Abstract
Two major isoforms of PML-RARα are associated with (15;17)-positive acute promyelocytic leukemia (APL); however, functional differences between these isoforms have been difficult to define, and the molecular mechanism by which each isoform contributes to the pathogenesis of APL is not fully understood. To address these issues, the ‘short’ (S) and ‘long’ (L) isoforms of PML-RARα were constitutively expressed in the factor-dependent human erythroleukemia cell line, TF1. Expression of the L, but not the S, isoform inhibited growth of these cells in the presence of granulocyte-macrophage colony-stimulating factor (GM-CSF). In the absence of GM-CSF, the S isoform partially protected against apoptosis, while the L isoform accelerated cell death. Treatment with all-trans retinoic acid (ATRA) inhibited cell growth and caused apoptosis only in PML-RARα–expressing cells, and these effects of ATRA were more marked in cells expressing the L isoform. ATRA treatment also led to downregulation of bcl-2 and endogenous RARα in PML-RARα–expressing cells, but had little effect on the level of exogenously expressed PML-RARα. We conclude that (1) subtle differences exist in the biologic activities of the L and S isoforms of PML-RARα, and (2) both isoforms are capable of transducing an ATRA-mediated signal that leads to downregulation of bcl-2 and induction of programmed cell death.
ACUTE PROMYELOCYTIC LEUKEMIA (APL) represents a prototype among human leukemias, and indeed among human cancers in general, in its sensitivity to the differentiating properties of specific retinoids such as all-trans retinoic acid (ATRA; reviewed in Fenaux et al1 and Warrell2). The addition of ATRA to a standard chemotherapeutic regimen of daunorubicin and cytarabine effectively doubles the cure rate in APL, from less than 35% with chemotherapy alone to approximately 70% with the chemotherapy/retinoid combination.3,4 The central molecular defect in APL is the disruption of the α receptor for retinoic acid, RARα, and its reciprocal in-frame fusion with one of four partner genes, PML, PLZF, NPM, or NuMA.5-8 In greater than 99% of APL cases, the RARα fusion partner is the PML gene on chromosome 15; the resulting PML-RARα chimeric transcript is present in malignant promyelocytes, and the PML-RARα fusion protein accumulates to high levels in these cells.5 In contrast, the reciprocal RARα-PML fusion transcript is found in only 80% of APL patients.9 Recent studies in transgenic mice have confirmed that the PML-RARα protein is integrally involved in the pathogenesis of APL.10-12 The two unique features of APL, ie, the dramatic response to ATRA and the expression of a modified retinoic acid receptor (eg, PML-RARα), are undoubtedly related, but the nature of this relationship is not yet clear, and the precise molecular mechanisms by which ATRA causes differentiation of APL blasts remain largely unknown. The delineation of these mechanisms will clarify whether APL is a fortunate medical curiosity or whether it will serve as a paradigm for the development of effective differentiation therapies in other types of human cancers.
There are two major isoforms of PML-RARα that are found in patients with (15;17)-positive APL.5 As shown in Fig1, the so-called “Short” (S) isoform results from a genomic break in intron 3 of the PML gene, while the “Long” (L) isoform is a consequence of a breakpoint in PML intron 6; the type S isoform of PML-RARα is found in approximately 35% of adult APL patients, while the L isoform is found in the remaining 55% to 60%.13 In both cases, and indeed in all cases of (15;17)-positive APL, the RARα gene is broken in a large intron separating the A and B domains of RARα.5 A small subset (approximately 8%) of APL patients have PML breakpoints located within exon 6, and express a so-called V (variable) PML-RARα isoform.14 In a recent large retrospective study, a statistical association was noted between the PML-RARα S isoform and high presenting white blood cell (WBC) count, high peripheral blast plus promyelocyte count, and M3v morphology.13 Furthermore, we have reported a statistical relationship between the S isoform and secondary cytogenetic abnormalities in APL.15 Despite these clinical differences between type S and L APL patients, isoform type per se does not appear to be a significant prognostic factor for prediction of long-term disease-free survival.13 16Nevertheless, the statistical associations noted above suggest that there are differences in the biology of APL blasts expressing either the type S or type L PML-RARα isoform, and further insight into the basic pathophysiology of APL could be obtained by definition of such differences in vitro.
(A) Schematic diagram of the 5′ region of the PML gene showing the two major breakpoint regions (designated by vertical arrows) involved in formation of the chimeric PML-RARα fusion gene. PML exons (rectangles) are numbered. (B) Schematic drawing of the PML-RARα ‘L’ and ‘S’ isoforms, created by fusion of the RARα B through F domains with PML exon 6 or PML exon 3, respectively. Motifs in the PML gene common to each isoform include a Ring finger (Ring) followed by two B-boxes (B1 and B2) and a coiled-coil domain consisting of four distinct coils. A putative nuclear localization signal (NLS) and a serine/proline-rich region (S/P) are located in PML exon 6 and are not present in the PML-RARα S isoform.
(A) Schematic diagram of the 5′ region of the PML gene showing the two major breakpoint regions (designated by vertical arrows) involved in formation of the chimeric PML-RARα fusion gene. PML exons (rectangles) are numbered. (B) Schematic drawing of the PML-RARα ‘L’ and ‘S’ isoforms, created by fusion of the RARα B through F domains with PML exon 6 or PML exon 3, respectively. Motifs in the PML gene common to each isoform include a Ring finger (Ring) followed by two B-boxes (B1 and B2) and a coiled-coil domain consisting of four distinct coils. A putative nuclear localization signal (NLS) and a serine/proline-rich region (S/P) are located in PML exon 6 and are not present in the PML-RARα S isoform.
The central paradox in APL is the apparent dual function of the PML-RARα fusion protein. In the absence of ATRA, it can inhibit differentiation of myeloid cells17-20 and, either alone or in concert with other genetic abnormalities, cause APL; however, in the presence of ATRA, PML-RARα actually appears to facilitate myeloid differentiation,17 and thus seemingly contributes to the cure of the disease it is believed to cause. Two recent reports suggest a possible explanation of this paradox. Both Yoshida et al21 and Raelson et al22 have shown that the PML-RARα protein is rapidly and specifically degraded in response to pharmacological concentrations of ATRA. The loss of PML-RARα protein presumably allows an intrinsic differentiation program to proceed, a program which may or may not be dependent on ATRA. Despite its attractiveness, this model does not fully explain the apparent hypersensitivity of PML-RARα–expressing cells to ATRA. For example, only blasts from PML-RARα–positive APL patients are uniformly ATRA responsive23: blasts from APL patients with t(11;17) do not appear to respond to ATRA, despite a block at the promyelocyte stage of differentiation, and no other subtype of AML is as consistently sensitive to ATRA as is APL. It is not known if the failure of other subtypes of AML to respond to ATRA represents their ‘lack’ of PML-RARα, or whether their ATRA resistance simply reflects different inherent mechanisms (or stages) of differentiation arrest. If, in the presence of ATRA, PML-RARα is truly a pro-differentiative or pro-apoptotic molecule, then its over-expression in hematopoietic cells should promote differentiation and/or apoptosis in an ATRA-dependent manner.
In the current study, our goals were the following: (1) to define biologic differences, if any, between cells expressing the type S and type L PML-RARα isoforms in vitro; and (2) to develop an in vitro model to evaluate the function of the PML-RARα molecule, in particular its ability to transduce an ATRA-mediated differentiative or apoptotic signal. We show that TF1 erythroleukemia cells constitutively expressing PML-RARα, but not isogenic control cells, are hypersensitive to ATRA-induced apoptosis, an effect that is particularly pronounced in cells expressing the type L PML-RARα isoform. Further, we show that ATRA downregulates bcl-2 expression in a PML-RARα–dependent manner. These results suggest that gene expression programs important in the regulation of differentiation and/or apoptosis in APL cells are directly regulated by ATRA through the PML-RARα protein.
MATERIALS AND METHODS
Chemicals and reagents.
Except as noted, chemicals and other routine laboratory reagents were purchased from Sigma (St Louis, MO). ATRA (tretinoin) was dissolved in absolute ethanol at 10−3 mol/L and stored at −20°C. Fresh solutions were made weekly. Camptothecin was dissolved in dimethyl sulfoxide (DMSO) at 10 mmol/L and stored at −20°C. Final concentrations used were 10−6 mol/L (ATRA) and 0.15 μmol/L (camptothecin).
Plasmid construction.
The cDNAs for the S and L isoforms of PML-RARα24 were kind gifts from P. Chambon (Université Louis Pasteur). Using standard recombinant DNA procedures, each cDNA was subcloned into the mammalian expression vector pCI-neo (Promega Corp, Madison, WI), which contains the cytomegalovirus immediate-early promoter/enhancer, as well as the neomycin phosphotransferase gene. The integrity of the plasmids was confirmed by extensive restriction enzyme digestion and ultimately by visualization of correctly sized proteins in cell lines.
Cell culture and transfection.
HL-60, KG1, and NB425 cells were cultured at 37°C in a humidified 5% CO2 incubator in RPMI 1640 supplemented with 10% fetal calf serum, 2 mmol/L L-glutamine, and penicillin/streptomycin. TF1 cells26 were cultured in the same medium plus 5 ng/mL granulocyte-macrophage colony-stimulating factor (GM-CSF) (complete medium). For transfections, 10 × 106 TF1 cells were washed, resuspended at 2 × 107 cells/mL in serum-free RPMI 1640, and incubated with 25 μg of linearized plasmid DNA at room temperature for 5 minutes. Cells were transfected by electroporation (300 V, 950 μF), incubated on ice for 15 minutes, and transferred to prewarmed complete medium. After 48 hours, geneticin (Life Technologies, Gaithersburg, MD) was added to a final concentration of 800 μg/mL (active). At this concentration, essentially 100% of mock-transfected TF1 cells died within 3 weeks. Populations were screened for expression of PML-RARα using reverse transcriptase-polymerase chain reaction (RT-PCR) as described,15 and were then subjected to limiting dilution cloning. The clonal lines were also screened for PML-RARα mRNA expression using RT-PCR and subsequently for PML-RARα protein expression by Western blot.
Cell proliferation assays.
Cell growth and viability were quantitated using a colorimetric assay that detects cleavage of the tetrazolium salt WST-1 to formazan by mitochondrial dehydrogenases present in viable cells (Boehringer Mannheim, Indianapolis, IN). The amount of water-soluble formazan dye correlates directly with the number of metabolically active cells and was quantified by measuring absorbance at 450 nm using a Dynatech 5000 microplate reader (Dynatech Laboratories, Chantilly, VA). In some experiments, proliferation was assessed using standard 3H thymidine assays; excellent correlation was observed between the colorimetric and 3H thymidine assays.
Western blotting.
Total cell extract was prepared by lysing washed, precooled cells in ice-cold RIPA buffer (1× phosphate-buffered saline [PBS], 1% NP-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate) containing the following protease inhibitors added immediately before cell lysis: pefabloc (1.0 mg/mL); EDTA (1 mmol/L); leupeptin (25 μg/mL); pepstatin (10 μg/mL); and aprotinin (2.5 μg/mL). The protease inhibitors were purchased from Boehringer Mannheim. The lysate was incubated on ice for 20 minutes, then centifuged at 15,000gfor 30 minutes at 4°C. The supernatant (total cell lysate) was recovered and stored at −20°C. Protein concentration was determined using the BioRad protein assay kit (BioRad, Hercules, CA) according to the manufacturer's instructions. Equal amounts of total cell protein were electrophoresed using standard procedures and transferred to Hybond-ECL nitrocellulose (Amersham, Arlington Heights, IL). Membranes were blocked in tris-buffered saline containing 5% dried milk and 0.1% Tween 20, then incubated with primary and (horseradish peroxidase–conjugated) secondary antibodies. Detection of immunoreactive bands was performed using enhanced chemiluminescence and exposure to Hyperfilm-ECL (Amersham).
Antibodies.
The following antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA) and used at dilutions suggested by the supplier or at empiric dilutions determined by experimentation: rabbit polyclonal anti-RARα (cat. no. sc-551); mouse monoclonal anti–Bcl-2 (cat. no. sc-509); rabbit polyclonal anti-MEK2 (cat. no. sc-524); goat polyclonal anti-actin (cat. no. sc-1616); rabbit polyclonal anti-Bax (cat. no. sc-930); and goat polyclonal anti-Bcl-xS/L (cat. no. sc-634-G).
Apoptosis assays.
Two different flow cytometric assays were used to detect and quantitate apoptosis. Details of the TUNEL assay (see Fig 4) have been published.27 Briefly, cells were resuspended at 2 × 106 cells/mL and fixed for at least 18 hours at 4°C in PBS, pH 7.1, containing 5% ultrapure formaldehyde (Polysciences Inc, Warrington, PA). For end-labeling 3′ DNA strand breaks, cells (1 × 106) were washed in 1× TdT buffer (200 mmol/L sodium cacodylate, 25 mmol/L Tris-HCl, 250 μg/mL bovine serum albumin fraction V) and subsequently incubated for 30 minutes at 37°C in 1× TdT buffer plus 2.5 mmol/L CoCl2, 0.0125 nmol fluorescein-12-dUTP, and 12.5 U terminal deoxynucleotidyl transferase (TdT; all purchased from Boehringer Mannheim). After washing, samples were analyzed on a FACScan flow cytometer (Becton Dickinson, San Jose, CA) equipped with a 488-nm argon excitation laser. Fluorescein-12-UTP was detected using a 530/30-nm bandpass filter (FL1 channel). In the second assay (see Fig 5), apoptosis was quantitated by measuring membrane redistribution of phosphatidylserine, as detected by binding to fluorescein isothiocyanate (FITC)-conjugated Annexin-V antibody.28 Briefly, 1 × 106 cells were washed with PBS and incubated for 10 minutes in 200 μL binding buffer containing FITC-conjugated Annexin-V antibody (10 μL of 20 μg/mL stock) and propidium iodide (10 μL of 50 μg/mL stock). Reagents for use in this assay were purchased as a kit (ApoAlert Annexin V apoptosis kit; Clontech Laboratories, Palo Alto, CA). Cells were analyzed by flow cytometry at 488 nm as discussed above.
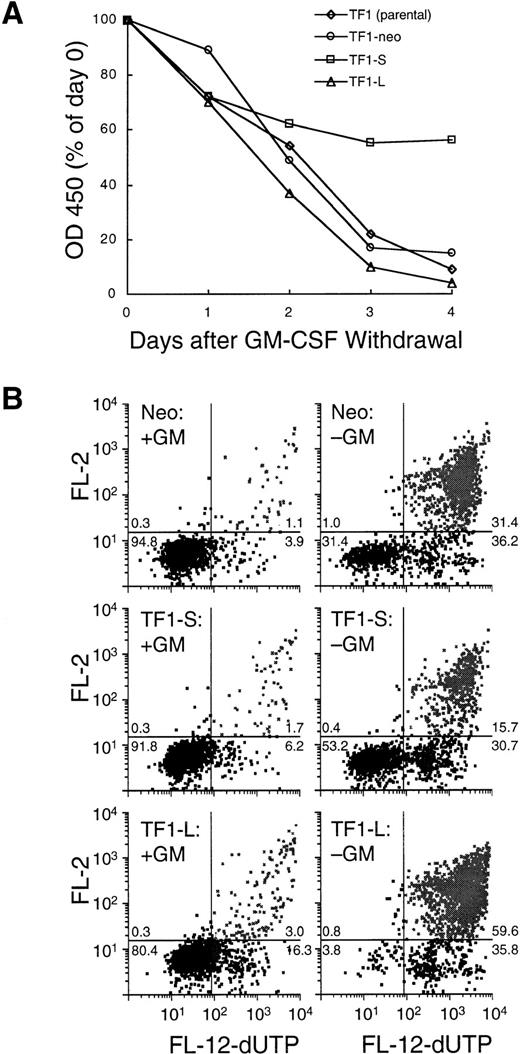
(A) Growth and viability in the absence of GM-CSF. TF1 parental cells, TF1-neo clones (n = 4), TF1-S clones (n = 9), and TF1-L clones (n = 8) were washed extensively and replated at 2.5 × 105 cells/mL in medium lacking GM-CSF. Daily determinations of viable cell number were performed as in Fig 3. The final plotted value is the average for all clones tested. The day 0 value was set at 100%, and represents a colorimetric determination performed 1 hour after plating. (B) Apoptosis determination using a TUNEL assay (see Materials and Methods) after 72 hours of culture in the presence (left panels) or absence (right panels) of GM-CSF. Top panels: TF1-neo; middle panels: TF1-S; bottom panels: TF1-L. Viable cells are represented in the left lower quadrant of each panel, early (live) apoptotic cells are in the right lower quadrant, and late-stage apoptotic (dead) cells are displayed in the right upper quadrant. The number in each quadrant represents the percent of events in that quadrant.
(A) Growth and viability in the absence of GM-CSF. TF1 parental cells, TF1-neo clones (n = 4), TF1-S clones (n = 9), and TF1-L clones (n = 8) were washed extensively and replated at 2.5 × 105 cells/mL in medium lacking GM-CSF. Daily determinations of viable cell number were performed as in Fig 3. The final plotted value is the average for all clones tested. The day 0 value was set at 100%, and represents a colorimetric determination performed 1 hour after plating. (B) Apoptosis determination using a TUNEL assay (see Materials and Methods) after 72 hours of culture in the presence (left panels) or absence (right panels) of GM-CSF. Top panels: TF1-neo; middle panels: TF1-S; bottom panels: TF1-L. Viable cells are represented in the left lower quadrant of each panel, early (live) apoptotic cells are in the right lower quadrant, and late-stage apoptotic (dead) cells are displayed in the right upper quadrant. The number in each quadrant represents the percent of events in that quadrant.
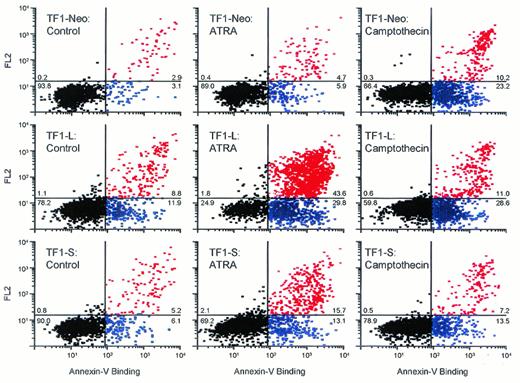
Apoptosis in response to ATRA or camptothecin. TF1-neo cells (top panels) and TF1 cells expressing the type L (middle panels) or S (lower panels) PML-RARα isoforms were plated at 1 × 105 cells/mL in complete medium (+GM-CSF) and cultured with vehicle alone (left panels), ATRA (10−6 mol/L, 96 hours; middle panels), or camptothecin (0.15 mol/L, 16 hours; right panels). Apoptosis was evaluated by flow cytometry after staining cells with an FITC-conjugated Annexin-V antibody (1 μ/mL) and propidium iodide (2.5 μg/mL) as discussed in Materials and Methods. Black dots, viable cells; blue dots, early stage (live) apoptotic cells; red dots, late stage (dead) apoptotic cells. The percentages of events in each quadrant are shown.
Apoptosis in response to ATRA or camptothecin. TF1-neo cells (top panels) and TF1 cells expressing the type L (middle panels) or S (lower panels) PML-RARα isoforms were plated at 1 × 105 cells/mL in complete medium (+GM-CSF) and cultured with vehicle alone (left panels), ATRA (10−6 mol/L, 96 hours; middle panels), or camptothecin (0.15 mol/L, 16 hours; right panels). Apoptosis was evaluated by flow cytometry after staining cells with an FITC-conjugated Annexin-V antibody (1 μ/mL) and propidium iodide (2.5 μg/mL) as discussed in Materials and Methods. Black dots, viable cells; blue dots, early stage (live) apoptotic cells; red dots, late stage (dead) apoptotic cells. The percentages of events in each quadrant are shown.
Cell-cycle analysis.
Cells were stained with propidium iodide (50 μg/mL) in the presence of RNase A (1 mg/mL) and analyzed by flow cytometry as described.29
Statistical analysis.
The Student's t-test was used to test for statistically significant differences between groups.
RESULTS
Generation of cell lines expressing the short (S) and long (L) isoforms of PML-RARα.
Complementary DNAs encoding the type S and L isoforms of PML-RARα (see Fig 1) were subcloned into the mammalian expression vector pCIneo. These plasmids, as well as the pCIneo vector itself, were transfected into the human factor-dependent erythroleukemia cell line, TF126; single-cell clones (confirmed by Southern blotting) were derived by limiting dilution in the presence of both GM-CSF (5 ng/mL) and geneticin (800 μg/mL). These lines are hereafter referred to as TF1-S (clones expressing the PML-RARα S isoform), TF1-L (clones expressing the PML-RARα L isoform), and TF1-neo (TF1 clones containing vector only).
Nine randomly chosen clones expressing either the PML-RARα S or L isoform were expanded and characterized. In addition, 6 TF1-neo clones were derived and used as controls. Expression of exogenous PML-RARα and endogenous RARα proteins was demonstrated using a polyclonal RARα antibody (Fig 2), which detects both endogenous RARα (E; approximately 55 kD), as well as the hybrid PML-RARα proteins (Fig 2: L-isoform, approximately 110 kD; S isoform, approximately 95 kD). The level of expression of endogenous RARα was similar in all TF1-neo (data not shown), TF1-S, and TF1-L clones (Fig2). The PML-RARα S isoform was expressed at a higher level than the L isoform in 8 of the 9 clones (Fig 2). This was not a technical artifact, because similar results were seen with different electrophoretic and transfer conditions (data not shown); stripping and reprobing the membranes with two different control antibodies confirmed equivalence of loading and transfer (Fig 2).
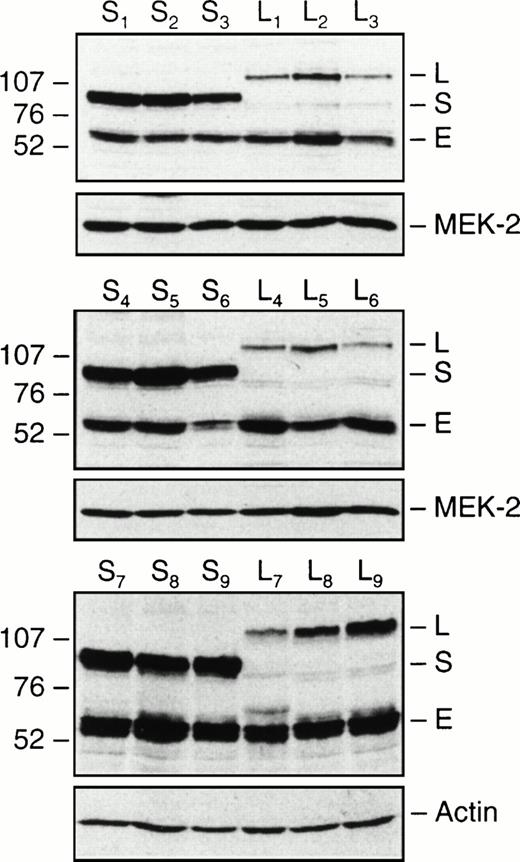
Expression of PML-RARα in TF1-S and TF1-L clones. Equal amounts of total cellular protein from nine independent TF1-S and TF1-L clones were immunoblotted with an antibody to human RARα, as detailed in Materials and Methods. Molecular weights are indicated to the left of the gel in kilodaltons (×10−3). The polyclonal RARα antibody detects both endogenous RARα (E, at approximately 55 kD), as well as the fusion PML-RARα proteins (S isoform, approximately 95 kD; L isoform, approximately 110 kD). The blots were stripped and reprobed with either MEK-2 or actin antibodies as a control for differences in loading and transfer.
Expression of PML-RARα in TF1-S and TF1-L clones. Equal amounts of total cellular protein from nine independent TF1-S and TF1-L clones were immunoblotted with an antibody to human RARα, as detailed in Materials and Methods. Molecular weights are indicated to the left of the gel in kilodaltons (×10−3). The polyclonal RARα antibody detects both endogenous RARα (E, at approximately 55 kD), as well as the fusion PML-RARα proteins (S isoform, approximately 95 kD; L isoform, approximately 110 kD). The blots were stripped and reprobed with either MEK-2 or actin antibodies as a control for differences in loading and transfer.
Expression of PML-RARα inhibits growth of TF1 cells and confers responsiveness to the growth inhibitory effects of ATRA.
Growth of TF1-neo, TF1-S, and TF1-L clones was assessed using a colorimetric cell proliferation assay; at each time point, the values for independent clones were averaged, and composite growth curves were constructed as shown in Fig 3A. The growth of TF1-neo clones was indistinguishable from that of parental TF1 cells (data not shown), and was also similar to that of the TF1-S clones (Fig3A). After 96 hours of culture, there were significantly fewer TF1-L cells compared with either TF1-S or TF1-neo cells (Fig 3A; P = .003, comparing mean optical density [OD] value of L clones to neo controls at 96 hours. The difference between TF1-S and TF1-L viable cell number at 96 hours was also statistically significant (Fig 3A; P = .004). Thus, in this system, PML-RARα functioned as an isoform-specific inhibitor of cell growth.
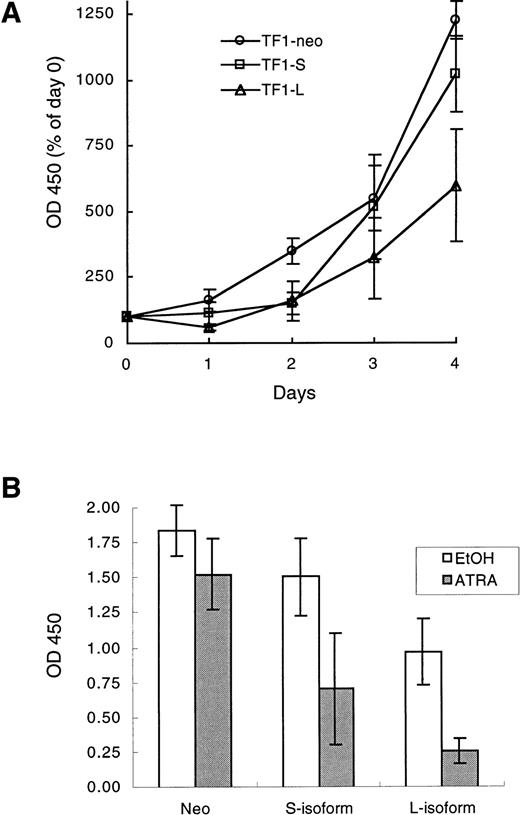
(A) Growth curves of TF1-neo, TF1-S, and TF1-L clones. Cells were plated at 7 × 104 cells/mL in 96-well plates in complete medium (GM-CSF at 5 ng/mL). Viable cell number was determined daily using a colorimetric assay (see Materials and Methods) with the day 0 value set at 100%. The results are the averages (±SEM) of values for 3 TF1-neo, 6 TF1-S, and 5 TF1-L clones. (B) Growth inhibition in response to ATRA. Cells were plated in 96-well plates at 5,000 cells/well in 100 μL of complete medium, and cultured for 96 hours in the presence of ATRA (10−6 mol/L) or an equal volume of vehicle control (ethanol, EtOH). Viable cell number at 96 hours was quantitated as in (A). The final plotted value is the average (±SEM) for 4 TF1-neo, 9 TF1-S, and 8 TF1-L clones.
(A) Growth curves of TF1-neo, TF1-S, and TF1-L clones. Cells were plated at 7 × 104 cells/mL in 96-well plates in complete medium (GM-CSF at 5 ng/mL). Viable cell number was determined daily using a colorimetric assay (see Materials and Methods) with the day 0 value set at 100%. The results are the averages (±SEM) of values for 3 TF1-neo, 6 TF1-S, and 5 TF1-L clones. (B) Growth inhibition in response to ATRA. Cells were plated in 96-well plates at 5,000 cells/well in 100 μL of complete medium, and cultured for 96 hours in the presence of ATRA (10−6 mol/L) or an equal volume of vehicle control (ethanol, EtOH). Viable cell number at 96 hours was quantitated as in (A). The final plotted value is the average (±SEM) for 4 TF1-neo, 9 TF1-S, and 8 TF1-L clones.
ATRA treatment (10−6 mol/L for 96 hours) of parental TF1 cells (data not shown) or TF1-neo clones caused minimal inhibition of cell growth (OD 450, 84% ± 15% of control; P = .13; Fig3B). This was not due to lack of expression of RARα, because endogenous RARα was abundantly expressed in these cells (eg, see Fig7; and data not shown). In cells expressing the PML-RARα S isoform, viable cell number was decreased by ATRA to 47% ± 24% of control (Fig 3B; P = .015 compared with TF1-neo cells), while in TF1-L cell lines, viable cell number after ATRA treatment was reduced to 27% ± 8% of control (Fig 3B; P < .001 compared with TF1-neo cells). The difference in response to ATRA between clones expressing the type L and type S PML-RARα isoforms was significant (P = .04). The effect of ATRA on cell-cycle status was also examined in representative TF1-neo, TF1-S, and TF1-L clones. There was a small increase in the percentage of cells in G2 in the TF1-S and TF1-L clones treated with ATRA for 96 hours (from 4.6% to 7.1% and 5.7% to 9.7%, respectively), which was accompanied by a slight decrease in the percentage of cells in S-phase in each case (data not shown). There were no significant changes in cell-cycle parameters in TF1-neo cells treated with ATRA in a similar fashion (data not shown).
Regulation of RARα and PML-RARα by ATRA. (A) The indicated cell types were treated with vehicle control (ethanol [−]) or 10−6 mol/L ATRA (+) for 96 hours; total cellular protein was obtained, and equal amounts were electrophoresed and immunoblotted successively with RARα (top panel) and actin (bottom panel) antibodies. Molecular weight markers are indicated to the left of the gel (in kilodaltons × 10−3). The migration of the PML-RARα L isoform (L), PML-RARα S isoform (S), and endogenous RARα (E) are indicated. (B) Quantitation of the results shown in (A) including data from additional independent experiments. The control level of expression of either endogenous RARα or PML-RARα was set at 100% in each case, and the relative level of expression of these proteins in response to ATRA was quantitated by densitometric analysis of Western blots such as those shown in (A). Values were corrected for differences in loading and transfer by incorporating the actin control in the calculation.
Regulation of RARα and PML-RARα by ATRA. (A) The indicated cell types were treated with vehicle control (ethanol [−]) or 10−6 mol/L ATRA (+) for 96 hours; total cellular protein was obtained, and equal amounts were electrophoresed and immunoblotted successively with RARα (top panel) and actin (bottom panel) antibodies. Molecular weight markers are indicated to the left of the gel (in kilodaltons × 10−3). The migration of the PML-RARα L isoform (L), PML-RARα S isoform (S), and endogenous RARα (E) are indicated. (B) Quantitation of the results shown in (A) including data from additional independent experiments. The control level of expression of either endogenous RARα or PML-RARα was set at 100% in each case, and the relative level of expression of these proteins in response to ATRA was quantitated by densitometric analysis of Western blots such as those shown in (A). Values were corrected for differences in loading and transfer by incorporating the actin control in the calculation.
The PML-RARα S isoform, but not the L isoform, partially protects TF1 cells from apoptosis in response to growth factor withdrawal.
To evaluate the effect of exogenously expressed PML-RARα on cell number and viability in response to growth factor withdrawal, TF1-neo, TF1-S, and TF1-L clones were washed and cultured for 96 hours in the absence of GM-CSF. As shown in Fig 4A, most TF1-S clones remained relatively viable after 4 days of growth factor deprivation (OD 450, 56% ± 35% of day 0 value; range, 8.5% to 124%), whereas TF1-L clones uniformly died (OD 450, 4% ± 3% of day 0 value; range, 1.8% to 11%; P < .001 compared with S clones). TF1-neo clones showed an intermediate pattern of cell viability (OD 450, 15% ± 7% of day 0 value; range, 8.6% to 25%), similar to that of parental TF1 cells (9.1%). The difference in viable cell number between TF1-L and TF1-neo clones was statistically significant (P < .001). To confirm that the loss of viability was due to apoptosis, cells were cultured for 72 hours in the presence or absence of GM-CSF and apoptosis was assessed using a flow cytometric assay based on incorporation of fluorescently labeled dUTP into DNA strand breaks.27 After 72 hours of growth factor deprivation, virtually all (95.4%) TF1-L cells had begun to undergo, or had completed, apoptosis, compared with only 46% of TF1-S cells (Fig 4B; compare middle and lower panels). The corresponding percentages of live, nonapoptotic cells after 72 hours of culture in the absence of GM-CSF were 4.6% for TF1-L and 53.6% for TF1-S (Fig4B). For TF1-neo cells, the percentage of apoptotic cells after 72 hours of growth factor deprivation was 67.6% (Fig 4B, top right panel).
ATRA treatment of PML-RARα-expressing, but not control, cells causes apoptosis.
To evaluate the effects of ATRA on apoptosis in PML-RARα–expressing and control cells, TF1-neo, TF1-L, and TF1-S clones were treated with vehicle control (ethanol) or ATRA (10−6 mol/L) for 96 hours. As a positive control, cells were also treated with the topoisomerase I inhibitor camptothecin (0.15 mol/L, 16 hours), a known inducer of apoptosis in hematopoietic cell lines.30Apoptosis was evaluated by flow cytometry after staining cells with an FITC-labeled antibody to Annexin-V.28 Under control conditions (Fig 5, left panels) there was a small but significant degree of spontaneous apoptosis in the TF1-L cells (20.7% apoptotic) and, to a lesser extent, in the TF1-S cells (11.3% apoptotic, v 6% spontaneous apoptosis in the TF1-neo cells). On a relative basis, the TF1-neo cells were most sensitive to camptothecin (Fig 5; percent apoptotic/dead cells increased from 6.0% [control] to 33.4% [camptothecin-treated]). For both TF1-L and TF1-S, the percent apoptotic/dead cells increased by approximately twofold in response to camptothecin (Fig 5; TF1-L 20.7% to 39.6%; TF1-S 11.3% to 20.7%). The final percentage of apoptotic cells was similar for TF1-neo and TF1-L (33.4% and 39.6%, respectively), but somewhat lower for the TF1-S cells (20.7%). Thus, camptothecin caused apoptosis in all three cell types, although expression of either PML-RARα isoform may have afforded a small degree of protection. In contrast, ATRA caused significant apoptosis only in cells expressing PML-RARα, and the degree of apoptosis in response to ATRA was much more marked in TF1-L (73.4% apoptotic) than in TF1-S cells (28.8% apoptotic; Fig 5, middle panels). Eighty-nine percent of TF1-neo cells remained nonapoptotic after ATRA treatment, versus 24.9% of TF1-L and 69.2% of TF1-S cells. These results were qualitatively confirmed using DNA laddering assays and different TF1-neo, L, and S clones (data not shown).
ATRA decreases bcl-2 expression in a PML-RARα–dependent fashion.
bcl-2 is known to protect a variety of cell types, including myeloid cells, from apoptosis.31 To explore the relationship, if any, between bcl-2 expression and PML-RARα, bcl-2 levels were measured by immunoblotting in multiple TF1-neo, TF1-S, and TF1-L clones. The basal level of bcl-2 expression in TF1-neo clones was essentially identical to that seen in parental TF1 cells grown under the same conditions (data not shown). Basal bcl-2 levels were approximately twofold higher in TF1-S clones compared with neo controls (Fig 6A and B). In contrast, basal bcl-2 expression in TF1-L clones was significantly lower than that seen in TF1-neo cells (average, 34% of the level in TF1-neo clones; Fig 6A and B). When TF1-neo clones were treated with ATRA (10−6mol/L, 96 hours), there was no significant change or, in some experiments, a slight increase in the level of bcl-2 protein (Fig 6). However, identical ATRA treatment of TF1-S and TF1-L clones resulted in a significant decline in bcl-2 protein levels. In TF1-L cells, there was a sixfold decrease in bcl-2 protein levels in response to ATRA, whereas in TF1-S cells, the decline in bcl-2 in response to ATRA was approximately threefold (from a higher baseline; see Fig 6B and the representative Western blot in Fig 6A). The regulation of bcl-2 by ATRA in these cells occurred at a pretranslational level, as documented by Northern blot analysis (data not shown). ATRA treatment had no effect on expression of bcl-x or bax in TF1-neo, TF1-S, or TF1-L clones (Fig6A).
Expression of bcl-2, bax, and bcl-x in response to ATRA in TF1-neo, TF1-L, and TF1-S cells. (A) Cells were treated with vehicle alone (ethanol [−]) or ATRA (10−6 mol/L; [+]) for 96 hours; equal amounts of total cellular protein were electrophoresed and immunoblotted successively with antibodies to bcl-2, bcl-x, or bax. The blots were then reprobed with an actin antibody to control for variations in loading and transfer. A representative Western blot is shown. (B) Quantitation of data from four independent experiments with two different TF1-neo, TF1-S, and TF1-L clones. Shown is the average level of bcl-2 expression (±SEM) under control conditions (EtOH; ▪) or after 96 hours of ATRA treatment (ATRA; □), compared in all cases to the bcl-2 expression level in TF1-neo clones under control conditions (value arbitrarily set at 100%). Values were corrected for differences in loading and transfer by incorporating the actin control in the calculation.
Expression of bcl-2, bax, and bcl-x in response to ATRA in TF1-neo, TF1-L, and TF1-S cells. (A) Cells were treated with vehicle alone (ethanol [−]) or ATRA (10−6 mol/L; [+]) for 96 hours; equal amounts of total cellular protein were electrophoresed and immunoblotted successively with antibodies to bcl-2, bcl-x, or bax. The blots were then reprobed with an actin antibody to control for variations in loading and transfer. A representative Western blot is shown. (B) Quantitation of data from four independent experiments with two different TF1-neo, TF1-S, and TF1-L clones. Shown is the average level of bcl-2 expression (±SEM) under control conditions (EtOH; ▪) or after 96 hours of ATRA treatment (ATRA; □), compared in all cases to the bcl-2 expression level in TF1-neo clones under control conditions (value arbitrarily set at 100%). Values were corrected for differences in loading and transfer by incorporating the actin control in the calculation.
Regulation of PML-RARα and endogenous RARα by ATRA.
It has been suggested22 that ATRA contributes to the cure of APL by selectively downregulating the expression of PML-RARα, with little or no effect on endogenous RARα. To address this issue in the current model system, TF1 parental cells, TF1-neo clones, and TF1-S and -L clones were treated with ATRA (10−6 mol/L for 96 hours) and the level of expression of both endogenous RARα and transfected PML-RARα was assayed by Western blot (Fig7). Control experiments were performed with the cell lines KG1 (a non-APL human myeloid cell line) and NB4 (a human promyelocytic cell line which expresses the PML-RARα L isoform). The Western blots were quantitated by densitometry, and values for relative PML-RARα and/or endogenous RARα expression were normalized for actin expression. In KG1 (data not shown), TF1 parental (data not shown), and TF1-neo cells, treatment with ATRA had a minimal effect on the level of expression of endogenous RARα (Fig 7A and B). In contrast, endogenous RARα was consistently downregulated in all PML-RARα–expressing cell lines (TF1-S, TF1-L, NB4) in response to ATRA (Fig 7A and B). The extent of this decrease in endogenous RARα expression was similar in all three PML-RARα–expressing cell types (TF1-S clones: 17% of control, range 7% to 31% in three experiments; TF1-L clones: 13% of control, range 3.5% to 22% in four experiments; NB4 cells: 10% of control, range 2.9% to 17% in two experiments). In NB4 cells, the endogenous type L PML-RARα protein was also downregulated by ATRA (average 13% of control level in two experiments; Fig 7A and B), in agreement with Raelson et al.22 In TF1-L clones, there was some variability in the level of expression of exogenous PML-RARα in response to ATRA; however, averaging four experiments in which quantitative values were obtained after controlling for actin expression, the relative level of PML-RARα (L) expression after ATRA treatment was 63% of control (range, 36% to 117%; see Fig 7A and B). The relative level of exogenous PML-RARα (S) expression after ATRA treatment was 88% of control (range, 76% to 103%; Fig 7A and B).
DISCUSSION
One of the major aims of this study was to define biologic differences, if any, between the type S and type L PML-RARα isoforms in vitro. To achieve this goal, the two PML-RARα isoforms were constitutively expressed in TF1 cells, a human erythroleukemia cell line that is resistant to the differentiative and antiproliferative effects of ATRA.26 It is noteworthy that the type S PML-RARα isoform was expressed at significantly higher levels that the type L isoform in 8 of the 9 clones examined (Fig 2). This finding is likely due to differences in the physiologic properties of the two PML-RARα molecules in TF1 cells because our data show that the PML-RARα type L isoform, even when expressed at a lower level than the S isoform, inhibited growth and promoted apoptosis to a much greater degree. Thus, it is probable, although not experimentally proven, that TF1 cells can express only a finite amount of the PML-RARα L isoform and remain viable and clonable. This interpretation is consistent with the difficulty other investigators have encountered in establishing cell lines which constitutively express PML-RARα, and with our own difficulty in constitutively expressing the PML-RARα L isoform in other hematopoietic cell lines, including HL-60.
There are significant structural differences between the PML-RARα S and L isoforms which may account for the observed differences in biological activity reported in the present study. The two major structural motifs missing in the S isoform are a serine-proline rich region and a putative nuclear localization signal (NLS), both encoded by PML exon 6 (see Fig 1). The importance of these and other structural elements to the subcellular location and function of the PML protein has been examined in 3T3 cells.32 In that system, a mutant PML protein which lacked the NLS was localized to the cytoplasm, did not participate in nuclear body formation, and was functionally inactive.32 In contrast, a mutant PML protein that lacked the serine-proline rich region was found in the nucleus in normal-appearing nuclear bodies, and was fully functional.32 Although some nuclear localization must be provided by the NLS in the RARα moiety, the PML-RARα S isoform may nevertheless be less able to enter the nucleus than the L isoform, and therefore less able to transmit a retinoid-mediated signal; this hypothesis is supported by experimental immunofluorescence data,24 and by data presented in the present report (Figs3B and 5) showing that the S isoform, despite being expressed at a higher level than the L isoform, was less effective at transducing an ATRA-mediated growth inhibitory/apoptotic signal. Despite the differences in structure and in vitro biologic activity between the L and S PML-RARα isoforms, both molecules readily cause APL in humans. Furthermore, when cultured in vitro, blasts from patients with type S and type L APL were reported to differentiate to a similar degree in response to ATRA.33 However, it is interesting to note that there are several clinical differences between APL patients who express either the S or L isoforms. For example, type S APL patients have a higher incidence of secondary chromosomal abnormalities,15and also present with higher median WBC and peripheral blast counts.13 However, after correcting for presenting WBC count, type S and type L APL patients have essentially identical clinical outcomes.13 16 In summary, the combined clinical and in vitro data suggest that there are subtle biologic differences between the L and S isoforms of PML-RARα, but that these differences do not affect clinical outcome in patients who receive aggressive, modern treatment with ATRA/anthracycline combination therapy.
In the absence of ATRA (or other retinoids), PML-RARα has been reported to inhibit myeloid cell differentiation17-20,34and to suppress or delay apoptosis.17,35,36 These effects have generally been relieved by the addition of ATRA, and PML-RARα has been reported to specifically enhance retinoic-acid induced differentiation of U937 cells.17 The growth inhibitory and pro-apoptotic properties of the PML-RARα L isoform reported here, even in the absence of ATRA, are somewhat difficult to reconcile with a previous study,35 and with the presumed oncogenic function of PML-RARα, but are consistent with another previous report which examined PML-RARα expression in lymphoid cell lines.37 In contrast to data reported by Rogaia et al,35 we observed no protection from growth factor withdrawal-induced apoptosis in TF1 cells expressing the PML-RARα L isoform. However, cells expressing the S isoform were partially protected from apoptosis induced by growth factor withdrawal, and underwent less apoptosis (compared with TF1-neo or TF1-L cells) in response to camptothecin; these results confirm that PML-RARα may have anti-apoptotic activity, but in the present system this activity is seen primarily with the S isoform. The data reported here with the S isoform are consistent with those previously reported by Fu et al,36 who also observed protection from apoptosis after expression of the PML-RARα S isoform in TF1 cells. Although it is possible that the difference between isoforms observed in the present study is due to the consistently higher expression levels of the S isoform in the TF1 clones, this seems unlikely, because the presence of the PML-RARα L isoform actually accelerated apoptosis in response to growth factor withdrawal (Fig 4), and clearly led to decreased cell growth and viability even under ideal growth conditions (Fig 3). Thus, it seems unlikely that higher levels of expression of the L isoform would confer a growth advantage to these cells. The reasons for the differences between the results reported here and those of Rogaia et al35 are therefore unknown, but may be due to differences in experimental conditions (eg, different expression plasmids and potentially different methods to culture cells and isolate clonal cell lines).
In the presence of pharmacologic doses of ATRA (10−6mol/L), the type L (and to a lesser extent type S) PML-RARα protein mediated rapid and extensive programmed cell death (Fig 5), which was also accompanied by signs of erythroid differentiation (manuscript in preparation). Essentially no apoptosis was observed in control TF1-neo cells in response to ATRA. It should be emphasized that the degree of apoptosis of TF1-neo and TF1-L clones in response to camptothecin, a topoisomerase I inhibitor, was similar, if not somewhat higher for the TF1-neo cells (Fig 5). This result shows that the apoptosis seen in the TF1-L clones in response to ATRA is not due to a generalized increase in susceptibility to apoptosis, but rather is specific for the PML-RARα and RARα ligand ATRA. The combined results indicate that PML-RARα, particularly the L isoform, sensitizes cells to ATRA-induced, but not camptothecin-induced, apoptosis; we hypothesize that ATRA binds to the RARα moiety of PML-RARα and modulates the transcriptional activity of this protein, with activation (or repression) of as yet uncharacterized genes. The apoptotic effect of ATRA is not mediated by the normal, endogenous RARα, because parental TF1 cells and TF1-neo cells express RARα at high levels and yet do not undergo significant apoptosis in response to ATRA.
ATRA treatment of PML-RARα–expressing, but not control, TF1 cells caused a significant decrease in expression of bcl-2, but not bax or bcl-x (Fig 6). The effects of ATRA on bcl-2 levels and apoptosis in cells expressing PML-RARα and other myeloid cell types have been investigated by others.38-40 Calabresse et al38reported that ATRA treatment of fresh, cultured APL blasts resulted in a rapid decrease in bcl-2 expression, whereas no modulation of bcl-2 was observed in response to ATRA in non-APL cells; however, the fresh APL cells did not undergo apoptosis in response to ATRA alone.38 Treatment of NB4 cells (a patient-derived PML-RARα–positive APL cell line) with ATRA also leads to a significant decrease in bcl-2 expression39 (and our unpublished observations, November 1996). Although bcl-2 can be downregulated by retinoids in myeloid cells that do not express PML-RARα,40,41 the data presented by Calabresse et al38 and in the current report strongly suggest that PML-RARα facilitates bcl-2 downregulation by ATRA and suggests that bcl-2 is a direct molecular target of retinoids in PML-RARα–positive cells. Because bcl-2 can protect cells from chemotherapy-induced apoptosis,42 it is possible, as suggested in other systems,40,43 that the decrease in bcl-2 levels in response to ATRA in APL blasts and other PML-RARα–expressing cells sensitizes these cells to the subsequent apoptotic effects of chemotherapy. Thus, the ability of PML-RARα to facilitate bcl-2 downregulation in response to ATRA, with a consequent increased susceptibility to chemotherapy-induced apoptotic cell death, may explain why ATRA and other RARα agonist retinoids44,45 are effective clinical agents in PML-RARα–positive APL, but not in other (PML-RARα–negative) AML subtypes. If downregulation of bcl-2 expression is critical to the effectiveness of chemotherapeutic and/or differentiative agents in AML and APL, then alternative methods of bcl-2 inhibition, such as the use of antisense oligodeoxynucleotides,43 46 may offer alternative strategies of treatment in AML patients or APL patients who are resistant to ATRA.
The effectiveness of ATRA in APL depends on the presence of the PML-RARα protein.1,4 One model to explain the success of ATRA in APL is suggested by recent studies which demonstrate that the PML-RARα protein is significantly and rapidly downregulated by ATRA, apparently at a posttranslational level.21,22 Because PML-RARα can inhibit myeloid differentiation,17-20 its downregulation by ATRA could allow normal differentiation to occur and lead to clinical complete remission, as seen both in vitro and in vivo. In this model, binding of ATRA to the RARα moiety may lead to a change in conformation of the PML-RARα molecule that results in its specific destruction via the proteasome pathway21; by extension, ATRA binding to PML-RARα would not necessarily directly activate expression of genes involved in myeloid differentiation or apoptosis. An alternative model suggests just the opposite, ie, that PML-RARα, on binding ATRA, becomes an active inducer of myeloid differentiation and apoptosis, presumably by activating or inhibiting expression of specific genes or gene programs. Results from the present study offer support primarily for the latter model. We have confirmed, as reported by others,22 that the PML-RARα protein is downregulated by ATRA in NB4 cells, but our results suggest that the endogenous RARα protein is downregulated to a similar degree (Fig 7); thus, there was no net change in the ratio of PML-RARα to RARα after ATRA treatment. Furthermore, we observed little (L-isoform) to essentially no (S-isoform) downregulation of exogenously expressed PML-RARα protein in response to ATRA. These results call into question the generality of a posttranslational pathway for downregulation of PML-RARα in response to ATRA. Finally, it is notable that a consistent and striking downregulation of endogenous RARα in response to ATRA was observed only in cells that expressed PML-RARα. This result suggests that endogenous RARα is a molecular target of ATRA in PML-RARα–positive cells.
Overall, our results support a model in which PML-RARα, in the presence of ATRA, inhibits cell growth, facilitates differentiation, and induces apoptosis. The apoptotic response appears to be general, because PML-RARα can mediate ATRA-induced apoptosis in lymphoid37 as well as erythroid cell lines (Fig 5). Identification of target genes that are modulated by ATRA specifically in PML-RARα–expressing cells will be an important avenue of further study, and at least one such gene, transglutaminase II, has been identified.47 Identification and functional characterization of such genes may lead to a fuller understanding of APL, and this knowledge may lead to the development of novel strategies for the treatment of both resistant APL and other AML subtypes. Ultimately, it is hoped that the knowledge gained in the study of APL, particularly the mechanisms by which this disease is sensitive to retinoids, can be translated to other AML subtypes, in which cure rates continue to be distressingly low.
ACKNOWLEDGMENT
We thank Earl Timm, Carlton Stewart, and Sigrid Stewart for help with flow cytometric assays and analysis of flow cytometric data.
Supported in part by grants from the National Cancer Institute to Roswell Park Cancer Institute.
Address reprint requests to James L. Slack, MD, Division of Medicine, Roswell Park Cancer Institute, Elm & Carlton Sts, Buffalo, NY 14263.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.


![Fig. 7. Regulation of RARα and PML-RARα by ATRA. (A) The indicated cell types were treated with vehicle control (ethanol [−]) or 10−6 mol/L ATRA (+) for 96 hours; total cellular protein was obtained, and equal amounts were electrophoresed and immunoblotted successively with RARα (top panel) and actin (bottom panel) antibodies. Molecular weight markers are indicated to the left of the gel (in kilodaltons × 10−3). The migration of the PML-RARα L isoform (L), PML-RARα S isoform (S), and endogenous RARα (E) are indicated. (B) Quantitation of the results shown in (A) including data from additional independent experiments. The control level of expression of either endogenous RARα or PML-RARα was set at 100% in each case, and the relative level of expression of these proteins in response to ATRA was quantitated by densitometric analysis of Western blots such as those shown in (A). Values were corrected for differences in loading and transfer by incorporating the actin control in the calculation.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/9/10.1182_blood.v91.9.3347/3/m_blod40928007w.jpeg?Expires=1767794259&Signature=BgM920KuNaHmpYdak1X9NYSmIb7snWGitvM8ku4hDuF~SlmHJbwlF5tLFrzTvgxUCFANrOlDu2Q3FEPUUE7USTFlaG-BiecTbMgtxrZ~NpDymbbyOR897OAZYOutVBXTz7Chh~qFi9Fit8onIzyOsMW7dV-SpPmaJyI8aWHuG~7-T7JJB~ag0d06Qf2kF1WyH3WWIQrgZZEX6rDMDW7eu1QWDlsW38kCkT441PcMSyBwmyZbmEEIE68ObK1J15DiGRZ55-fILFLiP8-43q7EYJuU6N5GmEEPKb~Mxk4J3tjdjOby3zMUEDo9FzLUANbPzFaMu0vS-gSZBlCQARIVQQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. Expression of bcl-2, bax, and bcl-x in response to ATRA in TF1-neo, TF1-L, and TF1-S cells. (A) Cells were treated with vehicle alone (ethanol [−]) or ATRA (10−6 mol/L; [+]) for 96 hours; equal amounts of total cellular protein were electrophoresed and immunoblotted successively with antibodies to bcl-2, bcl-x, or bax. The blots were then reprobed with an actin antibody to control for variations in loading and transfer. A representative Western blot is shown. (B) Quantitation of data from four independent experiments with two different TF1-neo, TF1-S, and TF1-L clones. Shown is the average level of bcl-2 expression (±SEM) under control conditions (EtOH; ▪) or after 96 hours of ATRA treatment (ATRA; □), compared in all cases to the bcl-2 expression level in TF1-neo clones under control conditions (value arbitrarily set at 100%). Values were corrected for differences in loading and transfer by incorporating the actin control in the calculation.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/9/10.1182_blood.v91.9.3347/3/m_blod40928006w.jpeg?Expires=1767794259&Signature=zVJ8R6BejaiDwd8Idn06xcQ7TySqyy6qBKnGIb9Vt0wdbJy5YS-eaGtbGmzF9LvjJbJUUep~fycvu-O8ou-PuBqDC2DU519lk6hNBSesRBW9uVM3Lf~TR2SMKyNvBXGXRoCllJvh4foAty-69s91A08jbno3l8abup9Q8VF0R8~GC3HZx4WOKI55CxY93sSHjAkRHxLuAMwM7p5VaROUU~yK5VPUyMv0wc7oc5OaBxtZZGz0-5NG9jdwlJWnDe8sBkbr7yE8GG-vtwso5nIlZy9x7SkR7O4f98X1qJCV6r2yzPWzcfXZtHsZ~bjBLb86ayM4p~40tZysFu0eOMSs4g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal