Abstract
Positron emission tomography (PET) is a whole-body imaging technique using 18 fluorine-fluorodeoxyglucose (FDG), whose uptake is increased in tumor cells. Published studies have shown PET to be an effective method of staging lymphoma and to be more sensitive than CT at detecting extranodal disease. The purpose of this study was to determine whether the increased marrow uptake of FDG observed in some lymphoma patients during routine staging PET scans represented marrow involvement by disease. PET scans of 50 patients with Hodgkin's (12) and non-Hodgkin's (38) lymphoma were analyzed by three independent observers and the marrow graded as normal or abnormal using a visual grading system. Unilateral iliac crest marrow aspirates and biopsies were performed on all patients. The PET scan and marrow histology agreed in 39 patients (78%), being concordant positive in 13 and concordant negative in 26 patients. In 8 patients the PET scan showed increased FDG uptake but staging biopsy was negative; in 4 of these 8 patients the PET scan showed a normal marrow background with focal FDG “hot spots” distant from the site biopsied. In 3 patients the marrow biopsy specimen was positive but the PET scan normal; 2 of these 3 patients had non-Hodgkin's lymphoma whose malignant cells did not take up FDG at lymph node or marrow disease sites. Therefore, there were only 5 patients (10%) in whom there was a difference between the PET scan and biopsy result which could not be fully explained. Visual interpretation of marrow FDG uptake during whole-body staging PET scans can correctly assess marrow disease status in a high proportion of lymphoma patients. PET has the potential to reduce the need for staging marrow biopsy.
BONE MARROW (BM) BIOPSY is an important part of the routine staging of Hodgkin's disease (HD) and non-Hodgkin's lymphoma (NHL). BM involvement by lymphoma confers advanced-stage disease and may affect both treatment and prognosis. Histological evidence of lymphoma in the BM is found in approximately 50% to 80% of patients with low-grade NHL, 25% to 40% of high-grade NHL, and 5% to 14% of HD patients at diagnosis.1
The need for a staging marrow biopsy in all cases of lymphoma is the subject of ongoing debate and has been questioned by some investigators.2 Studies in NHL have shown that the marrow biopsy findings may not affect management in patients with advanced clinical stages of certain disease subtypes,3 while a recent United Kingdom survey revealed widely differing practices amongst hematologists and oncologists in their use of staging biopsy in HD.4 Two factors make the marrow trephine biopsy an unsatisfactory diagnostic test: it is a painful and invasive procedure and, even if the volume of the biopsy is adequate, focal lesions can be missed. Several large studies have consistently shown that a unilateral iliac crest trephine biopsy is an unreliable method of detecting marrow lymphoma, especially in high-grade NHL, where accurate staging is particularly important. These studies have shown lymphoma to be present in only one sample of paired bilateral iliac crest trephines in 22% to 30% of NHL cases when all histological grades were included, and this discrepancy between biopsy sites may occur in as many as 31% to 50% of high-grade NHLs.5-8 In young patients with lymphoblastic and large cell lymphoma, disease was present in only one sample in 33% of paired biopsies, when both were taken from the same iliac crest.7 Likewise in HD, unilateral marrow disease was found in 43% of cases.5 Against this background a more reliable, noninvasive method of detecting lymphoma in the marrow would be welcome.
Positron emission tomography (PET) is a whole-body imaging technique that uses positron emitting isotopes of biological elements, such as carbon, oxygen, nitrogen, and fluorine, for the functional assessment of perfusion and metabolism in vivo. The most common tracer used in oncology is the glucose analogue 18 fluorine fluorodeoxyglucose (FDG), whose uptake is increased into tumor cells, by virtue of their increased glucose transfer and glycolysis.9,10 Once inside the cell it is phosphorylated by hexokinase, but is then trapped because it is effectively unable to enter the subsequent glycolytic pathways. PET has an advantage over computed tomography (CT) in its ability to detect extranodal disease and to visualize tumor when there is no anatomical abnormality on imaging.11,12 The ability of PET to provide high-quality whole-body images of nodal and extranodal disease at a single scanning session means that its use is increasing for the primary staging, remission assessment, and treatment monitoring of lymphoma.13-17
Studies comparing PET with CT for staging lymphoma have consistently shown a high degree of concordance in identifying nodal disease and have shown PET to have greater sensitivity for detecting small or borderline nodes and extranodal soft-tissue disease.16,18Using PET as a first-line investigation in place of conventional imaging techniques may improve the accuracy of staging as well as being more cost effective. In one study of 18 patients with NHL and HD, PET scanning increased the disease stage in three patients by detecting lesions not previously identified by a variety of clinical and imaging techniques.17
The role of PET for assessing marrow disease has not been addressed and may further add to its value in staging lymphoma. We postulated that increased uptake of FDG in marrow might correlate with the presence of disease in patients at initial diagnosis. The purpose of this study was to determine whether the intensity and distribution of FDG uptake in the marrow could be used to accurately identify marrow infiltration.
MATERIALS AND METHODS
Patients.
Fifty consecutive patients with HD or NHL who were staged before treatment by both PET scan and BM biopsy were prospectively recruited into the study. The routine lymphoma staging procedure at our institution involves thoracic and abdominal CT scan, whole-body FDG-PET, and a unilateral iliac crest marrow aspirate and trephine biopsy. In all patients the PET scan was performed within 4 weeks of the marrow biopsy.
Marrow histology.
Marrow aspirates were stained with May-Grünwald-Giemsa. Trephine biopsy samples were decalcified and stained with hematoxylin and eosin and Gordon and Sweet's reticulin method. All biopsy samples were stained with CD20 for B cells and CD3 for T cells. Where appropriate, specimens were stained with CD15, CD30, κ and λ light chains, and IgG, IgA, IgM heavy chains. The marrow biopsy samples were examined for lymphoma infiltration by two hematologists and a histopathologist, blinded to the PET scan result. The NHLs were classified according to the Kiel and Revised European-American Lymphoma (REAL) classifications.19
PET scanning.
18-Fluoride was produced in a Siemens RDS 112 cyclotron (Siemens CTI, Knoxville, TN) by proton bombardment of a high-pressure water target. FDG was synthesized by the method of nucleophilic substitution of a precursor by 18 − F−. PET scans were performed after a 6-hour fast using an ECAT 951R whole body scanner (Siemens CTI). The patients were injected with 350 MBq of [18F]-FDG and imaged 30 to 45 minutes later with half-body images obtained by acquiring 10 consecutive 5-minute images from the skull base to the mid thigh. The complete sets of 310 image planes were reconstructed by filtered back projection and smoothed in the axial direction to obtain a single 3D dataset with a spatial resolution of 12 mm. Images were viewed as a series of transaxial, coronal, and sagittal volume images.
Analysis of FDG uptake by marrow.
For the purposes of this study, the intensity and distribution of FDG activity within the marrow was visually scored by three nuclear medicine physicians independently. The marrow was assumed to be abnormal where the uptake was equal to or greater than uptake into the liver, provided the liver uptake was greater than background. In one patient where there was negligible uptake within the liver, marrow uptake was compared to uptake within soft tissue rather than using the liver as the reference organ. The pattern of increased uptake was also noted, with patients who appeared to have focal disease only within the marrow differentiated from those with diffusely abnormal marrow changes. Representative examples of cases with marrow uptake of differing intensity (Fig 1) and distribution (Fig 2) are shown.
Coronal images are shown from three patients with different degrees of marrow uptake on PET. FDG uptake within the marrow is seen on these sections in the thoracic spine (broken arrows) and within the liver (solid arrow). The intensity of uptake was graded as less than liver (A), equal to liver (B), or greater than liver (C), with the marrow deemed to be abnormal where uptake was equal to or greater than liver.
Coronal images are shown from three patients with different degrees of marrow uptake on PET. FDG uptake within the marrow is seen on these sections in the thoracic spine (broken arrows) and within the liver (solid arrow). The intensity of uptake was graded as less than liver (A), equal to liver (B), or greater than liver (C), with the marrow deemed to be abnormal where uptake was equal to or greater than liver.
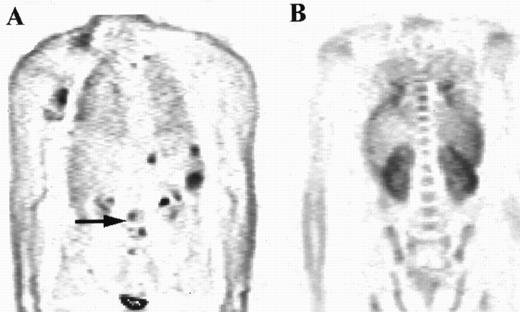
The distribution of uptake within the marrow was noted. Focal abnormality is seen within the lumbar spine in patient A (arrow), while patient B has diffuse marrow abnormalities.
The distribution of uptake within the marrow was noted. Focal abnormality is seen within the lumbar spine in patient A (arrow), while patient B has diffuse marrow abnormalities.
The kappa (κ) statistic20 was used to measure interobserver variability to assess the reproducibility of the visual method used to assess marrow disease on PET. Where there were differences in reporting between observers, if two reporters concurred this was taken as the final PET report.
RESULTS
Fifty patients with NHL (38) and HD (12) were recruited into the study. Forty-nine patients had lymph node, splenic, or soft-tissue extranodal lymphoma masses shown by CT or clinical examination. All but 2 of these 49 patients had their nodal and/or extranodal disease demonstrated by the FDG-PET scan. The 2 patients whose primary lymph node disease did not accumulate FDG are discussed below. The remaining patient, who had no clinical lymphadenopathy and a normal abdominal CT scan, had intense uptake of FDG within the peritoneum and bowel due to a diffuse infiltration by Burkitt's lymphoma cells. This patient has been previously reported.12
There were 29 PET scans in which the marrow was regarded as having normal FDG uptake. There were 21 PET scans in which the marrow was considered to be abnormal: 16 of these patients had diffusely abnormal marrow uptake, 3 of whom had focal areas of higher intensity within a diffusely abnormal marrow. Five patients (3 diffuse large B-cell NHL, 2 HD) had focal areas of abnormality only, with the remainder of the marrow showing normal FDG uptake.
Assessment of inter-observer agreement in interpretation of PET.
There was complete agreement between the three observers in 76% (22 of 29) of scans showing normal marrow uptake and 71% (15 of 21) of scans showing abnormal uptake, either in a focal or diffuse pattern. There was thus good overall agreement between observers, κ = 0.64.
In those patients who had PET scans that were discordant with biopsy histology, all three observers agreed on the classification of marrow uptake as normal or abnormal in 73% (8 of 11). Therefore, there is no evidence that discordant results were a consequence of PET scans that were more difficult to assess.
Patients with a concordant PET and routine iliac crest marrow biopsy result.
In 39 patients there was concordance between the PET scan and the routine iliac crest marrow biopsy (Table1). In 26 patients there was no increased FDG uptake within the marrow and the biopsy histology was normal. In 13 patients increased marrow FDG uptake was associated with histological evidence of marrow infiltration by lymphoma in the routine iliac crest biopsy (11 NHL, 2 HD). Two of these patients demonstrated the sensitivity of the PET technique: 1 patient with follicular centroblastic centrocytic lymphoma who had low-volume, nodular marrow disease (Fig 3A) and another patient with T-lymphoblastic lymphoma, who had only 15% blast cells on marrow aspirate and a modest interstitial infiltrate on trephine section (Fig3B). Both had the marrow disease identified by increased FDG uptake. Only 1 of the 5 patients with focal FDG uptake in an otherwise normal marrow had, by chance, an area of high FDG accumulation (“hot spot”) biopsied by the routine marrow trephine, which confirmed NHL.
Patients With Concordance Between PET Scan and BM Biopsy Result
| Lymphoma Histology . | Positive Marrow . | Negative Marrow . |
|---|---|---|
| NHL | ||
| Centroblastic centrocytic | 2 | 4 |
| Mantle cell | 2 | |
| Diffuse large B-cell | 3 | 12 |
| Burkitt's | 1 | |
| HIV-associated Burkitt-like | 2 | |
| Peripheral T-cell | 4 | |
| T-lymphoblastic | 1 | |
| HD | ||
| Nodular sclerosis | 1 | 5 |
| Mixed cellularity | 1 | 1 |
| Total | 13 | 26 |
| Lymphoma Histology . | Positive Marrow . | Negative Marrow . |
|---|---|---|
| NHL | ||
| Centroblastic centrocytic | 2 | 4 |
| Mantle cell | 2 | |
| Diffuse large B-cell | 3 | 12 |
| Burkitt's | 1 | |
| HIV-associated Burkitt-like | 2 | |
| Peripheral T-cell | 4 | |
| T-lymphoblastic | 1 | |
| HD | ||
| Nodular sclerosis | 1 | 5 |
| Mixed cellularity | 1 | 1 |
| Total | 13 | 26 |
Trephine biopsy sections. (A) A patient with follicular centroblastic centrocytic lymphoma stained with hematoxylin and eosin showing low-volume, nodular disease (original magnification ×100). (B) A patient with T-lymphoblastic lymphoma stained with anti-CD3; cells with dark cytoplasm are CD3+ (original magnification ×400). These photomicrographs show the low level of lymphoma infiltration which in both cases was detected by PET.
Trephine biopsy sections. (A) A patient with follicular centroblastic centrocytic lymphoma stained with hematoxylin and eosin showing low-volume, nodular disease (original magnification ×100). (B) A patient with T-lymphoblastic lymphoma stained with anti-CD3; cells with dark cytoplasm are CD3+ (original magnification ×400). These photomicrographs show the low level of lymphoma infiltration which in both cases was detected by PET.
Patients with a discordant PET and routine iliac crest marrow biopsy results.
In 11 patients the PET scan and marrow biopsy findings differed (Table2). In 8 patients the PET scan showed focal or diffuse increased FDG uptake, but the routine iliac crest marrow biopsy specimen contained no evidence of lymphoma.
Patients in Whom the PET Scan and Routine BM Biopsy Result Differed
| Diagnosis . | Comment . |
|---|---|
| PET+, biopsy− | |
| Focal FDG uptake | |
| 1. HD (ns) | Normal FDG uptake at biopsy site |
| 2. HD (mc) | Normal FDG uptake at biopsy site |
| 3. Diffuse large B-cell lymphoma | Normal FDG uptake at biopsy site |
| 4. Diffuse large B-cell lymphoma | Normal FDG uptake at biopsy site |
| Diffuse FDG uptake | |
| 5. HD (ns) | |
| 6. HD (mc) | |
| 7. Peripheral T-cell (high grade) lymphoma | |
| 8. Anaplastic large cell (Ki-1+) lymphoma | |
| PET−, biopsy+ | |
| 1. Centroblastic centrocytic lymphoma | No FDG uptake by lymph nodes |
| 2. Mantle cell lymphoma | No FDG uptake by lymph nodes |
| 3. Mantle cell lymphoma (leukemic variant) |
| Diagnosis . | Comment . |
|---|---|
| PET+, biopsy− | |
| Focal FDG uptake | |
| 1. HD (ns) | Normal FDG uptake at biopsy site |
| 2. HD (mc) | Normal FDG uptake at biopsy site |
| 3. Diffuse large B-cell lymphoma | Normal FDG uptake at biopsy site |
| 4. Diffuse large B-cell lymphoma | Normal FDG uptake at biopsy site |
| Diffuse FDG uptake | |
| 5. HD (ns) | |
| 6. HD (mc) | |
| 7. Peripheral T-cell (high grade) lymphoma | |
| 8. Anaplastic large cell (Ki-1+) lymphoma | |
| PET−, biopsy+ | |
| 1. Centroblastic centrocytic lymphoma | No FDG uptake by lymph nodes |
| 2. Mantle cell lymphoma | No FDG uptake by lymph nodes |
| 3. Mantle cell lymphoma (leukemic variant) |
Abbreviations: PET−/+, normal/increased FDG uptake within the BM; biopsy−/+, absent/present lymphoma infiltration on marrow biopsy; ns, nodular sclerosis; mc,mixed cellularity HD.
All four patients who had focal marrow disease on the PET scan but histologically normal iliac crest marrow had normal FDG uptake at the site of the biopsy. In that respect these patients could be classed as concordant. In one of these patients subsequent biopsy of a “hot spot” localized within the left humeral head confirmed nodular sclerosing HD in the marrow (Fig 4). The other 3 patients did not have additional biopsies, but the 2 patients with high-grade NHL had other evidence of stage IV disease, with pulmonary lymphoma deposits shown by PET and CT.
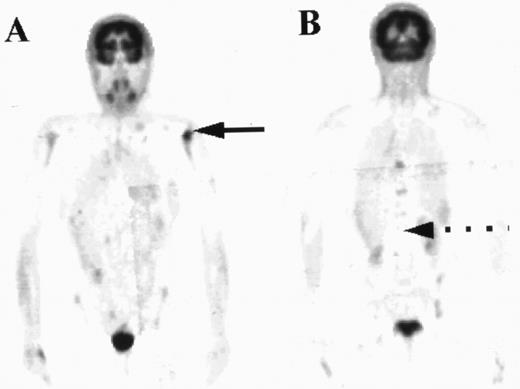
PET images of a patient with areas of focal increased uptake in marrow, most notably in the left humerus (solid arrow, A) and in the thoracic spine (B). The marrow was not diffusely abnormal and much of the axial skeleton exhibited FDG uptake less than in liver (broken arrow).
PET images of a patient with areas of focal increased uptake in marrow, most notably in the left humerus (solid arrow, A) and in the thoracic spine (B). The marrow was not diffusely abnormal and much of the axial skeleton exhibited FDG uptake less than in liver (broken arrow).
In the 4 patients with diffusely positive marrow on PET but normal biopsies there was no other evidence indicating marrow infiltration. The 2 patients with HD had the reactive myeloid hyperplasia characteristic of some HD patients; this in itself may have increased FDG uptake. The patients with T-cell and Ki-1+ lymphoma had clinical stage IV and stage II disease, respectively. None of these 4 patients had additional marrow biopsies to confirm the absence of disease.
Three patients had histologically demonstrated marrow disease that was not detected by the PET scan. Two of these patients, with low- and intermediate-grade NHL, had no FDG uptake into the site of their primary lymph node disease. Therefore, it was predictable that the PET scan would be unable to detect similar cells in the BM. In only one patient with histologically demonstrated marrow lymphoma was the marrow negative on PET when there was FDG uptake into another disease site, in this case the spleen. This patient had mantle cell lymphoma confined to spleen, marrow, and peripheral blood and is discussed further below.
DISCUSSION
In 39 of the 50 patients studied the PET scan correctly predicted the result of the unilateral staging marrow biopsy. In an additional 6 patients, in whom the PET scan and marrow histology differed, it was possible to predict, through careful interpretation of the PET scan, that either the scan or the biopsy would be an unreliable tool for diagnosing marrow disease. These included the 2 patients who had primary lymph node sites which did not accumlate FDG and the 4 patients with only focal marrow FDG uptake at sites distant from the iliac crest biopsy (Table 2). These latter 4 patients all had normal FDG uptake at the routine iliac crest biopsy site and all might well have had lymphoma confirmed by a PET guided biopsy. This assumption is supported by the one patient who had an initial iliac crest biopsy that was negative, followed by a positive biopsy from a focal “hot spot” within the left humeral head.
In the remaining 5 patients, representing 10% of the total studied, the cause of the discrepancy between PET and biopsy was less certain. In the 4 patients with diffuse PET+, biopsy− marrows the explanation may be either reactive hematopoietic changes within the marrow or genuine marrow disease that was missed by the single, unilateral biopsy. There was histological evidence to support myeloid hyperplasia as the cause of increased FDG uptake in the two HD patients. The alternative explanation, sampling error of patchy disease, has been recognized to occur in between one third and one half of high-grade NHL and HD patients.5-8
A unilateral staging marrow biopsy is in line with standard practice in the United Kingdom.4 As this study is the first to examine the ability of PET to identify marrow lymphoma it was not felt appropriate to perform additional biopsies unless it would have altered the intended treatment. This was the case in only one study patient.
In only one subject was the marrow negative with PET in the presence of histological marrow disease, when there was FDG uptake into another extranodal disease site. This patient, who represents the only “false negative” PET result, had a number of unusual features. He presented as a leukemic variant of mantle cell lymphoma21 22 with a high peripheral white blood cell count and splenomegaly but no clinical or CT detectable lymphadenopathy to assess FDG uptake at lymph node disease sites. The marrow had an interstitial infiltrate with B cells accounting for approximately half the cell population. The spleen, which appeared to be the primary disease site, was grossly enlarged (15 cm by CT scanning) and displayed high FDG uptake. The presence of increased FDG uptake within the spleen but not within the marrow might be explained by the high density of malignant lymphocytes in the spleen compared to the much lower volume and density within the marrow, in a tumor with an inherently low FDG uptake.
Although PET is increasingly being recognized as a valuable technique for the primary staging of patients with HD and NHL, the assessment of marrow infiltration by lymphoma using PET has not previously been investigated. Since the earliest studies of FDG uptake by lymphoma,23 FDG-PET has been found to be an effective method of staging these diseases which compares favorably with other conventional imaging techniques and is more sensitive at detecting extranodal disease than CT.12,16-18 In addition, the uptake of FDG appears to correlate with histological grade as defined by the International Working Formulation14,24-27 and the proliferative rate of the malignant cells,15,26 thus providing a potential index of prognosis. It has been previously noted that some low-grade NHLs may have low or absent FDG uptake,25,26 which may limit the use of PET in these lymphoma types. This was the case in the present study in two patients who had no FDG uptake into their primary disease sites. One of these patients, who had bulky lymph node disease, had mantle cell (diffuse centrocytic) lymphoma, a lymphoma type that has been shown to have highly variable FDG uptake.26 The other had centroblastic centrocytic lymphoma.
Other imaging techniques used to assess primary diseases of BM and infiltration by lymphoma have included magnetic resonance imaging (MRI) and BM scintigraphy. MRI is able to diagnose marrow infiltration as well as image nodal disease in lymphoma and thus may have an advantage over CT,28 although direct comparison of CT with MRI for nodal staging of lymphoma has been very limited.29 MRI is more sensitive than biopsy in detecting BM infiltration by lymphoma28 30 and is probably the imaging technique of choice in the regional assessment of marrow. However, it is not feasible to routinely assess the entire marrow with MR as is possible with PET.
BM scintigraphy has the ability to image the entire marrow, but the low uptake of conventional radioisotopes such as 99mTc colloids in the reticuloendothelial cells in the marrow relative to the liver and spleen create areas that are difficult to interpret. MRI appears to be superior to colloid imaging in the detection of marrow disease in lymphoma,30 but the use of either of these imaging modalities in combination with biopsy is better than biopsy alone. Specific antibodies directed against granulocytes and myeloid precursors labeled with radioisotopes have recently been developed to image BM in patients with primary BM disease, leukemias, and lymphoma as well as patients with metastases from solid tumors.31 32Antibody imaging has lower uptake in liver and spleen than colloid scintigraphy and may therefore be more sensitive. In one study in patients with lymphoma, antibody imaging appeared to correlate with the presence of marrow infiltration on biopsy, but as all the patients included had known BM disease, the specificity of the technique is unknown. As with MRI and PET, increased uptake is also likely to occur with marrow hyperplasia. There may be a problem with antibody response to repeated injections of murine antibody with serial imaging, although the incidence of this appears low and would not be a consideration in the initial staging of patients.
The shortcomings of these existing noninvasive techniques to image the BM mean that staging biopsy continues to be used despite its drawbacks.
Our data would suggest that additional information regarding marrow disease may be obtained from the PET scan at diagnosis of lymphoma. The scoring system used, although subjective, appears to be reproducible with good agreement between three independent observers interpreting the scans. Because the study was performed in patients undergoing whole-body nonattenuated scans for the purpose of staging lymphoma, quantitative estimates of marrow uptake of FDG were not possible. Absolute quantitation requires arterial blood sampling, which is not feasible or desirable in routine clinical imaging. Semi-quantitative estimates such as standardized uptake values (SUV), which are derived from attenuation-corrected images, may improve the definition of abnormal areas by reducing subjective assessment. Furthermore, attenuation correction may reduce reconstruction artifacts, including an apparent decrease in tracer accumulation in central structures such as axial marrow when compared with a superficial organ such as the liver, which is a potential source for error in the present study. However, attenuation correction requires extra transmission data to be acquired which, for each 10-cm axial field of view, would take an additional 10 to 20 minutes using current technology. Therefore, it can only be applied over selected regions and would be subject to the same sampling errors as marrow biopsy. The ability to acquire simultaneous transmission and emission data is currently being evaluated33 and whole-body attenuation correction methods are likely to be in clinical use shortly. This will enable SUVs to be calculated for sites of abnormal marrow uptake during retrospective analysis of the whole-body scan and should further improve the specificity of PET for detecting marrow disease.
This study suggests that visual interpretation of marrow FDG uptake during whole-body PET scanning can identify marrow infiltration by lymphoma with an accuracy which may be at least as reliable as unilateral iliac crest biopsy. Furthermore, these data are acquired as a byproduct of a minimally invasive procedure when PET is used for routine lymphoma staging. On the basis of the data reported here we conclude that, in patients whose involved lymph nodes accumulate FDG, normal marrow appearances indicate normal marrow histology. On the other hand, confirmatory marrow biopsies are indicated in all patients with abnormal FDG uptake, using the PET scan to direct the site of biopsy where appropriate.
Address reprint requests to Robert Carr, FRCP FRCPath, Department of Haematology, United Medical and Dental Schools of Guy's and St Thomas's, St Thomas' Hospital Campus, Lambeth Palace Rd, London, SE1 7EH UK.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal