Abstract
Primary graft rejection after marrow transplantation occurs more frequently in patients receiving HLA-haploidentical compared with HLA-identical sibling transplants. Both human and experimental animal data suggest that the cells responsible for this phenomenon are either host natural killer (NK) cells, T cells, or both. To investigate the mechanisms of graft rejection, we have developed a canine model of marrow transplantation, which uses DLA-nonidentical unrelated donors in the absence of postgrafting immunosuppression. In this model most animals rejected their marrow grafts after a preparative regimen of 9.2 Gy total body irradiation (TBI). However, engraftment of DLA-nonidentical marrow can be facilitated when the recipients are pretreated with monoclonal antibody (MoAb) S5, which recognizes CD44. In this report, we extended these observations by first cloning the canine CD44 and, next, mapping the epitope recognized by S5, which was located in a region conserved among human and canine CD44 and was distinct from the hyaluronan binding domain. However, in vitro binding of S5 caused a conformational change in CD44, which allowed increased hyaluronan binding. Then, we reexamined the in vivo model of marrow transplantation and compared results with MoAb S5 to those with two other anti-CD44 MoAbs, IM7 and S3. Only MoAb S5 significantly increased the engraftment rate of DLA-nonidentical unrelated marrow, whereas the two other anti-CD44 MoAbs were ineffective. The enhanced in vivo effect was not related to differences in the MoAbs' avidities, since both S5 and IM7 had equivalent binding to CD44, but most likely related to the specific epitope that S5 recognizes. Thus, this study shows that the effect of the anti-CD44 MoAb S5 in facilitating engraftment is epitope specific and if one is to use an anti-CD44 to facilitate engraftment of marrow in humans, one cannot assume that any anti-CD44 would work.
THE INCIDENCE OF rejection of HLA-haploidentical related marrow grafts increased in direct proportion to the number of mismatched HLA antigens on the nonshared haplotype.1 At our center, the overall incidence of graft rejection was 12%, ranging from 7% with marrow grafts from phenotypically HLA-identical donors to 20% with donors who were mismatched for two or three HLA loci. Graft rejection has been associated with a high likelihood of subsequent death.
To better understand the mechanisms involved in graft rejection and to test new therapies, we developed a preclinical canine model of graft rejection involving marrow transplants from DLA-nonidentical unrelated donors after conditioning by 9.2 Gy total body irradiation (TBI). In this setting, 80% to 90% of the grafts fail.2-4 We previously showed that the risk of rejection could be significantly decreased when recipients were treated with six daily intravenous injections of an anti-CD44 monoclonal antibody (MoAb), S5, at 0.2 mg/kg/d before 9.2 Gy TBI and marrow infusion.5 Sixty-seven percent of dogs administered MoAb S5 showed successful engraftment as compared with only 8% of control dogs not administered MoAb therapy or given an irrelevant MoAb. The antigen recognized by S5, CD44,6 is a cellular adhesion molecule expressed on both hematopoietic and nonhematopoietic tissues. CD44 is an integral membrane glycoprotein and serves as the principle receptor for hyaluronan,7 a glycosaminoglycan that is abundantly distributed in extracellular spaces. CD44 acts as a modulator of various immune functions including those involving CD2 and CD3,8-10 such as T-cell activation trigger via cross-linking of CD2. MoAbs to CD44 can also induce release of interleukin (IL)-1, tumor necrosis factor (TNF)α,11 and macrophage colony-stimulating factor (M-CSF)12 from human monocytes. In addition, we have shown a CD44-dependent increase of canine natural killer (NK)-cell activity, which is an indirect effect mediated both by an increase in effector/target cell conjugate formation and the elaboration of TNFα that, additionally, makes the cells more sensitive to radiation.13-15
In this report, we extended the previous observations with the anti-CD44 MoAb S5 by first cloning the canine CD44 and next, mapping the epitope recognized by S5, which was located in a region conserved among humans and dogs CD44. Then, we reexamined the in vivo model of marrow transplantation and compared results with MoAb S5 to those with two other anti-CD44 MoAbs, IM7 and S3, one directed against an immunologically overlapping epitope and the other recognizing a discreet epitope on CD44. The endpoints of the in vivo study, graft rejection, and graft-versus-host disease (GVHD) are mutually exclusive events in this model. Dogs that reject their graft do not show clinical or histologic evidence of GVHD. Conversely, because no postgrafting immunosuppression for prevention of GVHD was used in the present study in the interest of a simple and rapid study readout, all dogs with engraftment uniformly developed severe GVHD.16-18 Only MoAb S5 significantly increased the engraftment rate of marrow from DLA-nonidentical unrelated donor dogs, whereas the two other anti-CD44 MoAbs were ineffective, suggesting that the epitope recognized by S5 is important for the graft enhancing effect.
MATERIALS AND METHODS
MoAbs.
The murine anti-CD44 MoAbs S5 (subclass IgG1) and S3 (IgG2b) were generated against canine marrow cells.5,6 The rat anti-CD44 MoAb IM7 (IgG2b), generated against mouse cells,19 was cross-reactive with canine cells; MoAb MEM-85 (murine IgG1) recognized human CD44. Negative control murine MoAbs 6.4 (IgG2b) and 31A (IgG1)20 (which recognized murine Thy-1) did not cross-react with canine cells. All MoAbs used in vivo were protein A or protein G affinity purified and were endotoxin free. Protein concentrations were determined using the Bradford reaction (BioRad Labs, Hercules, CA).
Cloning of canine CD44.
Peripheral blood and splenic mononuclear cells were isolated from a Ficoll Hypaque gradient. The cells were cultured for 48 hours in RPMI-1640 supplemented with 5% fetal calf serum (FCS) in the presence of 1% phytohemagglutinin (PHA) and 5% pokeweed mitogen. RNA was extracted on a cesium chloride gradient.21 A cDNA library in the Lamda Zap cloning vector (Stratagene, La Jolla, CA) was constructed and packaged.22 A baboon BamH1 fragment containing 600 bp of the 5′ portion of the baboon CD44 cDNA was32P-labeled by extension random hexamers by DNA polymerase 1 in the presence of 32P deoxy cytidine triphosphate (dCTP) and was hybridized in 35% formamide/5XSSPE/0.1% Ficoll/0.1% polyvinyl pyrrolidine/0.1% bovine serum albumin/0.5% sodium dodecyl sulfate (SDS) at 42°C for 18 hours. Plasmids (pBluescript SK[-]) containing the canine CD44 inserts were subcloned from the Lambda Zap phage by an in vivo excision process in the presence of helper virus.22 Clones were sequenced in pBluescript SK(–) using the dideoxynucleotide method23 as described in the Sequenase (US Biochemicals, Cleveland, OH) protocol for single-stranded and double-stranded DNA. cDNA clones were initially sequenced from the flanking T3 and T7 primers. Specific primers for the insert were constructed to enable sequencing the complete clone. Sequence analysis was performed with GenePro 4.2 software (Riverside Scientific Enterprises, Bainbridge Island, WA).
Preparation of full-length and truncated extracellular domain of CD44.
As MoAb S5 recognized human CD44, available constructs of full-length and truncated mutants of the extracellular domain of human CD44 were used to map the MoAb recognition sites. Both wild-type human CD44H receptor globulins (Rg)7 and mutant CD44 Rg24that were ligated into a HindIII/BamHI-cut CDM7 vector containing genomic sequence encoding the hinge, CH2 and CH3 domains of human IgG1,25 were transfected into Cos cells as previously described.7,24 A similar construct of the extracellular domain of canine CD44 was produced. The fusion proteins were purified from the culture supernatants using Protein-A-Sepharose as previously described,7 24 and protein concentration determined.
Binding of MoAb to CD44 Rgs.
Binding of the anti-CD44 MoAb to the purified CD44 Rgs was assessed using an enzyme-linked immunosorbent assay (ELISA). Wells of polystyrene ELISA plates were coated with 0.2 μg/mL of the CD44 Rg. After blocking with nonfat milk in phosphate-buffered saline (PBS), the plates were incubated with 50 μL of 10 μg/mL of the MoAb. After washing, the plates were coated with goat antimouse (or antirat) IgG horseradish peroxidase (HRP) (1/2,000 dilution), followed by ABTS color solution (Sigma, St Louis, MO). Plates were read on a Vmax microtiter plate reader (Molecular Devices, Menlo Park, CA) at 405 nm.
Marrow transplantation.
Beagles and beagle/hound crossbreeds were bred and raised at the Fred Hutchinson Cancer Research Center (FHCRC) or purchased from commercial kennels. They were vaccinated against distemper, leptospirosis, hepatitis, and parvovirus and monitored to be disease free before start of the experimental protocol. The median age of the recipients was 9 months, and the median weight was 10.5 kg at the time of transplantation. The experimental protocols and husbandry for all dogs was approved by the FHCRC Institutional Animal Care and Use Committee per guidelines stipulated by the National Academy of Sciences/National Research Council.
Unrelated, DLA-nonidentical recipients and donors were selected on the basis of mutual reactivity of their cells in mixed leukocyte culture, serotypic differences for dog lymphocyte antigens (DLA-A and -B), and phenotyping for DLA-D alleles using homozygous typing cells as previously described.26-29 When possible, donors and recipients were sex mismatched to differentiate between donor versus host reconstitution of hematopoiesis. Marrow recipients were conditioned for transplantation by 9.2 Gy of TBI at 7 cGy/min delivered from two opposing 60Co sources.30,31Immediately after irradiation, they received an intravenous infusion of ≤4 × 108 nucleated marrow cells per kg body weight. No postgrafting immunosuppression was administered and, thus, no attempt was made to prevent rapidly fatal acute GVHD. Postgrafting immunosuppression was omitted because the drugs used for that purpose have been shown in other studies to be also capable of suppressing host-versus-graft reactions and, thereby, to enhance engraftment.16-18 The day of TBI and marrow infusion was designated as day 0. Supportive care was given after transplant as described.32 33 Complete peripheral blood counts were obtained before transplant and daily thereafter. Autopsies were performed on all dogs at the end of the study to evaluate marrow cellularity and the presence or absence of acute GVHD. Marrow engraftment was defined as rising and sustained white blood count (>500 per mm/m3) after the postirradiation nadir, marrow cellularity at autopsy (≥5% of normal) as estimated by light microscopy of marrow sections, cytogenetics in some cases where the donor/recipient were sex mismatched, and by clinical and histologic evidence of GVHD in allogeneic recipients.
In vivo infusion of MoAbs.
The recipient dogs were treated with daily intravenous injections of MoAb (0.2 mg/kg/day) for 6 days before TBI. Recipients of the irrelevant control MoAb 6.4 received the MoAb on days -5 to 0. For the recipients of MoAb S5, the initial five animals were administered MoAb on days -5 to 0 and the subsequent 16 animals from day -7 to -2. Three of the S5-treated animals were given 131I-labeled S5 (1 mCi/kg) for the first dose on day -7 followed by unlabeled MoAb S5 for the subsequent five doses. The estimated radiation dose delivered via the 131I administered was 100 cGy,34 which when combined with 920 cGy TBI, raised the radiation dose to the marrow to 1,020 cGy. Given the previous observation that raising the TBI dose to 1,000 cGy failed to decrease graft rejection in this model,35 we combined the results in these three dogs with those in the 18 dogs not administered radiolabeled MoAb for the purposes of analysis.
The animals that were administered MoAb IM7 and S3 received the MoAb on the same dose and schedule as the majority of the MoAb S5–treated dogs (0.2 mg/kg/d, day -7 to -2).
Flow cytometry.
To detect the presence of antibody on the surface of the peripheral blood mononuclear cells (PBMC) from animals infused with MoAb, cells were isolated from blood over a Ficoll-Hypaque density gradient (d = 1.074), washed, and incubated with an fluorescein isothiocyanate (FITC)-conjugated goat antimouse antibody (Biochemica, Indianapolis, IN). To evaluate whether the CD44 expressed on the cell surface of PBMC was saturated with MoAb from the in vivo infusion, a one-step procedure was used where the cells were incubated with a direct FITC conjugate of the same anti-CD44 MoAb that was infused into the animal to detect unbound CD44.
Serum level of antibody.
A four-step ELISA procedure was used to detect the level of antibody in the serum of MoAb-infused dogs. Briefly, the method consisted of the following: (1) polyvinyl 96-well plates (VWR, Seattle, WA) were coated with 50 μL of 10 μg/mL goat antimouse (or rat) IgG (Biosource International, Camarillo, CA); (2) after blocking with 5% nonfat milk in PBS, plates were incubated with 50 μL of sera from the infused dogs; (3) goat antimouse (or rat) IgG HRP (Tago Inc) was added (1/2,000 dilution); and (4) 100 μL of ABTS color solution was added to each well. Plates were read with a Vmax microtiter plate reader at 405 nm. As controls, sera from the dogs before infusion were used, and standard curves with known amounts of MoAb were established for each MoAb.
NK cell assay.
NK cell assays were performed as previously described.13 In brief, PBMC from either S5-infused dogs or control dogs were used as the effectors against canine thyroid adenocarcinoma cells (CTAC), which is an NK-sensitive target. Triplicate (100 μL) aliquots of the effector single cell suspensions were pipetted into round bottom 96-well plates with 100 μL of medium (for the spontaneous release) or 100 μL of 2% Triton-X (for maximum release). The target cells were suspended in 500 μL of medium with 50 μL of Chromium-51 (5 mCi/mL), and incubated for 1 hour at 37°C, 5% CO2, washed three times with 5 mL of cold medium, resuspended in desired concentration (5 × 104 to 105 cells/mL), and added (100 μL) to the plated effector cells. The plates were incubated at 37°C, 5% CO2 for 16 hours. After incubation, the plates were spun, 100 μL supernatant obtained, and the released radioactivity measured by a gamma scintillation counter. Percent specific lysis was calculated by the formula:
Iodination and avidity determination.
Iodination was performed in 20-mL glass scintillation vials coated with 100 μg Iodogen (Pierce Chemical, Rockford, IL).36Antibody was diluted in PBS to a 1-mL volume in the Iodogen-coated vial, and radioiodine (125I-labeled Na; ICN Biomedicals, Irvine, CA) was added. The vial was incubated at room temperature with intermittent agitation for 10 minutes. Unbound iodine was removed by passage over a Sephadex PD-10, G-25 column (Pharmacia Fine Chemicals, Piscataway, NJ).
Avidity was determined from Scatchard plots of the binding of labeled antibody to viable ML3 canine myelomonocytic leukemia cells.36 Known quantities of antibody were diluted in tissue culture media (RPMI 1640 and 1% bovine serum albumin) and incubated with 1 × 106 ML3 or Raji cells (human leukemia cell line used as a negative control) in microfuge tubes in a total volume of 1 mL for 1 hour at room temperature on a rotating stand. The cells were washed three times and bound radioactivity was counted.
RESULTS
Cloning canine CD44.
Approximately 1 × 106 recombinant clones from the canine PBMC and spleen cDNA library were screened by hybridization with a 32P-labeled baboon CD44 cDNA fragment. Three positive clones were identified. The complete DNA sequences of these three clones were determined. In one clone, only the last 2 bp before the termination codon at the 3′ end were missing, whereas another clone started approximately 200 bp from the 5′ end. A full-length clone was constructed by excising the truncated 3′ end and inserting the correct fragment from the second clone to make a full-length clone, including both the 5′ and 3′ untranslated regions. The amino acid sequences translated from nucleotide sequences for dog, mouse, and human CD44 are shown in Fig 1. The transmembrane regions as indicated by underlining, has the highest level of conservation between the species. The overall homology between canine and human CD44 was 85% at the sequence level and 84% at the amino acid level and the homology between canine and murine CD44 was similar. The membrane proximal region extracellularly has the greatest divergence in the three species. This confirms and completes the partial sequence of canine CD44 that was previously published.37
Comparison of the protein sequences of CD44. The protein sequence determined from nucleotides of dog is shown on the top line and amino acid differences found in human and mouse (C3H) are presented in the next two lines. Dashes (—) are deletions and the putative transmembrane segment is underlined. Also indicated are the locations of the truncation variants used for peptide mapping with the 5′ end of all the peptides initiating at * and terminating 3′ as indicated (F1-B, F1-C, F2, F3).
Comparison of the protein sequences of CD44. The protein sequence determined from nucleotides of dog is shown on the top line and amino acid differences found in human and mouse (C3H) are presented in the next two lines. Dashes (—) are deletions and the putative transmembrane segment is underlined. Also indicated are the locations of the truncation variants used for peptide mapping with the 5′ end of all the peptides initiating at * and terminating 3′ as indicated (F1-B, F1-C, F2, F3).
Epitope mapping.
Available constructs of full-length and truncated mutants of the extracellular domain of human CD44 were used to map the MoAb recognition sites. Wild-type human CD44 receptor globulins (Rg)7 along with truncated human CD44 Rg (CD44H-Rg) fusion proteins were used in ELISA to map for CD44 MoAbs S5, S3, IM7, MEM-85, and a negative control MoAb, 31A. MEM-85 was used as a positive control for the truncation proteins, as it recognized all the variants, but did not cross-react with canine CD44. CD44D-Rg contained the canine extracellular domain and was recognized by the three anti-CD44 MoAbs used in the in vivo transplant studies (S5, IM7, S3) (Table 1). CD44H-Rg contained the entire human extracellular domain of the hematopoietic form of CD44. It was also recognized by all of the CD44 MoAbs tested. Constructs F1-B Rg and F1-C Rg, which encoded an NH2 terminal 131 and 145 amino acids, respectively, were bound only by MoAb MEM-85. F2 Rg and F3 Rg, composed of the first 186 and 210 amino acids, were bound equivalently by S5, S3, and IM7, indicating that they bound between amino acids 145 and 186 (Fig 1). This region included the NH2-distal domain consisting of basic amino acids that were required for CD44/HA interactions,24 although most of the HA binding domain was located more proximally.
Binding of Anti-CD44 MoAbs to Plastic-Bound CD44 Rgs
| CD44 Construct* . | MoAb-151 . | ||||
|---|---|---|---|---|---|
| 31A . | S5 . | IM7 . | S3 . | MEM-85 . | |
| CD44D-Rg | − | + | + | + | − |
| CD44H-Rg | − | + | + | + | + |
| F1-B Rg | − | − | − | − | + |
| F1-C Rg | − | − | − | − | + |
| F2 Rg | − | + | + | + | + |
| F3 Rg | − | + | + | + | + |
| CD44 Construct* . | MoAb-151 . | ||||
|---|---|---|---|---|---|
| 31A . | S5 . | IM7 . | S3 . | MEM-85 . | |
| CD44D-Rg | − | + | + | + | − |
| CD44H-Rg | − | + | + | + | + |
| F1-B Rg | − | − | − | − | + |
| F1-C Rg | − | − | − | − | + |
| F2 Rg | − | + | + | + | + |
| F3 Rg | − | + | + | + | + |
*CD44D-Rg encoded the full-length extracellular domain of canine CD44. CD44H-Rg encoded the full-length extracellular domain of human CD44. F1-B Rg encoded the initial 131 amino acids of CD44, which includes the hyaluronan binding domain. F1-C Rg, F2 Rg, and F3 Rg encoded successively larger proteins (amino terminal 145, 186, and 210 amino acids), with F1 and F2 encoding the region of CD44 that is highly conserved among species. The F3 Rg protein encoded a 24-amino acid region of nonconserved residues.
31A was a negative isotype control MoAb that recognized murine Thy-1 and did not cross-react with human or canine cells. IM7, S3, and MEM-85 were other anti-CD44 MoAbs. MEM-85 was a positive control that recognized both the mature human CD44 and all truncation variants, but did not cross-react with canine CD44.
HA binding.
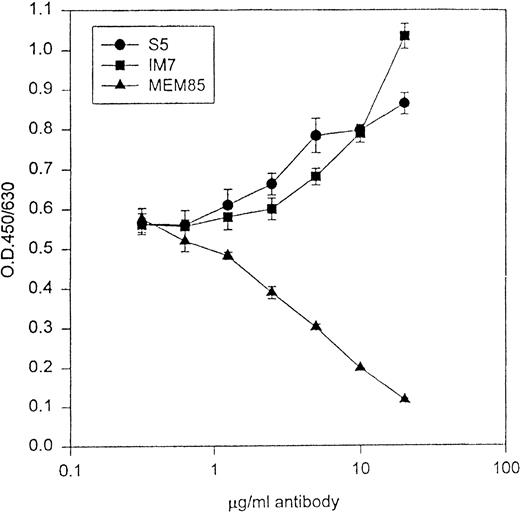
Because IM7 and S5 mapped to one of the HA binding domains of CD44, we explored CD44/HA interactions in the presence of the MoAbs to evaluate for possible functional differences. Zheng et al38 have shown that IM7 could block CD44 binding to HA in some, but not all cell lines. Using the CD44H-Rg fusion protein in an in vitro HA binding assay, both S5 and IM7 augmented CD44/HA interactions, whereas MEM-85 blocked CD44 binding to HA (Fig 2).
The abscissa represents increasing concentrations of MoAb, and the ordinate represents binding of CD44H-Ig to HA in the presence of antibodies. MoAb MEM-85 blocked the binding of CD44H to its ligand HA, whereas both S5 and IM7 augmented CD44/HA interactions.
The abscissa represents increasing concentrations of MoAb, and the ordinate represents binding of CD44H-Ig to HA in the presence of antibodies. MoAb MEM-85 blocked the binding of CD44H to its ligand HA, whereas both S5 and IM7 augmented CD44/HA interactions.
Marrow transplant.
To evaluate whether the previously observed graft enhancing effect of MoAb S5 was representative of other anti-CD44 MoAbs, MoAbs IM7 and S3 were tested concurrently with MoAb S5 in the DLA-nonidentical unrelated marrow transplant model. Results are shown in Tables 2 and 3. Of 57 historical and concurrent control dogs administered 9.2 Gy TBI and not treated with any MoAb, 47 rejected marrow grafts from DLA-nonidentical unrelated donors. Only 10 dogs (17%) engrafted and, because no postgrafting immunosuppression was administered, all 10 died with complications of hyperacute GVHD. Seven dogs were treated with intravenous injection of an irrelevant MoAb 6.4 for 6 days before TBI.5 Of the seven, six failed to show sustained hematopoietic engraftment and died of infectious complications. One dog engrafted and died of GVHD. This result was not statistically different from the results in control animals not given MoAb (P = 1.0). Fourteen of 21 dogs treated with S5 engrafted, as determined by increases in peripheral blood counts and by marrow cellularity at autopsy. All of the animals that engrafted developed clinical GVHD, which was confirmed histologically. Of the five animals that were treated with MoAb IM7 (Table 2), only one showed sustained hematopoietic engraftment, whereas the remaining four failed to engraft. Twelve dogs were treated with MoAb S3 (Table 2), three engrafted and nine had graft failure. In cases where cytogenetic studies were performed, clinical assessment of engraftment or rejection was confirmed by karyotype analysis of marrow or peripheral blood cells (Table 3). Only MoAb S5 significantly enhanced engraftment of DLA-nonidentical unrelated marrow grafts as compared with controls (P < .001) (Table 4). The two other anti-CD44 MoAbs (IM7 and S3) had no significant effect on engraftment, and results were not significantly different from controls (P = 1.0 and .69, respectively).
Data in Dogs Administered 9.2 Gy TBI and Infusion of DLA-Nonidentical Unrelated Bone Marrow Grafts After Treatment of Marrow Recipients With Intravenous Injection of MoAbs S5, IM7, or S3
| MoAb . | Recipient No. . | Marrow Cell No. Infused (×108/kg) . | Increase in WBC ≥500/mm3 After Transplant . | Marrow Cellularity at Autopsy (% of normal) . | GVHD . | Sustained Graft . | Survival (d) . | Cause of Death . | |
|---|---|---|---|---|---|---|---|---|---|
| Clinical . | Histological . | ||||||||
| S5 | C202*,† | 2.4 | No | 0 | No | No | No | 11 | Graft failure, sepsis |
| C209*,† | 2.6 | No | 0 | No | No | No | 10 | Graft failure, sepsis | |
| B821*,† | 4.2 | Yes | 100 | Skin | Skin | Yes | 30 | GVHD, sepsis | |
| C171*,† | 3.2 | Yes | 20 | Skin, gut | Skin, gut | Yes | 16 | GVHD, pneumonia | |
| B864*,† | 6.5 | Yes | 70 | Skin, gut | Skin, gut, liver | Yes | 14 | GVHD, sepsis | |
| C231*,‡ | 3.8 | No | 0 | No | No | No | 14 | Graft failure, pneumonia | |
| C253*,‡ | 4.1 | Yes | 0 | No | No | No | 13 | Graft failure, pneumonia | |
| C249*,‡ | 4.3 | No | 0 | No | Skin, gut | No | 13 | Graft failure, sepsis | |
| C213*,‡ | 2.6 | Yes | 25 | Skin, gut | Skin, liver | Yes | 9 | GVHD, sepsis | |
| C197*,‡ | 4.2 | Yes | 5 | Skin, gut, liver | Skin, liver | Yes | 19 | GVHD, pneumonia | |
| C226*,‡ | 4.1 | Yes | 80 | Skin, gut | Skin, gut, liver | Yes | 11 | GVHD, pneumonia | |
| C218*,‡ | 1.6 | Yes | 60 | Skin, gut, liver | Skin | Yes | 18 | GVHD, pneumonia | |
| C254*,‡ | 2.8 | Yes | 60 | No | Gut | Yes | 10 | GVHD, pneumonia | |
| C258*,‡ | 4.8 | Yes | 100 | Skin | Skin, gut | Yes | 11 | GVHD, pneumonia | |
| C247*,‡ | 2.3 | Yes | 60 | Skin, gut | Skin, gut, liver | Yes | 8 | GVHD, pneumonia | |
| D291‡ | 4.0 | Yes | 60 | No | Gut | Yes | 10 | Intussusception | |
| D444‡ | 3.0 | No | 0 | No | No | No | 13 | Graft failure, pneumonia | |
| D450‡ | 1.9 | No | 0 | No | Skin, gut | No | 14 | Graft failure, pneumonia | |
| C5981-153 | 4.4 | Yes | 25 | Skin, gut | Skin, gut | Yes | 13 | GVHD, dehydration | |
| C6461-153 | 3.2 | Yes | 100 | Skin, gut, liver | No | Yes | 21 | GVHD | |
| C7121-153 | 4.5 | Yes | 50 | Skin, liver | Skin, gut | Yes | 20 | GVHD | |
| IM71-155 | D109 | 4.3 | Yes | 70 | Skin, gut | Skin, gut | Yes | 14 | GVHD |
| D115 | 4.0 | No | <2 | No | No | No | 10 | Graft failure, pneumonia | |
| D153 | 4.1 | Yes | <2 | No | Skin | No | 20 | Graft failure | |
| D161 | 4.0 | No | 0 | No | Skin, gut | No | 13 | Graft failure, pneumonia | |
| D173 | 3.2 | No | 1 | No | No | No | 13 | Graft failure, pneumonia | |
| S31-155 | C388 | 3.7 | No | 0 | No | Gut | No | 15 | Graft failure, pneumonia |
| C433 | 1.3 | No | Early focal | No | No | No | 14 | Graft failure, pneumonia | |
| C436 | 4.1 | Yes | 25 | Skin, gut | Skin, gut, liver | Yes | 21 | Pneumonia | |
| C439 | 4.1 | No | 0 | No | No | No | 17 | Graft failure, pneumonia | |
| C441 | 4.1 | Yes | 0 | No | No | No | 12 | Graft failure | |
| C445 | 4.0 | No | 0 | No | No | No | 12 | Graft failure, pneumonia | |
| C454 | 4.6 | Yes | 25 | Skin, gut | Gut | Yes | 17 | GVHD, pneumonia | |
| C952 | 3.2 | No | 0 | No | No | No | 11 | Graft failure, pneumonia | |
| C962 | 1.8 | Yes | 25 | Skin | Skin, liver | Yes | 13 | Pneumonia | |
| C968 | 4.0 | Yes | 0 | No | No | No | 12 | Graft failure | |
| C983 | 4.0 | Yes | 0 | No | No | No | 28 | Graft failure, sepsis | |
| MoAb . | Recipient No. . | Marrow Cell No. Infused (×108/kg) . | Increase in WBC ≥500/mm3 After Transplant . | Marrow Cellularity at Autopsy (% of normal) . | GVHD . | Sustained Graft . | Survival (d) . | Cause of Death . | |
|---|---|---|---|---|---|---|---|---|---|
| Clinical . | Histological . | ||||||||
| S5 | C202*,† | 2.4 | No | 0 | No | No | No | 11 | Graft failure, sepsis |
| C209*,† | 2.6 | No | 0 | No | No | No | 10 | Graft failure, sepsis | |
| B821*,† | 4.2 | Yes | 100 | Skin | Skin | Yes | 30 | GVHD, sepsis | |
| C171*,† | 3.2 | Yes | 20 | Skin, gut | Skin, gut | Yes | 16 | GVHD, pneumonia | |
| B864*,† | 6.5 | Yes | 70 | Skin, gut | Skin, gut, liver | Yes | 14 | GVHD, sepsis | |
| C231*,‡ | 3.8 | No | 0 | No | No | No | 14 | Graft failure, pneumonia | |
| C253*,‡ | 4.1 | Yes | 0 | No | No | No | 13 | Graft failure, pneumonia | |
| C249*,‡ | 4.3 | No | 0 | No | Skin, gut | No | 13 | Graft failure, sepsis | |
| C213*,‡ | 2.6 | Yes | 25 | Skin, gut | Skin, liver | Yes | 9 | GVHD, sepsis | |
| C197*,‡ | 4.2 | Yes | 5 | Skin, gut, liver | Skin, liver | Yes | 19 | GVHD, pneumonia | |
| C226*,‡ | 4.1 | Yes | 80 | Skin, gut | Skin, gut, liver | Yes | 11 | GVHD, pneumonia | |
| C218*,‡ | 1.6 | Yes | 60 | Skin, gut, liver | Skin | Yes | 18 | GVHD, pneumonia | |
| C254*,‡ | 2.8 | Yes | 60 | No | Gut | Yes | 10 | GVHD, pneumonia | |
| C258*,‡ | 4.8 | Yes | 100 | Skin | Skin, gut | Yes | 11 | GVHD, pneumonia | |
| C247*,‡ | 2.3 | Yes | 60 | Skin, gut | Skin, gut, liver | Yes | 8 | GVHD, pneumonia | |
| D291‡ | 4.0 | Yes | 60 | No | Gut | Yes | 10 | Intussusception | |
| D444‡ | 3.0 | No | 0 | No | No | No | 13 | Graft failure, pneumonia | |
| D450‡ | 1.9 | No | 0 | No | Skin, gut | No | 14 | Graft failure, pneumonia | |
| C5981-153 | 4.4 | Yes | 25 | Skin, gut | Skin, gut | Yes | 13 | GVHD, dehydration | |
| C6461-153 | 3.2 | Yes | 100 | Skin, gut, liver | No | Yes | 21 | GVHD | |
| C7121-153 | 4.5 | Yes | 50 | Skin, liver | Skin, gut | Yes | 20 | GVHD | |
| IM71-155 | D109 | 4.3 | Yes | 70 | Skin, gut | Skin, gut | Yes | 14 | GVHD |
| D115 | 4.0 | No | <2 | No | No | No | 10 | Graft failure, pneumonia | |
| D153 | 4.1 | Yes | <2 | No | Skin | No | 20 | Graft failure | |
| D161 | 4.0 | No | 0 | No | Skin, gut | No | 13 | Graft failure, pneumonia | |
| D173 | 3.2 | No | 1 | No | No | No | 13 | Graft failure, pneumonia | |
| S31-155 | C388 | 3.7 | No | 0 | No | Gut | No | 15 | Graft failure, pneumonia |
| C433 | 1.3 | No | Early focal | No | No | No | 14 | Graft failure, pneumonia | |
| C436 | 4.1 | Yes | 25 | Skin, gut | Skin, gut, liver | Yes | 21 | Pneumonia | |
| C439 | 4.1 | No | 0 | No | No | No | 17 | Graft failure, pneumonia | |
| C441 | 4.1 | Yes | 0 | No | No | No | 12 | Graft failure | |
| C445 | 4.0 | No | 0 | No | No | No | 12 | Graft failure, pneumonia | |
| C454 | 4.6 | Yes | 25 | Skin, gut | Gut | Yes | 17 | GVHD, pneumonia | |
| C952 | 3.2 | No | 0 | No | No | No | 11 | Graft failure, pneumonia | |
| C962 | 1.8 | Yes | 25 | Skin | Skin, liver | Yes | 13 | Pneumonia | |
| C968 | 4.0 | Yes | 0 | No | No | No | 12 | Graft failure | |
| C983 | 4.0 | Yes | 0 | No | No | No | 28 | Graft failure, sepsis | |
*Data on these dogs were previously reported.
MoAb S5 days −5 to 0, 0.2 mg/kg/d.
MoAb S5 days −7 to −2, 0.2 mg/kg/d.
MoAb S5 days −7 to −2, 0.2 mg/kg/d with the day −7 MoAb131I-iodinated (dose equivalent to 100 cGy external beam TBI).
MoAb was administered 0.2 mg/kg/d days −7 to −2.
Cytogenetic Results in Female Dogs Administered 920 cGy TBI, Pretreatment With MoAb, and Marrow Grafts From DLA-Nonidentical, Unrelated Male Donors
| Dog . | MoAb* . | Day . | Site . | Result† . |
|---|---|---|---|---|
| D109 | IM7 | 14 | PB | XY 18 |
| XX 3 | ||||
| 14 | BM | XY 18 | ||
| XX 3 | ||||
| D153 | IM7 | 20 | BM | XX 4 |
| C983 | S3 | 22 | PB | XX 10 |
| 25 | PB | XX 10 |
| Dog . | MoAb* . | Day . | Site . | Result† . |
|---|---|---|---|---|
| D109 | IM7 | 14 | PB | XY 18 |
| XX 3 | ||||
| 14 | BM | XY 18 | ||
| XX 3 | ||||
| D153 | IM7 | 20 | BM | XX 4 |
| C983 | S3 | 22 | PB | XX 10 |
| 25 | PB | XX 10 |
Abbreviations: BM, bone marrow; PB, peripheral blood after phytohemagglutinin stimulation.
MoAb used to treat recipient dog.
Only cells with 78 chromosomes were analyzed. Number of metaphases examined.
Results in Dogs Administered 9.2 Gy TBI and DLA-Nonidentical Marrow Grafts From Unrelated Donors With or Without MoAbs Before Transplant
| MoAb* . | No. of Dogs . | P Value3-151 . | ||
|---|---|---|---|---|
| Studied . | Sustained Engraftment No. (%) . | Graft Rejection No. (%) . | ||
| None | 57 | 10 (18) | 47 (82) | |
| 6.4 (irrelevant) | 7 | 1 (14) | 6 (86) | 1.0 |
| S5 (anti-CD44) | 21 | 14 (67) | 7 (33) | <.0001 |
| IM7 (anti-CD44) | 5 | 1 (20) | 4 (80) | 1.0 |
| S3 (anti-CD44) | 12 | 3 (25) | 9 (75) | .69 |
| MoAb* . | No. of Dogs . | P Value3-151 . | ||
|---|---|---|---|---|
| Studied . | Sustained Engraftment No. (%) . | Graft Rejection No. (%) . | ||
| None | 57 | 10 (18) | 47 (82) | |
| 6.4 (irrelevant) | 7 | 1 (14) | 6 (86) | 1.0 |
| S5 (anti-CD44) | 21 | 14 (67) | 7 (33) | <.0001 |
| IM7 (anti-CD44) | 5 | 1 (20) | 4 (80) | 1.0 |
| S3 (anti-CD44) | 12 | 3 (25) | 9 (75) | .69 |
*MoAb was administered 0.2 mg/kg/d × 6 days before TBI.
Fisher's exact test was used to compare experimental results designated “none.”
Serum level of MoAb.
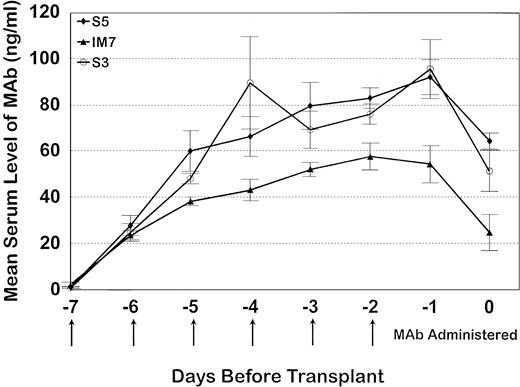
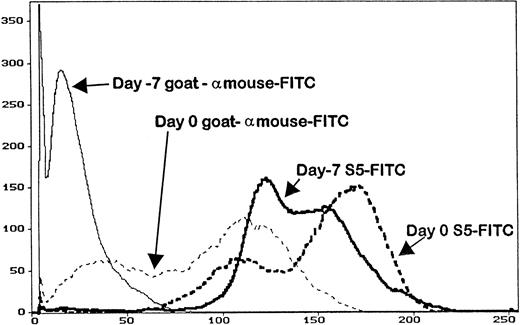
Serum trough levels were determined for seven dogs administered MoAb S5, five dogs administered MoAb S3, and five dogs given IM7. Figure 3 shows the mean MoAb serum levels, which peaked on day -1 for MoAbs S5 and S3 and day -2 for MoAb IM7. Overall, serum levels between S5 and S3 were not significantly different from each other because the curves were overlapping, although MoAb IM7 serum levels were lower than those of MoAbs S5 and S3 throughout the period of injections. In this regard, the single IM7-treated dog that engrafted (D109) had a very low serum MoAb level at the time of marrow infusion, indirectly implying that failure to engraft in the four other MoAb IM7-treated dogs was not due to insufficient MoAb serum levels. PBMC obtained from the S5-treated dogs on day 0 had detectable antibody on their cell surfaces, as measured by goat antimouse FITC, although not at saturating levels, as additional S5-FITC was able to bind to the cells, increasing the fluorescence intensity as detected by flow cytometry (Fig 4). Similar results were observed for the IM7 and S3-treated dogs.
The mean serum trough MoAb levels (± standard error) for seven dogs that received S5, all five dogs that received IM7, and five dogs that received S3. All animals received MoAb from day -7 through day -2 where day -7 represents the baseline, day -2 is the last day of MoAb administration, and day 0 is the day of transplant.
The mean serum trough MoAb levels (± standard error) for seven dogs that received S5, all five dogs that received IM7, and five dogs that received S3. All animals received MoAb from day -7 through day -2 where day -7 represents the baseline, day -2 is the last day of MoAb administration, and day 0 is the day of transplant.
The abscissa represents the mean fluorescence intensity of the cells analyzed from the animals on day -7 (before MoAb administration), and on day 0 (day of transplant) of an animal that received MoAb S5. Cells were stained with either goat antimouse FITC (to detect surface MoAb) or S5-FITC, which is the direct FITC conjugate of MoAb S5. Cells removed from the animal on day 0 had surface MoAb, as shown by staining with goat antimouse FITC, although not saturation levels, as additional MoAb S5 binds to cells (S5-FITC).
The abscissa represents the mean fluorescence intensity of the cells analyzed from the animals on day -7 (before MoAb administration), and on day 0 (day of transplant) of an animal that received MoAb S5. Cells were stained with either goat antimouse FITC (to detect surface MoAb) or S5-FITC, which is the direct FITC conjugate of MoAb S5. Cells removed from the animal on day 0 had surface MoAb, as shown by staining with goat antimouse FITC, although not saturation levels, as additional MoAb S5 binds to cells (S5-FITC).
Effect of S5 on NK activity of PBMC retrieved from dogs after in vivo injection of MoAb.
PBMC were obtained from the animals on day -7 (before any MoAb treatment) during various timepoints of the MoAb infusion, and on day 0 just before and after TBI. There were mild increases in NK activity among cells from dogs treated with MoAb S5, consistent with previous in vitro observations of increase of NK activity by S513 (data not shown). More remarkable was the NK activity in cells obtained on day 0 just before and just after TBI in two S5-treated dogs studied. Cells from both dogs showed, on average, 72% reduction in NK activity after TBI (Table 5). In four controls that received TBI but no antibody, the reduction in specific lysis was less pronounced on average (32%) (P = .06 by two sample Wilcoxon's rank-sum test).
NK Activity in Dogs Before and After TBI
| . | Reduction of Specific Lysis After TBI (9.2 Gy)4-150 . | . |
|---|---|---|
| Controls | ||
| D229 | 34% | |
| D269 | 17% | |
| D309 | 38% | |
| D447 | 40% | |
| 32% Average | ||
| S5 Treated | ||
| D291 | 59% | |
| D450 | 85% | |
| 72% Average |
| . | Reduction of Specific Lysis After TBI (9.2 Gy)4-150 . | . |
|---|---|---|
| Controls | ||
| D229 | 34% | |
| D269 | 17% | |
| D309 | 38% | |
| D447 | 40% | |
| 32% Average | ||
| S5 Treated | ||
| D291 | 59% | |
| D450 | 85% | |
| 72% Average |
Comparison of NK activity of dogs in control- and S5-treated (in vivo) animals.
Data are reported as percent decrease in NK activity pre- and post-TBI.
Cell binding.
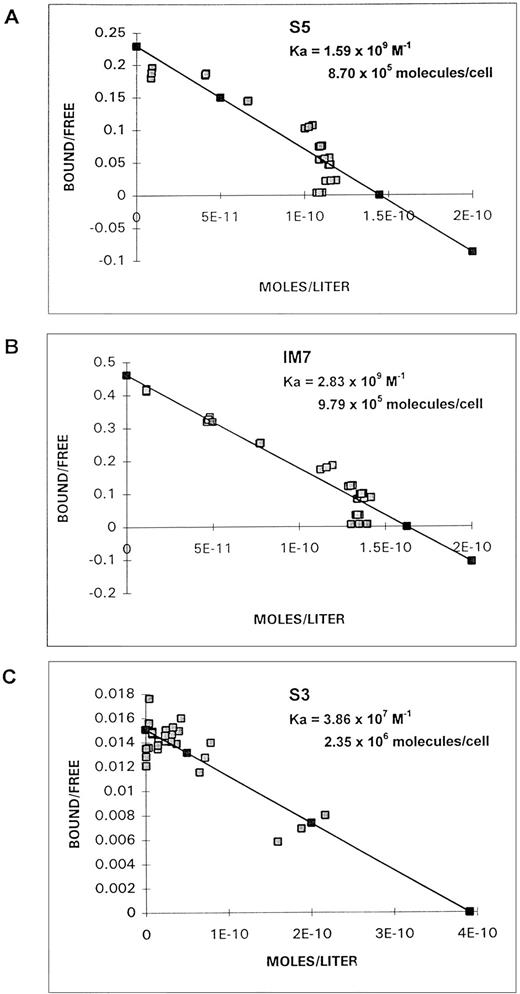
Scatchard analyses of S5, IM7, and S3 binding to ML3 cells are shown in Fig 5. Based on the molecular weight of the MoAbs and the number of cells in the incubation, for S5 the association constant (Ka) was 1.5 × 109 (mol-1) and the number of molecules bound per cell was 8.7 × 105; for IM7, the association constant was 2.83 × 109(mol-1) and the number of molecules bound was 9.79 × 105; and for S3, the association constant was 3.86 × 107 (mol-1) and the number of molecules bound was 2.35 × 106.
Scatchard plots of binding of MoAb S5 (A), IM7 (B), or S3 (C) to ML3 cells.
DISCUSSION
The current study extended previous observations5 and confirmed that treatment of recipients of DLA-nonidentical marrow grafts with an anti-CD44 MoAb, S5, before TBI significantly decreased the incidence of graft rejection. In an attempt to better understand how MoAb S5 accomplished its in vivo effects in the canine graft rejection model and to ultimately develop an analogous antihuman CD44 MoAb, we cloned the canine CD44 gene, mapped MoAb S5, and tested other anti-CD44 MoAbs in the in vivo model to judge whether this was a phenomenon applicable to all CD44 MoAbs.
The high homology between canine and human CD44 at both the sequence and amino acid levels implied conservation of ligand binding regions and function. This also allowed us to use the human truncation constructs of CD44 for protein mapping. Although the initial mapping of S5 determined that S5 recognized a region highly conserved between human and canine CD44, both IM7 and S3 also mapped to the same general region of CD44. This region included the NH2-distal domain comprised of basic amino acids that were required for CD44/HA interactions, although most of the HA binding domain was located more proximally.
We previously showed in competitive binding assays that S5 and IM7 blocked each other's binding to CD44,6 whereas S3 (our unpublished observations, June 1989) did not. This suggested that S5 and IM7 saw overlapping epitopes. The observation that S5 recognized only canine CD44 and cross-reacts only with lower affinity with human CD44 (and not rodent or nonhuman primate CD44), whereas IM7 recognizes CD44 on mouse, dog, nonhuman primates, and humans indicated that the epitope recognized by the two MoAbs was not identical.
In an attempt to further localize the epitope bound by S5, peptides (15 amino acids in length) based on the canine sequence were constructed spanning amino acids 130-184. Using ELISA, multiple anti-CD44 MoAbs, including S5 and IM7, were tested on plates coated with the peptides, and all were negative (data not shown). This suggested that the epitope recognized by S5 might be conformational and/or involve noncontiguous regions of the glycoprotein.
In addition to extending the in vivo transplant results with S5, we examined other anti-CD44 MoAbs in vivo to see if the graft enhancement effect of S5 was a general phenomenon. We showed that only MoAb S5 facilitated engraftment (P < .0001 compared with control), whereas two other anti-CD44 MoAbs (IM7 and S3) were ineffective. This suggested that the specific epitope bound by S5 was relevant for the graft enhancement effect.
Because IM7 and S5 mapped to one of the HA binding domains of CD44, we explored CD44/HA interactions in the presence of the MoAbs to evaluate for possible functional differences. Zhang et al38 have shown that IM7 could block CD44 binding to HA in some, but not all cell lines. Using the CD44H-Rg fusion protein in an in vitro HA binding assay, both S5 and IM7 augmented CD44/HA interactions, whereas MEM-85 blocked CD44 binding to HA. This suggested that MoAb S5 and IM7 were providing a signal to cause a conformational change in the CD44 molecule, which allowed it to bind to a ligand, similar to what has been reported with T cells.39 These data confirmed that both S5 and IM7 mapped to a region that was distinct from the HA binding region. S5 and IM7 did not block HA binding, but caused the conformational change in CD44, which allowed HA binding as previously shown by one other antimurine CD44 MoAb.38 This finding could potentially play a role in the ability of S5 to enhance engraftment by improving interaction of hematopoietic cells with the extracellular matrix of the microenvironment, although it is not sufficient to explain the in vivo effect of engraftment because IM7, which also augmented HA/CD44 interactions, failed to affect engraftment of mismatched marrow.
Both determination of serum MoAb levels and saturation studies performed on cells from animals receiving MoAb were similar between the three different anti-CD44 MoAbs, which indicated that the differences seen between S5 and the other two anti-CD44 MoAbs (IM7 and S3) were not due to variation in pharmacokinetics of the MoAbs.
We have previously shown that S5-treated PBMC had a higher baseline of NK activity.13 In addition, the S5-treated PBMC were found to be more sensitive to radiation kill, as shown by the steeper decline in specific lysis with increasing radiation doses.14 To evaluate whether this observation was relevant in the in vivo model, PBMC from a dog that was infused with MoAb S5 were evaluated before and after 9.2 Gy TBI. The reduction in NK activity after TBI, as compared with control, was consistent with the in vitro findings of increased radiation sensitivity of S5-treated NK cells, suggesting that S5 facilitates engraftment by increasing the radiation sensitivity of recipient NK cells that may be responsible for marrow graft rejection.
To evaluate whether the in vivo differences observed with the three anti-CD44 MoAbs were the result of lower affinity, lower avidity, or overall diminished immunoreactivity, binding assays were performed with the three MoAbs. Although MoAb S3 had a significantly lower avidity to CD44, S5 and IM7 were indistinguishable, with IM7 having a slightly higher avidity than S5. These data suggest that the enhanced in vivo effect on engraftment of S5 is not on the basis of higher avidity, but rather is due to the epitope bound by S5.
In the canine system, we have determined that pretreatment of PBMC with MoAb S5 enhances NK activity.13-15 Both in vivo and in vitro data indicate that S5-treated NK cells are more sensitive to radiation than NK cells not treated by MoAb, indicating that S5 may facilitate marrow engraftment by increasing radiation-induced death of host cells capable of marrow graft rejection.13 We have also shown previously that S5 augments in vitro hematopoiesis in long-term marrow cultures (LTMC)40 and increased CD34 cell production (unpublished data, December 1995). Other anti-CD44 MoAbs tested did not have this effect. If this occurs in vivo, it is reasonable to postulate that pretreating transplant recipients with S5 could enhance engraftment in two ways, reducing host resistance and enhancing donor hematopoiesis via effects on host marrow stroma. Whether both mechanisms are required to facilitate engraftment is not yet known. There was no evidence in either the autologous setting (unpublished, February 1986) or in the DLA-identical setting41 that S5 augmented hematopoiesis. In all cases, the recoveries of neutrophils and platelets were normal, although the neutrophil recovery may not be sensitive enough to test the hypothesis that S5 can have a direct hematopoietic effect. Further studies are directed at developing antihuman CD44 MoAbs that are equivalent to MoAb S5 to prevent graft rejection in patients. To ensure that the epitope that S5 recognizes is conserved, we will convert the mouse anticanine CD44 MoAb S5 into a human antihuman CD44 MoAb using phage display technology and “chain shuffling.” This is a proven method of converting a low-affinity mouse antibody into a high-affinity human antibody with identical epitope specificity.42 43
Although the epitope mapping data does not discriminate between the three MoAbs that were used in vivo, it does provide the information that the functional MoAb, S5, binds to an area of CD44 highly conserved between the different species and does not involve the HA binding domain. The data supports the hypothesis that an antibody that will recognize the same epitope on human CD44 as S5 will have the same functional effect in human HLA-haploidentical marrow graft recipients.
ACKNOWLEDGMENT
We would like to acknowledge Dr Dana Matthews for assistance in analysis of binding assays, Dr Ted Gooley for assistance in statistical analyses, Dr Eileen Bryant and Robert Raff for cytogenetic studies, Laura Bolles and Jennifer Smith for technical assistance, and Harriet Childs and Bonnie Larson for their help in manuscript preparation.
Supported in part by Grants No. CA01483, CA18221, CA31787, HL36444, and DK42716 from the National Institutes of Health, Department of Health and Human Services, Bethesda, MD. Laboratory support was also available through a prize from the Josef Steiner Krebsstiftung, Bern, Switzerland (awarded to R.S.).
Address reprint requests to Brenda M. Sandmaier, MD, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, D1-100, PO Box 19024, Seattle, WA 98109.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal