother and father; clear area, affected children. The results from analysis of an additional three normal volunteers are shown by thedashed lines overlapping the normal sample in the shaded area. There is no detectable GPIbα on the platelets of the two children, whereas the samples from the two parents are similar to those of the normal controls.
Flow cytometric analysis of patients' and normal platelets. Analysis was performed on whole blood with monoclonal antibodies against GPIbα (AP1) and GPIIbIIIa (AP2). Platelets were then analyzed for their binding to the anti-GPIbα antibodies AP1 (solid bold line), 142.2 (solid line), and 142.11 (dashed line), the anti-GPIX antibody (hatched area), and an irrelevant mouse IgG (gray area). The anti-GPIbα antibodies bind to normal platelets, but not to those in this patient. There are similar amounts of GPIX on the surface of platelets from the patient and a normal volunteer.
Flow cytometric analysis of patients' and normal platelets. Analysis was performed on whole blood with monoclonal antibodies against GPIbα (AP1) and GPIIbIIIa (AP2). Platelets were then analyzed for their binding to the anti-GPIbα antibodies AP1 (solid bold line), 142.2 (solid line), and 142.11 (dashed line), the anti-GPIX antibody (hatched area), and an irrelevant mouse IgG (gray area). The anti-GPIbα antibodies bind to normal platelets, but not to those in this patient. There are similar amounts of GPIX on the surface of platelets from the patient and a normal volunteer.
Western blot analysis of GPIbα in platelet lysate. Platelet lysate from a normal volunteer, the parents, and the two affected children was analyzed by immunoblotting with the GPIbα-specific monoclonal antibody 142.6. GPIbα is readily detectable in the normal volunteer and in both parents, but not in the children.
Western blot analysis of GPIbα in platelet lysate. Platelet lysate from a normal volunteer, the parents, and the two affected children was analyzed by immunoblotting with the GPIbα-specific monoclonal antibody 142.6. GPIbα is readily detectable in the normal volunteer and in both parents, but not in the children.
Western blot analysis of GPIbβ in platelet lysate. Platelet lysate from a normal individual and the affected brother was analyzed by immunoblotting with an anti-GPIbβ polyclonal antibody. A total of 11 μg and 168 μg of protein was loaded on the gel from the normal individual and patient respectively. GPIbβ is readily detectable in the normal control but is significantly reduced in the patient.
Western blot analysis of GPIbβ in platelet lysate. Platelet lysate from a normal individual and the affected brother was analyzed by immunoblotting with an anti-GPIbβ polyclonal antibody. A total of 11 μg and 168 μg of protein was loaded on the gel from the normal individual and patient respectively. GPIbβ is readily detectable in the normal control but is significantly reduced in the patient.
Soluble GPIbα can be immunoprecipitated from the patient's plasma.
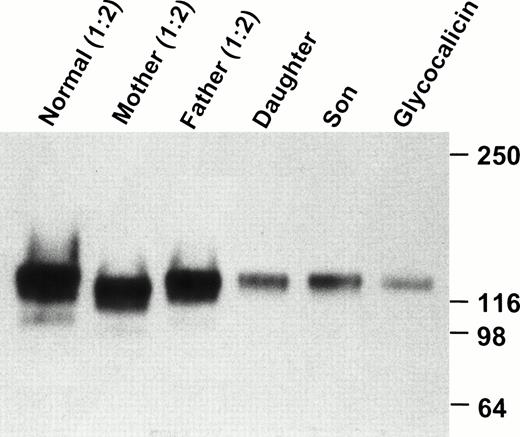
Immunoprecipitation of plasma with MBC 142.2 and immunoblotting with MBC 142.6 demonstrated the presence of a protein of approximately 130-kD in both siblings and their parents. This band had a mobility similar to that of purified glycocalicin. However, this was significantly reduced in both patients compared to their parents (Fig5). Thus, it seems unlikely that the absence of detectable GPIbα on the patient's platelets was caused by cleavage of glycocalicin from the platelet surface, as this would result in a greater amount of circulating glycocalicin.
Western blot analysis of plasma GPIbα. Platelet-poor plasma was immunoprecipitated with 142.2, analyzed by SDS-PAGE on an 8% to 16% exponential gradient in the presence of β-mercaptoethanol, and immunoblotted with MBC 142.6. Plasma samples from the parents and the normal volunteer were diluted 1:2. Each patient's plasma contains a soluble GPIbα, similar to glycocalicin, although there is significantly less in the two patients as compared with the parents and normal sample.
Western blot analysis of plasma GPIbα. Platelet-poor plasma was immunoprecipitated with 142.2, analyzed by SDS-PAGE on an 8% to 16% exponential gradient in the presence of β-mercaptoethanol, and immunoblotted with MBC 142.6. Plasma samples from the parents and the normal volunteer were diluted 1:2. Each patient's plasma contains a soluble GPIbα, similar to glycocalicin, although there is significantly less in the two patients as compared with the parents and normal sample.
Patients have compound heterozygous mutations within the GPIbα gene.
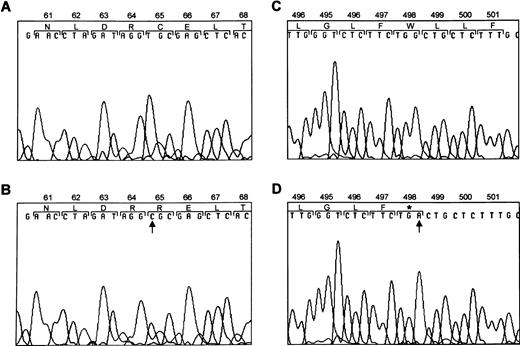
To determine the molecular basis for the absence of GPIbα, we amplified the entire coding region of GPIbα from both patients' genomic DNA. The PCR-amplified DNA of both patients were of the predicted size according to the published sequence.41Direct sequence analysis of PCR-amplified DNA showed a heterozygous T → C substitution at nucleotide 777 and a heterozygous G → A substitution at nucleotide 2078 (Fig6). The T → C substitution changes a free cysteine within the second leucine-rich repeat of GPIbα to an arginine. The G → A substitution results in a premature stop codon within the transmembrane region of GPIbα.
Mutations within GPIbα. DNA sequence analysis of GPIbα from a normal individual (A and C) and from the patients (B and D). Sequence analysis of PCR-amplified DNA showed a T → C substitution at nucleotide 777 (B) and a G → A substitution at nucleotide 2078 (D). Amino acids are numbered above.
Mutations within GPIbα. DNA sequence analysis of GPIbα from a normal individual (A and C) and from the patients (B and D). Sequence analysis of PCR-amplified DNA showed a T → C substitution at nucleotide 777 (B) and a G → A substitution at nucleotide 2078 (D). Amino acids are numbered above.
In addition to these mutations, a number of other differences were noted from the published sequence.6 41 Within the intron, there was an A → T polymorphism at nucleotide 497 and a T → C polymorphism at nucleotide 532 within the 5′ untranslated region of GPIbα. We followed the segregation of these mutations within the affected family by amplification and direct sequencing of the entire coding region of both parents. In addition, the entire coding region was also cloned and sequenced in both parents. An allele from the mother contained the T → C substitution at nucleotide 777; this allele also contained two of the variable number of tandem repeats in the macroglycopeptide region. The mother was homozygous for an A in the A497T polymorphism within the intron, and her WT allele contained only one of the tandem repeat polymorphisms. The allele from the father contained the G → A transversion at nucleotide 2078. The father was homozygous for the T polymorphism within the intron and the T → C polymorphism at nucleotide 532 within the 5′ untranslated region of GPIbα. Both fathers' alleles contained two of the variable number of tandem repeats within the macroglycopeptide region. Consequently, the affected children had inherited the T777 → C mutation from the mother and the G2078 → A mutation from the father.
The mutations are either nonfunctional or not expressed in a mammalian expression system.
Transfected alone, GPIbα is not expressed on the cell surface of CHO cells in appreciable quantities.16 Studies examining the role of GPIbβ and GPIX have shown that all three subunits are required for efficient expression of the ligand-binding subunit on the surface of transfected cells.16 Therefore, to investigate the effect of this mutation on surface expression, both the WT GPIbα and the constructs containing the mutations were transiently transfected individually into CHO cells that stably express both GPIbβ and IX.16 These cells were then incubated with vWF, botrocetin, and antibodies against GPIbα and vWF, and then analyzed by flow cytometry. Botrocetin was used in these investigations, as the patients' platelets failed to aggregate in the presence of ristocetin. As shown in Fig 7, a significant fraction (9.77%) of the cells transfected with the WT GPIbα construct exhibited an appreciable increase in surface fluorescence when reacted with the anti-GPIbα antibody 142.11. Furthermore, a significant number of the cells expressing GPIbα bound vWF (2.94%). Surprisingly, there was an increase in surface fluorescence of cells transfected with the C65 → R construct when reacted with the anti-GPIbα antibody 142.11, which was similar to the WT transfections, however none of these cells bound vWF. By contrast, surface expression of the construct containing the W498stop construct was barely detectable, and none of these cells bound vWF.
Analysis of vWF binding in Chinese hamster ovary (CHO) βIX cells expressing GPIbα, and the GPIbαC65 → R and GPIbαTrp498 → stop constructs. The binding of vWF in the presence of botrocetin was assessed in CHO βIX cells that were mock-transfected (A), in CHO βIX cells transiently transfected with the wild-type (WT) GPIbα (B), and in CHO βIX cells transfected with the constructs GPIbαCys65 → Arg (C) and GPIbαTrp498 → stop (D). The x-axis is fluorescence detected with an anti-GPIbα MoAb. The y-axis is fluorescence detected with a polyclonal anti-vWF antibody. A: Neither GPIbα expression or binding of vWF is detected in the mock-transfected cells. B: In cells transfected with the WT GPIbα, GPIbα is readily detectable on the cell surface (9.77% of cells) and binds vWF (2.94% of cells). C: Whereas the GPIbαCys65 → Arg construct results in a significant increase in fluorescence when detected with the anti-GPIbα antibody (6.66%) of cells, there is no binding of vWF. D: In the cells transiently transfected with the GPIbαTrp498→ stop construct, there is no significant increase in surface fluorescence when detected with the anti-GPIbα antibody, and there is no binding of vWF.
Analysis of vWF binding in Chinese hamster ovary (CHO) βIX cells expressing GPIbα, and the GPIbαC65 → R and GPIbαTrp498 → stop constructs. The binding of vWF in the presence of botrocetin was assessed in CHO βIX cells that were mock-transfected (A), in CHO βIX cells transiently transfected with the wild-type (WT) GPIbα (B), and in CHO βIX cells transfected with the constructs GPIbαCys65 → Arg (C) and GPIbαTrp498 → stop (D). The x-axis is fluorescence detected with an anti-GPIbα MoAb. The y-axis is fluorescence detected with a polyclonal anti-vWF antibody. A: Neither GPIbα expression or binding of vWF is detected in the mock-transfected cells. B: In cells transfected with the WT GPIbα, GPIbα is readily detectable on the cell surface (9.77% of cells) and binds vWF (2.94% of cells). C: Whereas the GPIbαCys65 → Arg construct results in a significant increase in fluorescence when detected with the anti-GPIbα antibody (6.66%) of cells, there is no binding of vWF. D: In the cells transiently transfected with the GPIbαTrp498→ stop construct, there is no significant increase in surface fluorescence when detected with the anti-GPIbα antibody, and there is no binding of vWF.
DISCUSSION
A critical event in hemostasis is the interaction of vWF with the platelet GPIb-V-IX receptor. Defects in the GPIb-V-IX complex result in the congenital acquired bleeding disorder Bernard-Soulier syndrome. In the present investigation, we have identified, to the best of our knowledge, the first patients with these compound heterozygous mutations within the coding region for the α-subunit of the GPIb-V-IX complex that cause Bernard-Soulier syndrome. Both Western blot and FACS analysis of platelets from both patients failed to demonstrate any GPIbα; however, FACS analysis clearly demonstrated GPIbα on the surface of both parents who are heterozygous carriers, similar to normal volunteers. The clinical diagnosis of Bernard-Soulier syndrome was suggested by giant platelets and an absence of ristocetin-induced platelet aggregation.34 The specific diagnosis was confirmed by detailed platelet membrane glycoprotein analysis and expression studies. In the present investigation, we used botrocetin as an agonist to induce binding of vWF to expressed GPIbα. Previous investigations, using either recombinant soluble GPIbα25or a chimera containing the vWF binding region of GPIbα,45 have demonstrated no difference in the binding of vWF to GPIbα induced by either ristocetin or botrocetin. Furthermore botrocetin, like ristocetin, fails to induce agglutination of platelets from a Bernard-Soulier syndrome patient.46Because botrocetin forms a soluble complex with vWF,47 a technical advantage over ristocetin48 when examining the binding of vWF to GPIbα in transfected cells, we used botrocetin as an agonist to study the role of vWF binding to the recombinant expressed GPIbα. Expression studies confirmed that the mutations identified in these affected patients resulted in expression of a nonfunctional protein and the decreased surface expression of GPIbα.
The consensus sequence for the leucine rich repeats for GPIbα and for the entire family of leucine-rich repeats is shown in Fig8. At the sixth residue within the consensus sequence shown in Fig 8, most proteins in the leucine-rich repeat family contain asparagine, but three have cysteine in this position.1 Within GPIbα, the cysteine at residue 65 is a free cysteine residue.49 The functional consequences of variations of consensus residues at this position are unknown. The results of our investigation demonstrate that this cysteine is critically important to the function of the receptor, because a mutation that alters the cysteine to an arginine results in the expression of a receptor that does not bind vWF “in response to the agonist botrocetin” in vitro. Whereas measurement of vWF binding in the presence of ristocetin in vitro may have provided useful confirmatory evidence of the platelet studies in vivo, other investigators have shown that platelets from Bernard-Soulier syndrome patients do not agglutinate in response to botrocetin when they do not respond to ristocetin.”
Consensus sequence for the leucine-rich motif. The free cysteine within the second LRM is double-underlined.
Consensus sequence for the leucine-rich motif. The free cysteine within the second LRM is double-underlined.
In contrast to the results demonstrated on the patients' platelet surfaces, the results of the expression studies demonstrate some surface expression, of the C65 → R mutation. This discrepancy has a number of potential explanations. In vivo disruption of the normal trafficking pathways of the complex by causing a conformational change within the protein, as a result of changing a free cysteine could explain the observed Bernard-Soulier syndrome phenotype. The results obtained in vitro, using a eukaryotic expression vector in which transcription is greatly exaggerated may account for the results seen in vitro. Platelets that lack a nucleus do not have the same ability to synthesize proteins; consequently, any protein that may be made in these two patients is below the physiologically relevant range and results in the phenotype described. However, even when expressed in an in vitro system the mutant protein clearly failed to bind vWF.
The precise mechanism whereby a mutation within the leucine-rich repeat causes Bernard-Soulier syndrome is unclear. Investigations using either overlapping synthetic peptides11 or site-directed mutagenesis10 have demonstrated that residues Ser251-Asp287 are critically important for the binding of vWF to GPIbα induced by the agonists ristocetin and botrocetin. Residues Ser251-Asp287 are located near the N-terminus of the receptor between the leucine-rich repeats and the macroglycopeptide region. Curiously, although there have been several reports of mutations within the leucine-rich region that resulted in Bernard-Soulier syndrome, to date no mutations described within the region bounded by residues 251–287 have resulted in Bernard-Soulier syndrome. Using overlapping synthetic peptides to delineate the vWF binding region of GPIbα, Vincente et al11 showed that peptides spanning the second and third leucine-rich repeat inhibited ristocetin-dependent binding of platelets to vWF to a similar degree as did synthetic peptides between residues 251–287. By contrast, peptides within the second and third leucine-rich repeat were marginally less effective in inhibiting botrocetin induced binding of vWF compared to peptides within residues 251–287. The corollary of ristocetin or botrocetin-induced binding of vWF to GPIb in vivo is unclear. However, the results of these in vitro experiments in conjunction with the mutations described in vivo would suggest that the structure of the leucine-rich repeats is essential for residues 251–287 to be able to function in the binding of vWF to GPIb. The results of the present investigation suggest that the in vivo mutations that affect C65 within the leucine-rich repeat not only inhibit adequate platelet surface expression but also disrupt function of the receptor when expressed in a mammalian cell line.
The postulated transmembrane region of GPIbα extends from Leu486 to Gly514. The transmembrane region is followed by two charged amino acids that may help anchor the protein within the platelet membrane; after this region is a cytoplasmic domain of approximately 100 amino acids. The second mutation described in this report converts the Trp498 to a stop codon approximately halfway within the transmembrane region. Although the coordinate expression of each of the three subunits is required for efficient surface expression of the complex, in the presence of a truncated transmembrane region it appears that GPIbα is synthesized but fails to anchor within the platelet membrane since GPIbα is found circulating in plasma and GPIX is found on the platelet surface. These results are similar to those described in a recent report by Holberg et al,50 who identified a patient with the W498stop. However, our expression studies demonstrate for the first time that this mutation results in the lack of surface expression of GPIbα. Although we have not demonstrated that the W498stop mutant is responsible for the soluble GPIbα found circulating in plasma, previous investigations both from our laboratory29 and from those of other investigators21,28,51 have demonstrated that in patients with Bernard-Soulier syndrome resulting from truncated versions of GPIbα that a soluble form of GPIbα is found circulating in plasma. These “experiments of nature” have been confirmed in expression studies which have demonstrated that truncated GPIbα is readily detectable in culture media.29 52 Together, these results strongly suggest that the W498stop mutant explains the soluble GPIbα found circulating in our patients plasma, albeit significantly reduced, as compared with normal.
These results are analogous to a case of Bernard-Soulier syndrome previously described by our group,29 in which a mutation within the transmembrane region caused failure of the protein to anchor within the platelet; however, a soluble form was found in the patient's plasma.
In the present investigation, GPIbα was not detectable on the platelet surface of the affected patients; however, platelet surface expression of GPIX was similar to that of a normal control. Transfection experiments have shown that at least three of the polypeptides (GPIbα, GPIbβ, and GPIX) are necessary for efficient surface expression of the GPIbα subunit, which is then capable of binding vWF.16 Therefore, a molecular genetic effect in either of these three subunits could cause Bernard-Soulier syndrome by a reduction in the vWF binding subunit. The interaction of GPIbα with GPIX is less clear. However, López et al42demonstrated that in cell lines expressing each combination of only two of the three subunits, that two polypeptides would only associate in cells containing GPIbβ. In the present investigation, we demonstrated the presence of GPIbβ in platelet lysate, albeit reduced compared with the normal condition. The results of the present investigation confirm these experiments of López et al., by demonstrating normal GPIX expression with detectable GPIbβ. de la Salle et al23 also reported similar findings of reduced levels of GPIbβ with normal GPIX in a patient with a mutation in the GPIbα subunit. It appears that GPIX can be expressed normally on the platelet surface with reduced amounts of GPIbβ and absent GPIbα.
In summary, we have identified and characterized a novel BSS resulting from compound heterozygous mutations within the GPIbα gene. One mutation changes a free cysteine within the leucine-rich motif and changes the functional surface expression of GPIbα, the other mutation causes a premature stop codon within the transmembrane region, so that the mutant peptide does not anchor within the plasma membrane and is found circulating in plasma. Each of these mutations, when inherited together with a normal allele in the family reported, does not cause any symptoms. However, compound heterozygotes for these mutations result in the observed phenotype of Bernard-Soulier syndrome.
ACKNOWLEDGMENT
The authors thank Dr Philip A. Kroner for providing glycocalicin, Dr Peter J. Newman for his critical review of the manuscript, and Dr Hannes Sigmarsson for assistance with the clinical samples.
Supported by US Public Health Service Grants No. HL56027 (D.K.), HL44612, and HL33721 (R.R.M.) and by American Heart Association Grant No. 95007200 (D.K.).
Address reprint requests to Dermot Kenny, MD, Center for Cardiovascular Science, The Royal College of Surgeons in Ireland, 123 St Stephen's Green, Dublin 2, Ireland.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal