Abstract
Two hundred and one patients (median age, 29 years) with acute myeloid leukemia (AML) underwent bone marrow transplantation (BMT) from HLA-identical sibling donors after conditioning with melphalan-total-body irradiation (TBI) (57%), cyclophosphamide-TBI (35%), or chemotherapy alone (8%). Graft-versus-host disease (GVHD) prophylaxis included cyclosporine alone (68%), cyclosporine-methotrexate (26%), or T-cell depletion (6%). The probability of relapse was calculated as a function of the absolute lymphocyte count (109/L) on days 27 to 30 posttransplant (<0.1 v ≥0.1, <0.2 v ≥0.2, and <0.3 v≥0.3). In each of these 12 comparisons, the probability of relapse was higher for the group with the lower lymphocyte count. Because the difference was most significant (P = .004) for an absolute lymphocyte count of <0.2 on day 29 (3-year relapse probability, 42%) versus ≥ 0.2 (16%), this variable was included in a Cox model to determine factors independently affecting relapse. Multivariate analysis showed that conditioning regimens other than melphalan-TBI, a low lymphocyte count on day 29, French-American-British (FAB) subtypes M4-7, and a nucleated cell dose of > 2.42 × 108/kg was associated with a higher risk of relapse. We conclude that slow lymphocyte recovery after allogeneic BMT, to < 0.2 × 109/L 29 days in this analysis, appears to be associated with a higher risk of relapse in patients with AML. This group of patients may benefit from posttransplant immune manipulations such as abbreviated GVHD prophylaxis, or donor cell or cytokine administration to enhance graft-versus-leukemia reactions to reduce relapse.
ALONG WITH transplant-related mortality, relapse is an important cause of failure of allogeneic bone marrow transplantation (BMT) for hematologic malignancies. Depending on various factors such as the chromosomal karyotype of the malignant clone, the stage of the disease, the conditioning regimen used, the graft-versus-host disease (GVHD) prophylaxis, and the development of GVHD, the risk of relapse after allogeneic BMT for acute myeloid leukemia (AML) in the first complete remission varies from 10% to 40%.1-15
The outcome of patients relapsing after allogeneic BMT is generally very poor.16 Although second allogeneic transplants and adoptive immunotherapy can result in long-term remission in a proportion of patients, both of these approaches are associated with considerable toxicity.16 17
Postallograft approaches aimed at exploiting graft-versus-leukemia reactions18 more effectively to prevent or minimize relapse could include administration of donor cells,19,20cytokines, such as interferon-α,21 or interleukin-2,22 or individualizing GVHD prophylaxis.23 All of these approaches are potentially risky because they can cause or aggravate GVHD and are best confined to patients with a high risk of relapse.
The aim of this analysis was to see if lymphocyte recovery 4 weeks after allogeneic BMT from HLA-identical sibling donors could identify patients with AML in first remission who were at a high risk of relapse, so that patients with poor lymphocyte recovery after allografting in the future could undergo immune manipulation to enhance graft-versus-leukemia.18
MATERIALS AND METHODS
The database of the Leukaemia Unit contains prospectively-collected information on over 1,600 patients treated in the Royal Marsden Hospital since 1978.24,25 This was searched to identify a group of 201 AML patients who underwent allogeneic BMT in the first complete remission (CR) from HLA-identical sibling donors between 1981 and 1995. Patients receiving blood-derived stem cells or blinded marrow/blood cells26 were excluded. Patient characteristics are shown in Table 1.
Patient Characteristics
| Sex | |
| Male | 102 (51%) |
| Female | 99 (49%) |
| Median age | 29 years (range, 1 to 51) |
| Median leukocyte count at pre sentation (109/L) | 5.1 (range, 0.2 to 300) |
| FAB subtypes | |
| M1 | 21 |
| M2 | 46 |
| M3 | 26 (M1-3: 46%) |
| M4 | 43 |
| M5 | 14 |
| M6 | 11 |
| M7 | 1 (M4-7: 34%) |
| Unknown | 39 (19%) |
| Median remission-transplant interval (weeks) | 16 (range, 1 to 96) |
| Conditioning regimen | |
| Melphalan-TBI | 114 (57%) |
| Cyclophosphamide-TBI | 70 (35%) |
| Chemotherapy only | 17 (8%) |
| GVHD prophylaxis | |
| Cyclosporine alone | 136 (68%) |
| Cyclosporine-methotrexate | 53 (26%) |
| T-cell depletion | 12 (6%) |
| Blood group match | 127 (63%) |
| Blood group mismatch | 74 (37%) |
| Median nucleated cell dose (108/kg) | 2.42 (range, 1.02 to 9.72) |
| Any grade acute or chronic GVHD | 173 (86%) |
| No GVHD | 28 (14%) |
| Median absolute lymphocytes on day 29 (109/L) | 0.49 (range, 0 to 5.87)* |
| Outcome | |
| Alive | 86 (43%)-151 |
| Relapse | 25 (12%)-151 |
| Toxic deaths | 92 (46%) |
| Sex | |
| Male | 102 (51%) |
| Female | 99 (49%) |
| Median age | 29 years (range, 1 to 51) |
| Median leukocyte count at pre sentation (109/L) | 5.1 (range, 0.2 to 300) |
| FAB subtypes | |
| M1 | 21 |
| M2 | 46 |
| M3 | 26 (M1-3: 46%) |
| M4 | 43 |
| M5 | 14 |
| M6 | 11 |
| M7 | 1 (M4-7: 34%) |
| Unknown | 39 (19%) |
| Median remission-transplant interval (weeks) | 16 (range, 1 to 96) |
| Conditioning regimen | |
| Melphalan-TBI | 114 (57%) |
| Cyclophosphamide-TBI | 70 (35%) |
| Chemotherapy only | 17 (8%) |
| GVHD prophylaxis | |
| Cyclosporine alone | 136 (68%) |
| Cyclosporine-methotrexate | 53 (26%) |
| T-cell depletion | 12 (6%) |
| Blood group match | 127 (63%) |
| Blood group mismatch | 74 (37%) |
| Median nucleated cell dose (108/kg) | 2.42 (range, 1.02 to 9.72) |
| Any grade acute or chronic GVHD | 173 (86%) |
| No GVHD | 28 (14%) |
| Median absolute lymphocytes on day 29 (109/L) | 0.49 (range, 0 to 5.87)* |
| Outcome | |
| Alive | 86 (43%)-151 |
| Relapse | 25 (12%)-151 |
| Toxic deaths | 92 (46%) |
*Excluding 9 patients who died before day 29. Lymphocyte counts were not available for 4 patients who were alive on day 29.
Two relapsing patients are alive in remission after donor leukocyte infusions.
Conditioning regimens.
GVHD prophylaxis.
Before the intravenous formulation of cyclosporine became available, the drug was administered intramuscularly at a dose of 25 mg/kg from day −1 for 5 days and then orally at a dose of 12.5 mg/kg. After the intravenous formulation of cyclosporine became available, the drug was administered intravenously at a dose of 3 mg/kg from day −1 until oral intake was resumed. The drug was then given orally at a dose of 12.5 mg/kg. Patients receiving a short course of methotrexate in addition to cyclosporine received 15 mg/m2of the drug on day 1 and 10 mg/m2 on days 3 and 6, or on days 3, 6, and 11.
The murine anti-CD52 monoclonal antibodies Campath-1G or Campath-1M (kindly provided by Drs G. Hale and H. Waldmann, Cambridge, UK) were used to deplete the donor marrow of T cells in a minority of patients.29
Acute GVHD was usually diagnosed and graded on the basis of clinical findings and was treated with conventional (2 mg/kg) or high (10 to 20 mg/kg) dose corticosteroids.
Donor marrow.
Marrow was harvested from the iliac crests of HLA-identical sibling donors under general anesthesia on the day of transplantation. The target cell number for the harvest was 2 × 108nucleated cells per kg recipient body weight. The marrow was usually not depleted of T cells and was infused into the patient essentially unmanipulated except as required for donor-recipient ABO blood group incompatibility.30
Supportive care.
All patients were treated in protective isolation in rooms with positive-pressure ventilation. Blood products transfused were not screened for cytomegalovirus (CMV) antibody before 1985. After that time, CMV-seronegative patients with CMV-seronegative donors received screened, CMV-negative blood products. Fever during the neutropenic phase was treated with broad-spectrum antibiotics and amphotericin as necessary. Antibiotic prophylaxis and therapy varied in accordance with prevalent practices and research programs. All research protocols were approved by the local institutional review board, and all patients and donors gave informed consent.
Statistical analysis.
Serial blood counts obtained by automated counters were analyzed to see if the lymphocyte counts 4 weeks after BMT (days 27 to 30; the day of marrow infusion being designated as day 0) were predictive of relapse. Although we would have liked to look at the effect of lymphocyte counts earlier (at the end of 3 weeks), as we have outlined elsewhere,31 32 differential counts are not very accurate with very low total leukocyte counts in the second and third weeks after BMT. Lymphocyte subset data were not available.
For each of these 4 days, patients were grouped on the basis of the absolute lymphocyte counts (109/L) in three ways (≤0.1v >0.1, ≤0.2 v >0.2, and ≤0.3 v >0.3), and the probability of relapse calculated using the method of Kaplan and Meier.
Patients were censored at the time of nonrelapse death (death in remission) or the last follow-up in continuous remission. The log-rank test was used to compare the differences.
The following factors were also analyzed in univariate and multivariate fashion to determine their effect on the probability of relapse: age (<20 v ≥20 years), conditioning regimen (melphalan-TBIv cyclophosphamide-TBI v chemotherapy only), donor-recipient ABO blood group compatibility (match vmismatch), French-American-British (FAB) subtype (M1-3 v M4-7v unknown), GVHD prophylaxis (cyclosporine alone vcyclosporine-methotrexate v T-cell depletion), leukocyte count at presentation (<5.1 × 109/L v ≥5.1), nucleated cell dose (≤2.42 v >2.42 × 108/kg), occurrence of acute or chronic GVHD (yes vno), remission-transplant interval (<4 months v ≥4 months), and the absolute lymphocyte count on day 29 after BMT (≤0.2 × 109/L v >0.2). The effect of the karyotype could not be analyzed because results were not available for more than half the patients (who were referred for BMT in remission after prior therapy elsewhere).
Among the different variables, a very strong correlation was seen between GVHD prophylaxis and conditioning regimen (r =.35;P < .0001) with 11 of 12 T-cell–depleted grafts being conditioned with cyclophosphamide-TBI and 47 of 53 cyclosporine-methotrexate grafts being conditioned with melphalan-TBI.
RESULTS
Twenty-five patients relapsed at 3 to 46 months (median, 10); 23 died of relapsed disease and 2 are alive after cell- and cytokine-mediated immunotherapy.17 Ninety-two patients died of transplant-related causes at 0.5 to 123 months (median, 3). Eighty-four patients are alive in continuous remission at 12 to 191 months (median, 91). Four of 12 patients receiving T-cell–depleted marrow relapsed, as did 14 of 136 patients receiving cyclosporine, and 7 of 53 patients receiving cyclosporine-methotrexate.
As Table 2 shows, for each of the lymphocyte levels compared on the 4 posttransplant days studied, patients with the lower lymphocyte counts had higher relapse rates. This difference was significant at the P < .01 level for 3 of the 12 comparisons. The significance was the highest (P = .004) for day 29 and lymphocytes <0.2 versus ≥0.2 × 109/L (Fig 1).
Probability of Relapse on the Basis of the Absolute Lymphocyte Count 4 Weeks After Bone Marrow Transplantation
| Absolute Lymphocyte Count (109/L) . | Day 27 (%) . | Day 28 (%) . | Day 29 (%) . | Day 30 (%) . |
|---|---|---|---|---|
| <0.1 | 45.3 | 44.4 | 43.8 | 68.8* |
| ≥0.1 | 19.3 | 17.9 | 18.0 | 17.6 |
| <0.2 | 29.6 | 37.6 | 42.0* | 36.0 |
| ≥0.2 | 19.0 | 17.0 | 15.9 | 17.3 |
| <0.3 | 24.3 | 29.9 | 34.1* | 29.0 |
| ≥0.3 | 17.8 | 15.9 | 15.3 | 17.0 |
| Absolute Lymphocyte Count (109/L) . | Day 27 (%) . | Day 28 (%) . | Day 29 (%) . | Day 30 (%) . |
|---|---|---|---|---|
| <0.1 | 45.3 | 44.4 | 43.8 | 68.8* |
| ≥0.1 | 19.3 | 17.9 | 18.0 | 17.6 |
| <0.2 | 29.6 | 37.6 | 42.0* | 36.0 |
| ≥0.2 | 19.0 | 17.0 | 15.9 | 17.3 |
| <0.3 | 24.3 | 29.9 | 34.1* | 29.0 |
| ≥0.3 | 17.8 | 15.9 | 15.3 | 17.0 |
*Indicates differences that are significant at the P < .01 level.
The effect of the day 29 lymphocyte count on relapse in 188 patients (13 of 201 patients died before day 29 or did not have lymphocyte counts available).
The effect of the day 29 lymphocyte count on relapse in 188 patients (13 of 201 patients died before day 29 or did not have lymphocyte counts available).
Table 3 shows that the mode of GVHD prophylaxis including the administration of methotrexate did not affect the lymphocyte count significantly.
Correlation Between GVHD Prophylaxis and Lymphocyte Counts on Day 29
| GVHD Prophylaxis . | Lymphocytes <0.2 × 109/L No. of Patients (%) . | Lymphocytes ≥0.2 × 109/L No. of Patients (%) . | Day 29 Lymphocytes (109/L) Median (range) . |
|---|---|---|---|
| T-cell depletion | 4 (33) | 8 (67) | 0.34 (0-1.52) |
| Cyclosporine alone | 25 (20) | 100 (80) | 0.48 (0-5.87) |
| Cyclosporinemethotrexate | 6 (12) | 45 (88) | 0.53 (0.08-2.82) |
| P | .18 | .50 (TCD v Cy) | |
| (Chi squared) | .14 (TCD vCy-MTX) | ||
| .19 (Cy vCy-MTX) (ANOVA) |
| GVHD Prophylaxis . | Lymphocytes <0.2 × 109/L No. of Patients (%) . | Lymphocytes ≥0.2 × 109/L No. of Patients (%) . | Day 29 Lymphocytes (109/L) Median (range) . |
|---|---|---|---|
| T-cell depletion | 4 (33) | 8 (67) | 0.34 (0-1.52) |
| Cyclosporine alone | 25 (20) | 100 (80) | 0.48 (0-5.87) |
| Cyclosporinemethotrexate | 6 (12) | 45 (88) | 0.53 (0.08-2.82) |
| P | .18 | .50 (TCD v Cy) | |
| (Chi squared) | .14 (TCD vCy-MTX) | ||
| .19 (Cy vCy-MTX) (ANOVA) |
Thirteen patients had either died by day 29 (n = 9) or had no lymphocyte counts available for that day (n = 4). None of the differences is significant.
Table 4 shows the results of the Cox analysis. Patients conditioned with melphalan-TBI had a significantly lower probability of relapse (Fig 2), and this factor dominated the risk of relapse. An absolute lymphocyte count of <0.2 × 109/L on day 29 was also significantly associated with a higher risk of relapse, as were FAB subtypes other than M1-3 and a higher infused cell dose. Because of the strong correlation between the conditioning regimen and GVHD prophylaxis, the effect of the latter, if any, was not discernible.
Multivariate Analysis of Factors Affecting Relapse
| Favorable Variable . | Unfavorable Variable . | Risk Ratio (95% CI) . | P . |
|---|---|---|---|
| Melphalan-TBI | Other | 3.2 (1.8-5.6) | .0001 |
| Day 29 lymphocyte count ≥0.2 × 109/L | Day 29 lymphocyte count <0.2 × 109/L | 2.9 (0.9-9.1) | .02 |
| FAB subtype M1-3 | Other | 1.6 (1.0-2.7) | .03 |
| Nucleated cell dose ≤2.42 × 108/kg | Nucleated cell dose >2.42 × 108/kg | 1.7 (1.1-1.8) | .05 |
| Favorable Variable . | Unfavorable Variable . | Risk Ratio (95% CI) . | P . |
|---|---|---|---|
| Melphalan-TBI | Other | 3.2 (1.8-5.6) | .0001 |
| Day 29 lymphocyte count ≥0.2 × 109/L | Day 29 lymphocyte count <0.2 × 109/L | 2.9 (0.9-9.1) | .02 |
| FAB subtype M1-3 | Other | 1.6 (1.0-2.7) | .03 |
| Nucleated cell dose ≤2.42 × 108/kg | Nucleated cell dose >2.42 × 108/kg | 1.7 (1.1-1.8) | .05 |
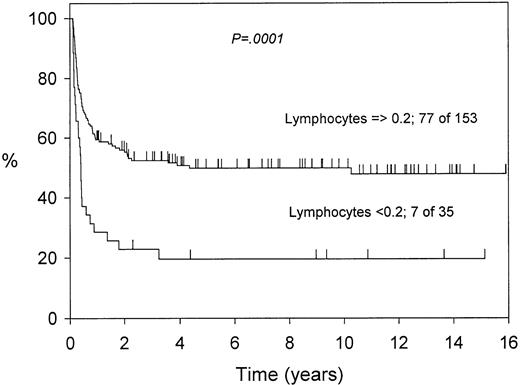
The higher lymphocyte count on day 29 was also associated with a significant survival benefit (Fig 3). This effect was partly also due to a lower toxic death rate among patients with the higher lymphocyte count (59 of 153 v 22 of 35; 1-year probabilities 35% v 65.5%; P = .003).
The effect of the day 29 lymphocyte count on survival in 188 patients (13 of 201 patients died before day 29 or did not have lymphocyte counts available).
The effect of the day 29 lymphocyte count on survival in 188 patients (13 of 201 patients died before day 29 or did not have lymphocyte counts available).
Finally, the beneficial effect of the higher lymphocyte count on relapse was independent of GVHD because, as Fig 4 shows, the lower lymphocyte count was associated with a slightly higherprobability of GVHD.
The relation between the day 29 lymphocyte count and the cumulative probability of acute or chronic GVHD. The lower count is associated with a higher risk of GVHD.
The relation between the day 29 lymphocyte count and the cumulative probability of acute or chronic GVHD. The lower count is associated with a higher risk of GVHD.
DISCUSSION
Our data show that slower lymphocyte recovery in the fourth week after allogeneic BMT for AML in first remission, as reflected by an absolute lymphocyte count of <0.2 × 109/L on day 29, is associated with an increased risk of disease recurrence independent of GVHD. This observation has practical significance because this identifies a group of patients who may be suitable candidates for early intervention to enhance graft-versus-leukemia.
Can factors operating so early in the course of an allograft really make a difference? In the early days or weeks after the allograft, the disease burden is at its lowest. At this time, the chances of eliminating it completely through immune mechanisms should logically be the highest. There is evidence to show that factors operating very early in the course of the transplant can affect relapse rates.33-35
A study from the International Bone Marrow Transplant Registry showed that the use of methotrexate for GVHD prophylaxis in patients with acute lymphoblastic leukemia decreased relapse independently of its effect on GVHD, possibly due to the direct antileukemic action of methotrexate.33 Blaise et al34 showed that the addition of an antibody against the interleukin-2 receptor (33B3.1) to standard GVHD prophylaxis early after transplantation delayed acute GVHD, but did not decrease its incidence. This delay was associated with an increased relapse rate and poorer leukemia-free survival. Jiang et al35 found an association between lower CD8+and natural killer cell numbers after allogeneic BMT in patients with chronic myeloid leukemia and an increased relapse rate.
Hancock et al36 in a retrospective study of children with acute lymphoblastic leukemia receiving T-cell–depleted marrow from unrelated donors, found that mixed chimerism on day 21 (persistent recipient hematopoiesis) was associated with a significantly higher probability of relapse. Although no data were available from their group of patients, it is possible that complete elimination of recipient hematopoiesis, and consequently a lower risk of relapse, may have been correlated with adequate lymphocyte reconstitution.
It would be interesting to look at lymphocyte subset data to see if there was any correlation between specific subsets and the observed outcome. Unfortunately, these investigations were either not available or were not routinely performed. A higher nucleated cell dose would be expected to contain a higher number of lymphocytes, and thus, possibly a lower relapse rate. Thus, the observation of a higher relapse rate with higher nucleated cell doses is difficult to explain. Although the difference is significant in the Cox analysis, it is not significant in univariate analysis (16 of 101 v 9 of 100; 3-year probabilities 24.5% v 13.4%; P = .11). Unlike those between marrow and blood,26 the differences between lymphocyte numbers at various nucleated marrow cell doses are likely to be rather modest and may not have any impact on immune recovery.
The risk of relapse as a result of slow lymphocyte recovery has to be interpreted in the context of other factors, which are known to (or would be expected to) increase relapse rates such as adverse chromosomal abnormalities.14 Thus, in a patient with a high-risk chromosomal abnormality, a low lymphocyte count could be a clear indication for action. In a patient without such abnormalities, it may simply call for careful monitoring for the earliest evidence of recurrence. It would be worthwhile to see if our data can be duplicated by other centers and in other diseases.
The potential therapeutic options in patients identified as being at a high risk of relapse a month after allografting could include administration of interferon-α, interferon-τ, interleukin-2 or more donor leukocytes, or modification (curtailment) of immunosuppression. Administration of interferon-α (in fact given for its potential antiviral effects) has been shown to reduce relapse rates,21 but has also been reported to increase the incidence and severity of GVHD.37 Interferon-τ can sometimes increase the CD4 count in patients with lymphopenia (unpublished observations). The administration of interleukin-2 after T-cell–depleted BMT has been shown to increase the natural killer cell numbers and reduce relapse without any effect on the lymphocyte numbers.22 Administration of graded increments of donor lymphocytes to acute leukemia patients after T-cell–depleted BMT decreased relapse of acute leukemia, but was associated with clinically significant GVHD.20 Curtailing the immunosuppression can reduce relapse rates,23 but may also give rise to severe GVHD, if tapered too rapidly.38 This could also be an appropriate setting to test active specific immunotherapy using inactivated malignant cells,39 possibly in combination with stimulatory cytokines.
In conclusion, our data suggest that poor lymphocyte recovery a month after allogeneic BMT is associated with an increased risk of relapse. Because the relapse risk is a combined function of the conditioning regimen and GVHD prophylaxis, it may be best for each transplant team to define their own criteria for identifying patients at risk of relapse. Patients identified on the basis of this could be candidates for prophylactic immunotherapy to enhance graft-versus-leukemia.
Supported by the Cancer Research Campaign, the Bud Flanagan Leukaemia Fund, and the Leukaemia Research Fund, UK.
Presented at 38th Annual Meeting of the American Society of Hematology, Orlando, FL, December 7-10, 1996.
Address reprint requests to Jayesh Mehta, MD, Division of Hematology/Oncology, University of Arkansas for Medical Sciences, 4301 West Markham, Slot 508, Little Rock, AR 72212.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal