Abstract
gp130 is a common signal-transducing receptor component for the interleukin-6 (IL-6) family of cytokines. To investigate the expression of gp130 in T-cell subsets and its regulation, anti-murine gp130 monoclonal antibody (MoAb) was used for flow cytometric analysis. In normal mice, gp130 was differentially expressed in thymocyte and splenic T-cell subpopulations defined by CD4/CD8 expression. In aged MRL/lpr mice, although gp130 expression was detectable in splenic CD4+ or CD8+ T cells, gp130 expression was significantly downregulated. Because serum levels of IL-6 and soluble IL-6 receptor (sIL-6R) are elevated in these mice, we examined the possibility that the downregulation of gp130 expression on splenic T cells might be produced in response to continuous activation of gp130 by high levels of serum IL-6. In transgenic mice overexpressing IL-6, gp130 expression in the splenic T cells was significantly decreased. After stimulation with IL-6 in vitro, the level of gp130 on CD4+ or CD8+ splenic T cells from normal mice was significantly decreased. These results suggest that the expression of gp130 in splenic T cells could be downregulated by the IL-6 stimulation under physiological or pathological circumstances.
COMMUNICATION BETWEEN cells in immune and hematopoietic systems is largely mediated by soluble factors called cytokines.1 gp130 was initially identified as a signal-transducing receptor component that associates with interleukin-6 receptor (IL-6R) when the receptor binds IL-6.2,3 Subsequently, gp130 has been shown to be shared by multiple cytokines in addition to IL-6, such as receptors for IL-11, leukemia inhibitory factor (LIF), oncostatin M, ciliary neurotrophic factor (CNTF), and cardiotrophin-1 (CT-1).4 5
It has been reported that gp130 mRNA is ubiquitously expressed in almost all the organs examined, including heart, spleen, kidney, lungs, liver, and brain.6 It has also been shown that the regulatory mechanism of gp130 expression may be somewhat different from that of IL-6R on the different cell lines.7-10 For example, IL-6R and gp130 mRNA levels are differentially regulated by IL-6 and by interferons in multiple myeloma cell lines.10 Other reports show that gp130 transcripts on various cells are upregulated in vitro by multiple factors, including IL-6.7 8
The existence of common signal transducers has been discovered not only in the IL-6R system, but in the other cytokine receptor systems as well. Like gp130, the common γ chain and the common β chain are also shown to be shared with multiple cytokine receptors. The cytokine receptors for at least IL-2, IL-4, IL-7, IL-9, and IL-15 use the same γ chain as an essential subunit.11 It has been shown that the common β-chain is shared with multiple cytokine receptors for IL-3, IL-5, and granulocyte-macrophage colony-stimulating factor (GM-CSF).12 Each cytokine has its own unique functions in certain cell types, and it has been considered that cellular responsiveness is largely determined by regulated expression of the ligand-specific receptors.1,4,11 12 Because the expression of common chains is believed to be relatively resistant to regulation, regulation of the common chain expression has been studied poorly to date. Thus, it would be of interest to examine the protein expression and the regulation of common signal transducer in vivo.
This report shows that with the use of a specific monoclonal antibody (MoAb) against murine gp130, we have examined the expression of gp130 on T-lineage cells in normal mice and MRL/lpr mice. To understand further the regulatory mechanism of gp130 expression in T cells by its ligands, we also investigated the expression of gp130 in T cells stimulated by IL-6 in vitro or those from IL-6 transgenic mice. Our results suggest that gp130 expression on T cells is downregulated by IL-6 both in vitro and in vivo.
MATERIALS AND METHODS
Animals.
C57BL/6, MRL/lpr, and MRL/n female mice were purchased from Shizuoka Laboratory Animal Center (Hamamatsu, Japan). IL-6, IL-6R single transgenic mice, and IL-6/IL-6R double transgenic mice were prepared as previously described.13 14 For these experiments, 4-month-old transgenic mice of both sexes were used.
Establishment of transfectants.
The murine gp130 cDNA was subcloned at a Xho I site of the BCMGNeo expression vector, kindly provided by Dr H. Karasuyama as previously described.15 Rat glioma C6G cells were transfected with BCMGNeo-containing murine gp130 cDNA. After selection with 1 mg/mL of the antibiotic G418 (Sigma, St Louis, MO), the gp130-expressing transfectant C6Gm130 was obtained.
Antibodies.
The preparation of anti-mouse gp130 MoAb (RX19) has been described elsewhere.16 RX19 and control rat myeloma IgG2a(Zymed, San Francisco, CA), were labeled with fluorescein isothiocyanate (FITC) (American Qualex, San Clemente, CA). Phycoerythrin (PE)-anti-CD4 and Cy-chrome-anti-CD8 were purchased from Pharmigen (San Diego, CA). PE-anti-B220 and PE-conjugated streptavidin were obtained from Becton Dickinson (San Jose, CA).
Preparation of T lymphocytes.
Cells were prepared from the spleen and thymus by passing them through a mesh. Lymphocytes were separated from murine spleens and thymus with standard procedures.17 The splenic cells were then labeled with anti-CD4, anti-CD8, and anti-Thy1.2 MoAb coupled with magnetic microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany) for 30 minutes on ice, and then were washed three times with a staining buffer (phosphate-buffered saline [PBS] containing 0.5% bovine serum albumin [BSA] and 0.001% sodium azide). The labeled cells were passed through MACS separation columns placed in the MACS separator (Miltenyi Biotec), the columns were then washed and removed from the magnet. The positive cells were washed out and collected.
Cell cultures and fluorescence-activated cell sorter (FACS) analysis.
Purified CD4+ or CD8+ splenic cells were cultured with or without IL-6 in RPMI 1640 supplemented with 10% fetal calf serum (FCS), penicillin (50 U/mL), streptomycin (50 mg/mL), 50 mmol/L 2-ME at an initial concentration of 5 × 106cells/mL in 96 round-bottomed well plates at 37°C in a humidified atmosphere with 5% CO2 in the air. After 2 days of culture, cells were stained with antibodies and analyzed with a FACScan flow cytometer (Becton Dickinson).
RESULTS
Specific recognition of murine gp130 by MoAb RX19.
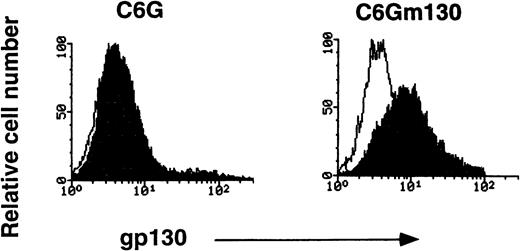
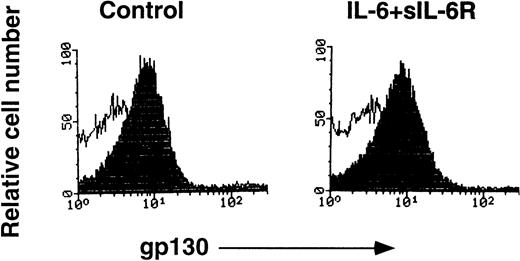
To examine the specificity of RX19, we used a rat glioma cell line, C6G, and its transfectant-expressing mouse gp130 (C6Gm130). As shown in Fig 1, C6Gm130 was positively stained with RX19, whereas the parent cells showed no staining, suggesting that RX19 specifically recognizes murine gp130. To analyze the expression of gp130 in lymphocytes freshly prepared from mice, we had to exclude the possibility that recognition of gp130 by RX19 might be influenced by the IL-6/IL-6R complex, which binds to gp130. RX19 was used for staining thymocytes, preincubated with or without an excess amount of IL-6 and sIL-6R and then analyzed by FACS. As shown in Fig2, the fluorescence intensity of cells stained by RX19 was not affected by 20-minute preincubation with IL-6 and sIL-6R, indicating that RX19 recognizes the epitope on gp130 as distinct from the IL-6R–binding site.
Specific recognition of murine gp130 by MoAb RX19. C6G cells and their transfectants with murine gp130 cDNA (C6Gm130 cells) were used. Closed and open histograms show the staining with RX19 and control rat myeloma IgG2a, respectively.
Specific recognition of murine gp130 by MoAb RX19. C6G cells and their transfectants with murine gp130 cDNA (C6Gm130 cells) were used. Closed and open histograms show the staining with RX19 and control rat myeloma IgG2a, respectively.
Recognition of murine gp130 by MoAb RX19 was not inhibited by the complex of IL-6 and sIL-6R. Thymocytes from C57BL/6 mice (12-week-old female) were incubated in PBS, 5 mmol/L EDTA, PH7.2, 0.5% BSA, with or without a combination of IL-6 and sIL-6R at 37°C for 20 minutes, the cells were then stained with RX19 (closed histograms) or control rat myeloma IgG2a (open histograms).
Recognition of murine gp130 by MoAb RX19 was not inhibited by the complex of IL-6 and sIL-6R. Thymocytes from C57BL/6 mice (12-week-old female) were incubated in PBS, 5 mmol/L EDTA, PH7.2, 0.5% BSA, with or without a combination of IL-6 and sIL-6R at 37°C for 20 minutes, the cells were then stained with RX19 (closed histograms) or control rat myeloma IgG2a (open histograms).
Distribution of gp130 on thymocytes and splenic T cells.
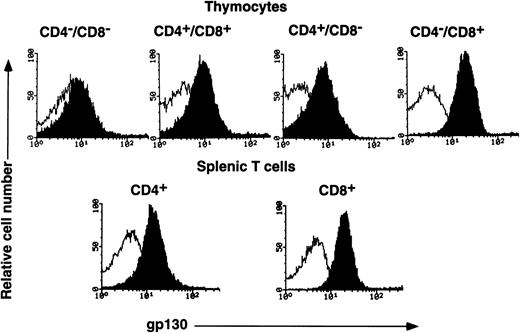
gp130 distribution on various T-lymphocyte subpopulations from C57BL/6 mice (12-week-old female) was examined by FACS analysis. As shown in Fig 3, gp130 was detectable on all thymocyte subpopulations fractionated in terms of CD4 and CD8 expression, although the expression levels were different among them. The CD4−/CD8− thymocytes, which are the most immature subset of T cells in the thymus, displayed the lowest gp130 expression. As for the gp130 expression level on CD4/CD8 single positive thymocytes, it was somewhat higher than those on the two other subpopulations. Among single positive thymocytes, the gp130 expression level on CD4+/CD8− thymocytes was slightly lower than that on CD4−/CD8+ thymocytes. The gp130 expression level of CD4+/CD8− or CD4−/CD8+ splenic cells was similar to that of CD4+/CD8− or CD4−/CD8+ thymocytes, respectively. T cells from younger (6-week-old) C57BL/6 and (6-week-old) Balb/c mice showed gp130 expression profiles similar to those of 12-week-old C57BL/6 (data not shown).
FACS analysis of gp130 on thymocytes and splenic T cells from C57BL/6 mice. Freshly isolated thymocytes with CD4−/CD8−, CD4+/CD8+, CD4+/CD8−, or CD4−/CD8+ phenotypes and splenic CD4+ or CD8+ T cells from C57BL/6 mice (12-week-old female) were analyzed for gp130 expression. Closed and open histograms show the staining with RX19 and control rat myeloma IgG2a, respectively.
FACS analysis of gp130 on thymocytes and splenic T cells from C57BL/6 mice. Freshly isolated thymocytes with CD4−/CD8−, CD4+/CD8+, CD4+/CD8−, or CD4−/CD8+ phenotypes and splenic CD4+ or CD8+ T cells from C57BL/6 mice (12-week-old female) were analyzed for gp130 expression. Closed and open histograms show the staining with RX19 and control rat myeloma IgG2a, respectively.
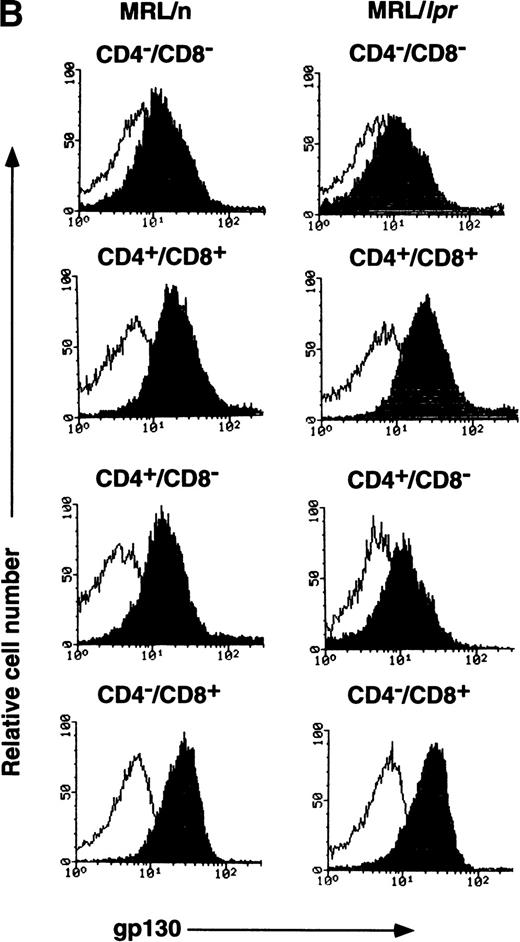
Decreased expression of gp130 on splenic T cells of aged MRL/lpr mice.
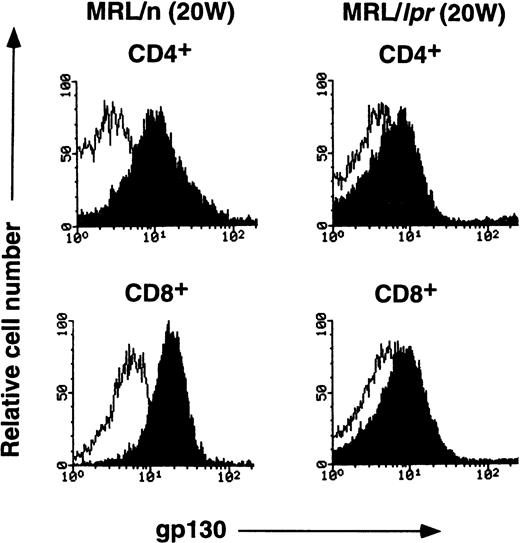
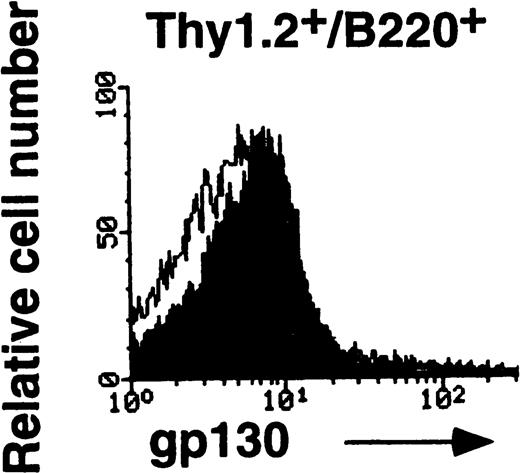
MRL/lpr mice carry the mutation of the Fas ligand gene and develop the lymphoproliferative disease associated with autoimmunity.18 IL-6 and sIL-6R levels were previously shown to be elevated in aged MRL/lpr mice.19,20 A previous report has demonstrated the elevated expression of IL-6R on B cells and reduced expression on CD4+ T cells in aged MRL/lpr mice.21 In this investigation, to determine whether the lpr mutation might have some influence on gp130 expression, either directly or indirectly, gp130 levels on T cells from MRL/lpr were examined. As shown in Fig4, the expression of gp130 in splenic T cells is significantly lower in 20-week-old MRL/lpr than in age-matched MRL/n and in 12-week-old C57BL/6 mice (Fig 3). By contrast, each subpopulation of thymocytes from MRL/lpr displays gp130 expression patterns similar to those of MRL/n and C57BL/6, although the gp130 level in CD4+/CD8− thymocytes of MRL/lpr was slightly decreased, as shown in Fig 4B. We further examined the gp130 expression on Thy1.2+/B220+T cells known to be abnormally proliferating in aged MRL/lpr.22 As shown in Fig 4C, T cells of this phenotype also showed a low level of gp130 expression. To analyze gp130 expression before onset of the disease, T cells of 6-week-old MRL/lpr were examined. In contrast with aged MRL/lpr, the gp130 expression levels in both splenic T cells and thymocytes in younger (6-week-old) MRL/lpr were not reduced (data not shown).
FACS analysis of gp130 on thymocytes and splenic T cells in aged MRL/lpr and MRL/n. (A) and (C) Cells were prepared from aged MRL/lpr and MRL/n mice (20-week-old female) spleens. CD4+, CD8+, and Thy1.2+ cells were purified by MACS system, and gated by B220 expression. (B) Cells were prepared from thymus. The cells were then analyzed for gp130 expression. Closed and open histograms show staining with RX19 and control rat myeloma IgG2a, respectively.
FACS analysis of gp130 on thymocytes and splenic T cells in aged MRL/lpr and MRL/n. (A) and (C) Cells were prepared from aged MRL/lpr and MRL/n mice (20-week-old female) spleens. CD4+, CD8+, and Thy1.2+ cells were purified by MACS system, and gated by B220 expression. (B) Cells were prepared from thymus. The cells were then analyzed for gp130 expression. Closed and open histograms show staining with RX19 and control rat myeloma IgG2a, respectively.
Reduction of gp130 expression on splenic T cells of IL-6 transgenic mice and IL-6/IL-6R double transgenic mice.
It is possible that reduced expression of gp130 on MRL/lprsplenic T cells is caused by overproduction of IL-6 in this strain of mice. To test this possibility, we further examined gp130 expression in T cells from transgenic mice overexpressing either IL-6 or IL-6R alone, or both. As previously reported,23 T cells normally develop in these transgenic mice. As shown in Fig 5and Table 1, the expression of gp130 on CD4+ T cells and CD8+ T cells in the spleen was significantly reduced in transgenic mice overexpressing either IL-6 alone or together with IL-6R. Such reduction was not observed in IL-6R single transgenic mice. By contrast, gp130 levels on each subpopulation of thymocytes in these transgenic mice were normal (data not shown) as was observed in MRL/lpr mice. These results suggest that the increased IL-6 level may lead to the down-regulation of gp130 expression on peripheral T cells in MRL/lpr, as well as transgenic mice overexpressing IL-6.
FACS analysis of gp130 on splenic T cells from transgenic mice. IL-6 single transgenic mice (IL-6+/R−Tg), IL-6R single transgenic mice (IL-6−/R+Tg), IL-6/IL-6R double transgenic mice (IL-6+/R+ Tg) and wild-type mice (IL-6−/R− Tg) were analyzed. Freshly isolated CD4+ T cells and CD8+ T cells from spleen were stained with RX19 and control rat myeloma IgG2a. Closed and open histograms show the staining with RX19 and control rat myeloma IgG2a, respectively.
FACS analysis of gp130 on splenic T cells from transgenic mice. IL-6 single transgenic mice (IL-6+/R−Tg), IL-6R single transgenic mice (IL-6−/R+Tg), IL-6/IL-6R double transgenic mice (IL-6+/R+ Tg) and wild-type mice (IL-6−/R− Tg) were analyzed. Freshly isolated CD4+ T cells and CD8+ T cells from spleen were stained with RX19 and control rat myeloma IgG2a. Closed and open histograms show the staining with RX19 and control rat myeloma IgG2a, respectively.
Expression of gp130 on Splenic T-Cell Subsets in 16-week-old Transgenic Mice
| Transgenic Mice . | T-Cell Subsets . | |
|---|---|---|
| CD4+ . | CD8+ . | |
| IL-6−/IL-6R− | 6.79 (9.22, 2.43)** | 11.07 (14.70, 3.63) |
| IL-6+/IL-6R− | 2.32 (5.68, 3.36) | 1.61 (5.74, 4.13) |
| IL-6−/IL-6R+ | 6.42 (8.83, 2.41) | 10.13 (13.93, 3.80) |
| IL-6+/IL-6R+ | 2.24 (5.50, 3.26) | 2.09 (6.32, 4.23) |
| Transgenic Mice . | T-Cell Subsets . | |
|---|---|---|
| CD4+ . | CD8+ . | |
| IL-6−/IL-6R− | 6.79 (9.22, 2.43)** | 11.07 (14.70, 3.63) |
| IL-6+/IL-6R− | 2.32 (5.68, 3.36) | 1.61 (5.74, 4.13) |
| IL-6−/IL-6R+ | 6.42 (8.83, 2.41) | 10.13 (13.93, 3.80) |
| IL-6+/IL-6R+ | 2.24 (5.50, 3.26) | 2.09 (6.32, 4.23) |
Numbers represent differences in mean fluorescence intensities (MFI) between RX19 and control staining (MFI of RX19-stained cells − MFI of control-stained cells); numbers in parentheses show MFI of RX19-stained cells and MFI of control-stained cells, respectively.
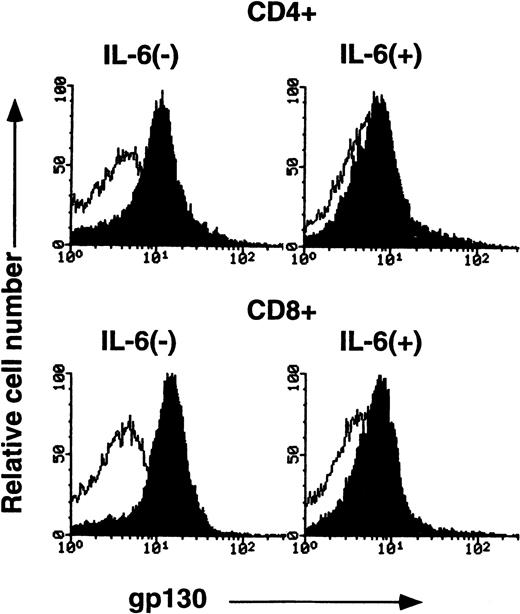
Downregulation of gp130 protein on splenic T cells after IL-6 stimulation in vitro.
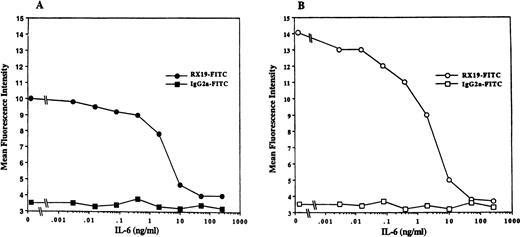
To further confirm IL-6–mediated downregulation of gp130 on peripheral T cells, CD4+ or CD8+ splenic T cells from C57BL/6 mice were cultured with or without IL-6 for 2 days and then gp130 expression on these cells was analyzed by FACS. As shown in Fig6, gp130 levels on both CD4+ T cells and CD8+ T cells that had been cultured with IL-6 were significantly reduced compared with those on T cells cultured without IL-6. Suppression of gp130 expression on T cells by IL-6 in vitro was observed in a dose-dependent manner, as shown in Fig7. These results indicate that IL-6 stimulation can lead to downregulation of the expression of gp130 on mature T cells in vitro as well as in vivo.
FACS analysis of gp130 on cultured CD4+ and CD8+ cells. Purified CD4+ and CD8+ cells from C57BL/6 spleen (12-week-old female) were cultured in RPMI 1640 medium supplemented with or without IL-6 (10 ng/mL) for 2 days. The cells were then analyzed for gp130 expression. Closed and open histograms show the staining with RX19 and control rat myeloma IgG2a, respectively.
FACS analysis of gp130 on cultured CD4+ and CD8+ cells. Purified CD4+ and CD8+ cells from C57BL/6 spleen (12-week-old female) were cultured in RPMI 1640 medium supplemented with or without IL-6 (10 ng/mL) for 2 days. The cells were then analyzed for gp130 expression. Closed and open histograms show the staining with RX19 and control rat myeloma IgG2a, respectively.
IL-6 downregulation of gp130 expression on T cells in a dose-dependent manner. Purified CD4+ and CD8+ splenic T cells from 6-week-old C57BL/6 mice were cultured with various concentrations of IL-6 for 2 days. Cells were washed and stained by RX19 or control rat myeloma IgG2a. (A) CD4+ T cells. (B) CD8+ T cells.
IL-6 downregulation of gp130 expression on T cells in a dose-dependent manner. Purified CD4+ and CD8+ splenic T cells from 6-week-old C57BL/6 mice were cultured with various concentrations of IL-6 for 2 days. Cells were washed and stained by RX19 or control rat myeloma IgG2a. (A) CD4+ T cells. (B) CD8+ T cells.
DISCUSSION
In our examination of the distribution of gp130 on splenic T cells and thymocytes from various mice, we found that although IL-6R was not detectable by flow cytometry in any thymocyte subpopulations,24,25 gp130 was readily detectable on all thymocyte populations, suggesting that immature T cells may use gp130-stimulatory cytokines other than IL-6. A recent report has shown that oncostatin M regulates the extrathymic T-cell development.26 It would be interesting to examine the expression of a receptor component of the functional oncostatin M receptor complex, which heterodimerizes with gp130, at various differentiation stages and in various subpopulations of T cells. Unlike fresh thymocyte, both CD4+/CD8− and CD4−/CD8+ cells in cultured thymocytes have been reported to express IL-6R, suggesting that thymocytes may have a potential to express IL-6R under certain circumstances.21Therefore, we cannot exclude the possibility that IL-6 can transduce the signal through gp130 on immature T cells under specific physiological circumstances.
Our results showed that gp130 was expressed at different levels on thymocytes of different maturation stages. gp130 was expressed at the lowest level on the most immature CD4−/CD8−thymocytes. Both CD4+/CD8+ and CD4+/CD8− thymocytes expressed the intermediate levels of gp130, and the gp130 expression level on CD4−/CD8+ cells was the highest. It has been reported that the cellular responsiveness to IL-6 varies among the thymocyte subsets; immature thymocytes are less responsive than mature subpopulations.27 This finding may be explained by the difference of gp130 expression among thymocyte subsets.
IL-6 acts on peripheral T cells at an early stage as both a proliferation factor and a cytotoxic T-cell differentiation factor.28-31 It has been previously shown that IL-6R is expressed on peripheral T cells before stimulation in vitro, and the levels of IL-6R expression on splenic CD4+ T cells and CD8+ T cells are almost identical.26 By contrast, gp130 expression on splenic CD8+ T cells was higher than that on splenic CD4+ T cells, as was observed in thymocytes. The responsiveness to IL-6 of CD4+/CD8− has been shown to be lower than that of CD4−/CD8+ T cells.27 This might reflect the differential gp130 expression levels on these T-cell subsets.
The MRL/lpr strain is one of the representative animal models of human autoimmune diseases. The immunologic abnormalities are usually first observed at the age of around 8 weeks, progressing with age.32 It has been reported that, in aged MRL/lprmice, IL-6R expression is elevated on B cells and absent on peripheral CD4+ cells. Our results showed that gp130 expression on splenic T cells of aged MRL/lpr decreased significantly. Serum IL-6 levels in 20-week-old MRL/lpr mice have been reported to reach up to 0.4 ng/mL.19 Elevated serum sIL-6R levels in aged MRL/lpr mice have also been shown,20suggesting that increased levels of IL-6–sIL-6R complex in MRL/lpr may cause a reduction in gp130 expression on splenic T cells. In support of this idea, gp130 expression on the splenic CD4+ cells and CD8+ cells was significantly decreased both in IL-6 single transgenic mice and in IL-6/IL-6R double transgenic mice. Serum hIL-6 levels are 0.1 to 5 ng/mL in IL-6 transgenic mice, whereas serum IL-6 levels in wild-type (WT) mice were only below 0.02 ng/mL.14 In IL-6R single transgenic mice, the gp130 distribution on splenic T cells was similar to that observed in WT mice. These results indicated that the expression of gp130 on splenic T cells was downregulated by continuous gp130 stimulation. This was confirmed by the in vitro experiment, showing that the gp130 expression on CD4+/CD8− and CD4−/CD8+ splenic T cells was significantly downregulated by 2 days of incubation with IL-6. In contrast to splenic T cells, gp130 expression on thymocyte subpopulations was relatively normal in IL-6 and IL-6/IL-6R transgenics as well as in MRL/lprmice. As IL-6R was previously shown to be undetectable on thymocytes of aged MRL/lpr by flow cytometry,21 it might be envisaged that failure of gp130 to receive IL-6 signals might explain the normal gp130 expression on thymocytes of aged MRL/lpr.However, this was probably not the case because normal gp130 expression was not affected, even on thymocytes from the transgenic mice expressing both IL-6 and IL-6R, in which IL-6R was abundantly expressed on thymocytes. These observations suggest that immature thymocytes may have a mechanism to regulate gp130 expression differently from that of peripheral T cells.
The precise molecular mechanism of IL-6–dependent downregulation of gp130 expression is unclear. It was previously shown that administration of IL-6 into mice can upregulate gp130 mRNA in various tissues, including spleen, kidney, heart, and liver.6 Such changes of gp130 mRNA were obtained only transiently at 1 to 5 hours after IL-6 administration in a kinetic study using liver.6Schoester et al9 also reported that gp130 mRNA is transiently upregulated 4 hours after stimulation by IL-6 in human monocytes and then reached the basal levels 21 hours later. It appears that regulation of cell-surface expression of gp130 protein is quite different from that of gp130 mRNA. These observations suggest that IL-6–dependent downregulation of gp130 expression might take place at a posttranscriptional level, although gp130 mRNA levels of lymphocytes freshly isolated from IL-6 transgenic mice and lymphocytes stimulated by IL-6 in vitro remain to be studied. Dittrich et al33 34 reported that the cytoplasmic region of gp130 is required for ligand induced endocytosis and downregulation of the IL-6R/gp130 complex. Therefore, internalization of the IL-6/IL-6R/gp130 complex may play a role, at least in part, in the IL-6–dependent downregulation of the gp130 protein observed in the present study.
Cellular responsiveness to cytokines has been considered to be largely determined by the regulated expression of the ligand-specific receptors, rather than the common signal-transducing chains, such as gp130. IL-2, IL-4, IL-7, IL-9, and IL-15 use the same γ chain as an essential subunit. IL-2R α chain and IL-2R β chain are shown to have restricted expression, whereas the common γ chain is expressed by monocytes and lymphocytes and is relatively resistant to regulation.11 The receptor complexes for IL-3, IL-5, and GM-CSF also contain the common β chain.12 Similar to the common γ chain, the expression of the common β chain is also relatively resistant to regulation. However, a previous report has shown that IL-2R γ chain expression on normal activated T cells was significantly suppressed by IL-2 stimulation.35IL-2-induced suppression of IL-2R γ chain was also demonstrated by IL-2Rγ promotor-driven luciferase assays.36 Our results have clearly demonstrated that the expression of the common signal-transducing chain gp130 in peripheral T cells can be directly regulated by one of ligands for receptors sharing gp130, IL-6. Cells in immune and hematopoietic systems often express multiple kinds of cytokine receptors, which share the same signal transducer; ligands for such receptors transduce similar intracellular signals through the same signal transducer, resulting in almost the same biological outcomes. Under inflammatory conditions, expression of multiple kinds of cytokines is often induced. Our finding in this study that gp130 expression on T cells is downregulated by IL-6 stimulation suggests that overexpression of IL-6 may make the target cells desensitized in response not only to IL-6, but also to the other gp130-stimulating cytokines. Therefore, the downregulation of gp130 expression by IL-6 may be one example of failsafe mechanisms of the cytokine receptor systems to avoid hyperstimulation. Although IL-6–induced downregulation of gp130 expression does not appear to be so dramatic (10-fold or less) in vivo, downmodulation of physiologic responses mediated by gp130 is considered significant. This is because the stimulation of gp130 has been shown to induce expression of inhibitory proteins that block gp130-mediated intracellular signals.37-40 Downregulation of gp130 and upregulation of such inhibitors would cooperatively downmodulate physiologic responses. To test this hypothesis, it may be necessary to examine the responsiveness of MRL/lpr and IL-6 transgenic mice to the IL-6 family of cytokines in various circumstances. Such studies are now in progress.
ACKNOWLEDGMENT
We are grateful to Drs K. Yoshida, J. Uchida, M. Kamanaka, I. Lee, and A. Kumanogoh for helpful discussion. We also thank K. Kubota for excellent technical assistance.
Supported by a Grant-in-Aid from the Ministry of Education Science and Culture, Japan, and by a grant from the Science and Technology Agency, Japan. X.J.W. is a fellow of the Osaka Foundation for Promotion of Clinical Immunology, Japan.
Address reprint requests to Tetsuya Toga, PhD, Department of Molecular Cell Biology, Medical Research Institute, Tokyo Medical and Dental University, 2-3-10, Kandasurugadai, Chiyoda-ku, Tokyo 101-0062, Japan.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal