Terminally differentiating erythrocytes degrade most of their RNA with subsequent release of mononucleotides. Pyrimidine mononucleotides are preferentially cleaved by an erythrocyte-specific pyrimidine 5′-nucleotidase; deficiency of this enzyme causes hemolytic anemia in humans. Details of the regulation of its activity during erythroid differentiation are unknown. The present study arose from the observation that the immature red blood cells (RBCs) of mid-term chick embryos contain high concentrations of uridine 5′-triphosphate (UTP) (5 to 6 mmol/L), which decline rapidly from days 13 to 14 onward. We analyzed two key enzymes of RBC pyrimidine nucleotide metabolism: pyrimidine nucleoside phosphorylase (PNP) and pyrimidine 5′-nucleotidase (P-5′-N), to evaluate if changes of enzyme activity during embryonic development are correlated with changes of RBC UTP. Secondly, we tested if these enzymes are under hormonal control. The results show that embryonic RBCs contain only minimal activity of PNP. In contrast, P-5′-N increases from day 13 on, suggesting that the enzyme is a limiting factor in UTP degradation. Activation of β-adrenergic and A2A-adenosine receptors causes transcription-dependent de novo synthesis of P-5′-N. Because β-adrenergic and adenosine receptors are also found on adult erythroid cells, P-5′-N might be an enzyme of differentiating RBCs whose expression is in part controlled by adenosine 3′:5′-cyclic monophosphate (cAMP).
IT IS WELL-KNOWN THAT, during the final steps of erythroid differentiation, the RNA content of mammalian and nonmammalian red blood cells (RBCs) is drastically reduced.1 Little attention has been paid to the metabolic fate of nucleotides liberated in this process. Studies on human RBCs have shown the presence of a 5′-nucleotidase specific for pyrimidine mononucleotides but with no affinity for purine mononucleotides2,3 whose molecular properties have been partially characterized.4 A reduction of pyrimidine 5′-nucleotidase (P-5′-N) activity due to genetic defects or lead poisoning causes hemolytic anemia in humans.2 5 The circulating RBCs show significant accumulation of pyrimidine nucleotides as well as incomplete degradation of RNA and ribosomes, which indicate the important role of the enzyme for RBC maturation, because the enzymatic step liberates cell-permeable nucleosides. Details of the regulation of P-5′-N activity and its influence on the nucleotide pattern during erythroid development are unknown.
Experimental evidence suggests that the P-5′-N activity of immature RBCs is substantially increased. In RBCs of human fetuses from the 17 to 23 weeks of gestation, the activity of P-5′-N was about threefold higher than in the RBCs of adults.6 Likewise, the RBCs of adult rabbits with an increased reticulocyte fraction contained significantly higher activity of the enzyme.7
The present investigation of the pyrimidine metabolism arose from the observation that circulating RBCs of midterm chick embryos (days 10 to 12) contain millimolar concentrations of uridine 5′-triphosphate (UTP),8 which decrease rapidly from about day 14 onwards. In the second week of incubation, circulating embryonic RBCs are predominantly (polychromatic/orthochromatic) erythroblasts that have concluded their terminal division but retained considerable transcriptional and protein synthetic activity.9 For the majority of the RBCs, the transition to mature definitive erythrocytes and shutdown of transcriptional activity is only accomplished in the last (third) week of incubation.9 Thus, the nucleated embryonic chick RBCs are a good experimental system to study pyrimidine metabolism in the penultimate stages of erythroid differentiation.
We have recently shown that adenosine 3′:5′-cyclic monophosphate (cAMP)-dependent processes control major aspects of the metabolism of embryonic RBCs in the second half of incubation, including the coordinated activation of 2,3-bisphosphoglycerate (2,3BPG) and carbonic anhydrase II (CAII) synthesis.8,10-12In late chick embryos, an increase of the RBC cAMP concentration is initiated by the rapid increase of plasma norepinephrine (NE), activating RBC adenylyl cyclase via β-adrenergic receptors.11 The physiologic stimulus for the NE release is hypoxia.11 These events occur at the time when the UTP concentration of embryonic RBCs decreases. Besides the β-adrenergic receptor, we have found an adenosine A2-receptor coupled to adenylyl cyclase. In vitro adenosine receptor activation induces the same metabolic processes we observed with β-adrenergic receptor activation.8 12
In addition, we could show that in vitro incubation of embryonic RBCs from day 11 with β-adrenergic or adenosine receptor agonists causes transcription-dependent stimulation of the synthesis of several other RBC proteins besides CAII. Therefore, we have analyzed the activity of two key enzymes of pyrimidine metabolism, pyrimidine nucleoside phosphorylase (PNP) and P-5′-N, to find out (1) if changes in the enzyme activity are correlated with the decrease of the RBC UTP concentration during terminal differentiation and (2) if the enzyme activities are under hormonal control by catecholamines and adenosine.
The results show that, during the second week of incubation, UTP is the second most abundant organic phosphate compound of the embryonic RBCs. The embryonic RBCs contain only minimal activities of PNP, precluding a use of uridine by embryonic RBCs. In contrast, the P-5′N activity increases significantly between days 13 and 15 of development. Incubation of embryonic RBCs of day 11 with β-adrenergic or adenosine receptor agonists or forskolin causes transcription-dependent de novo synthesis of the enzyme, which in turn increases the amount of released uridine into the incubation medium. The results show that P-5′N synthesis is partly controlled by cAMP during terminal erythroid differentiation and that the enzyme is rate limiting for the release of uridine during RBC maturation.
MATERIALS AND METHODS
Fertilized eggs of White Leghorn chickens were incubated at 37.5°C and 60% relative humidity in a commercial forced-draft incubator for up to 19 days of development.
Blood was sampled after a large extraembryonic vessel was cut. The effluent blood was aspirated and transferred to cold washing buffer [50 mmol/L tris(hydroxymethyl)aminomethane (Tris), 120 mmol/L NaCl, 4 mmol/L KCl, 5 mmol/L glucose, 1.5 mmol/L CaCl2, pH 7.4]. The RBCs were washed three times with cold washing buffer before use.
Determination of PNP activity.
The PNP activity of embryonic RBCs was analyzed by the method of Laurensse et al,13 which determines the degradation of uridine to uracil via analysis by reversed-phase high-performance liquid chromatography (HPLC).
For lysis, 50 μL of packed RBCs was diluted with 950 μL hypotonic Tris-EDTA buffer (50 mmol/L Tris, 1 mmol/L ethylenediamine tetraacetic acid (EDTA), pH 7.4). After 10 minutes on ice, the lysate was centrifuged for 10 minutes at 14,000g and 4°C to remove debris. Eight hundred microliters of supernatant was mixed with 280 μL Tris-EDTA buffer and 60 μL 0.8 mol/L KH2PO4, pH 7.4, and incubated for 10 minutes at 37°C. The reaction was started by the addition of 60 μL uridine stock solution (20 mmol/L). The reaction was stopped by heating 200 μL of the sample for 3 minutes at 95°C. After centrifugation, the supernatant was stored at −40°C until HPLC analysis with a Pharmacia HPLC system (Pharmacia, Uppsala, Sweden) and RP-18 column (LiChroSorb; 250 × 4 mm; 10-μm particle size; Merck, Darmstadt, Germany) according to Laurensse et al.13The hemoglobin (Hb) was converted to cyanmethemoglobin and measured spectrophotometrically. One unit of PNP is defined as the conversion of 1 μmol uridine to uracil per minute at pH 7.4 and 37°C.
Determination of P-5′-N activity.
P-5′-N activity was analyzed with the spectrophotometric method of Amici et al,14 which quantitates the metabolization of uridine 5′-monophosphate (UMP) to uridine by HPLC analysis. Samples for the test were prepared as follows: 100 μL of packed RBCs was resuspended in 100 μL of washing buffer and lyzed by two freeze-thaw cycles with liquid nitrogen/ice water. The lysate was centrifuged at 14,000g for 10 minutes. One hundred microliters of the supernatant was added to 900 μL of incubation buffer (50 mmol/L Tris, 10 mmol/L MgCl2, 10 mmol/L dithiothreitol, pH 7.5). After 10 minutes of preincubation at 37°C, the reaction was started by addition of 10 μL of 100 mmol/L UMP. Between days 4 and 11, RBCs from several embryos were pooled to obtain the necessary sample size for a single experiment. In intervals of 10 minutes (0 to 70 minutes), the reaction was stopped by adding 50 μL ice-cold HClO4 (1.2 mol/L) to a 100-μL sample. After centrifugation (5 minutes at 14,000g and 4°C), 130 μL of the supernatant was neutralized with 35 μL of 1 mol/L K2CO3 and the sample was stored at −40°C until HPLC analysis with a Pharmacia HPLC system and an RP-18 column (LiChroSorb; 250 × 4 mm; 10 μm; Merck). One unit of P-5′-N is defined as conversion of 1 μmol of UMP to uridine per minute at pH 7.5 and 37°C.
In vitro incubations.
To determine the effect of 10 μmol/L NE, 10 μmol/L epinephrine (E), 10 μmol/L 5′-(N-cyclopropyl)-carboxamidoadenosine (CPCA), 100 μmol/L forskolin, and 35 μmol/L actinomycin D on P-5′-N activity, erythrocytes from 11-day-old embryos were incubated for 16 hours at 37°C in a gyratory water bath [cytokrit 4%, Ham's medium F10 supplemented with 20 mmol/L N-(2-hydroxyethyl)piperazine-N′-2-ethanesulfonic acid (HEPES), and 10% fetal calf serum (FCS), pH 7.4] in the absence and presence of the tested substances. Following the dose-response curves for CPCA, NE, and E determined previously,11 12 the agonist concentrations were chosen to give maximal stimulation. Because FCS may contain small quantities of catecholamines, we added the β-adrenergic blocker propranolol during some control incubations.
We also measured the effect of the β-adrenergic agonists on RBC uridine release during 16 hours of incubation. Uridine concentration of the supernatant and RBCs and RBC UTP/uridine 5′-diphosphate (UDP) concentrations were determined at the end of the incubation period.
Nucleotide analysis.
RBC nucleotides were analyzed by reversed-phase HPLC following the method of Stocchi et al15 with slight modifications. Fresh whole blood for nucleotide analysis of RBCs from 7-day-old to 17-day-old chick embryos was taken using the following procedure: a short glass capillary with bevelled tip was inserted into a 2-mL pipette. The 2-mL pipette served as reservoir for the collected blood and was surrounded by a cooling jacket, which was perfused with a cold (−3°C) water-acetone mixture. The pipette was mounted onto a Leitz micromanipulator and the capillary inserted into an extraembryonic blood vessel under stereomicroscopic control. In general, collection of blood took less than 2 minutes. The collected blood was immediately transferred into ice-cold Eppendorf cups and centrifuged for 5 seconds at 14,000g. After removal of the plasma and buffy coat, RBCs were washed rapidly (twice for 5 seconds) with ice-cold washing buffer. Fifty μL of packed RBCs was added to 50 μL HClO4 (1 mol/L) and centrifuged (14,000g for 10 minutes at 4°C). The supernatant was neutralized with 10 μL of 5 mol/L K2CO3 and stored at −40°C until analysis.
cAMP determination.
RBC cAMP concentrations were determined using the fluorometric enzymatic test of Sugiyama and Lurie.16 For the cAMP determinations, RBCs were first preincubated for 30 minutes at 37°C. One hundred μL of packed RBCs was added to 400 μL of incubation buffer consisting of 10% FCS, 135 mmol/L NaCl, 4 mmol/L KCl, 5 mmol/L glucose, 1.5 mmol/L MgCl2, 1.5 mmol/L CaCl2, and 20 mmol/L HEPES, pH 7.4. The incubation was performed in a shaking water bath. After the preincubation period, the cells were incubated with agonists for 5 minutes. To stop the reaction, 100 μL of cell suspension was mixed with 1 mL of ice-cold ethanol. After 5 minutes on ice, the sample was centrifuged for 5 minutes at 13,000g at 4°C and the supernatant was transferred to an Eppendorf test tube. To remove the ethanol, the sample was dried at 50°C and stored at −80°C until analysis. For the cAMP determination, the sample was dissolved in 40 μL of ice-cold 0.5 mol/L HClO4 by mixing for 2 minutes and sonicating for 1 minute. After neutralization with 10 μL of 2 mol/L KOH and centrifugation for 10 minutes (13,000g and 4°C), the supernatant was used for the cAMP determination.16Fluorescence measurements were performed in microtitration plates (Nunc, Wiesbaden, Germany) at an emission wavelength of 460 nm and excitation wavelength at 360 nm, using the Perkin-Elmer spectrofluorometer LS 50B with attached microplate reader (Perkin-Elmer, Norwalk, CT).
Chemicals.
Analytical grade reagents, nucleotides, nucleosides, FCS, norepinephrine, epinephrine, and propranolol were purchased from Sigma Chemicals (Deisenhofen, Germany). CPCA and forskolin were obtained from RBI Biotrend (Köln, Germany), and Ham's F10-medium were obtained from Biochrom KG (Berlin, Germany).
RESULTS
Developmental changes of embryonic RBC ATP and UTP concentration, PNP, and P-5′-N activity.
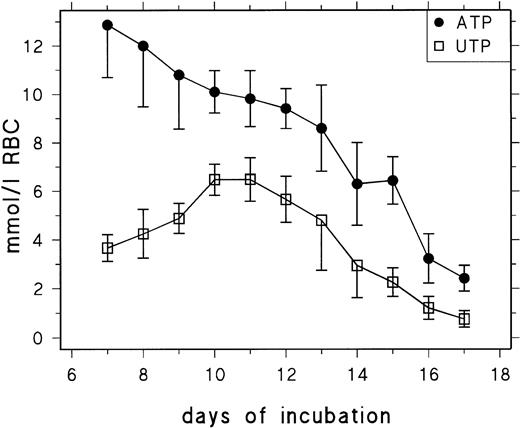
Figure 1 shows the ATP and UTP concentrations of RBCs between day 7 and day 17 of chicken development. In agreement with previous data,17 we find that early definitive RBCs contain excessively high concentrations of ATP (13 mmol/L RBCs at day 7), which decrease from 9 mmol/L at day 13 to 2.5 mmol/L at day 17. The UTP concentration increases between day 7 and day 10 from about 3.5 mmol/L to 6 mmol/L and decreases from 5 mmol/L at day 13 to 0.9 mmol/L at day 17. The UTP concentration profile suggests that, during the second week of incubation, UMP released from RNA degradation is to a considerable extent phosphorylated to yield UTP and retained in the RBCs rather than metabolized to uridine, indicating a limiting role of P-5′-N and/or PNP. Table1 contains data for UDP, cytidine 5′-diphosphate (CDP), and cytidine 5′-triphosphate (CTP) concentrations measured between days 11 and 15. They show that the CTP concentration decreases from 2.29 mmol/L RBCs at day 11 to 0.16 mmol/L RBCs at day 15 and that changes in UDP and CDP concentration are less conspicuous but follow the same trend.
Changes of RBC UTP and ATP concentration during chick embryonic development (day 7 to day 17). Data are presented as the mean and SD of 3 to 14 determinations at each point.
Changes of RBC UTP and ATP concentration during chick embryonic development (day 7 to day 17). Data are presented as the mean and SD of 3 to 14 determinations at each point.
CTP, CDP, and UDP Concentrations of Erythrocytes From 11- to 15-Day-Old Chick Embryos
| Nucleotide . | CTP . | CDP . | UDP . |
|---|---|---|---|
| Day 11 | 2.29 (0.7) | 0.37 (0.07) | 1.15 (0.17) |
| Day 14 | 1.27 (0.44) | 0.43 (0.14) | 1.1 (0.28) |
| Day 15 | 0.16 (0.06) | 0.14 (0.03) | 0.54 (0.03) |
| Nucleotide . | CTP . | CDP . | UDP . |
|---|---|---|---|
| Day 11 | 2.29 (0.7) | 0.37 (0.07) | 1.15 (0.17) |
| Day 14 | 1.27 (0.44) | 0.43 (0.14) | 1.1 (0.28) |
| Day 15 | 0.16 (0.06) | 0.14 (0.03) | 0.54 (0.03) |
Mean values and SD (in parentheses) for embryonic RBC concentrations of CTP, CDP, and UDP in moles per liter. Data are from three different experiments.
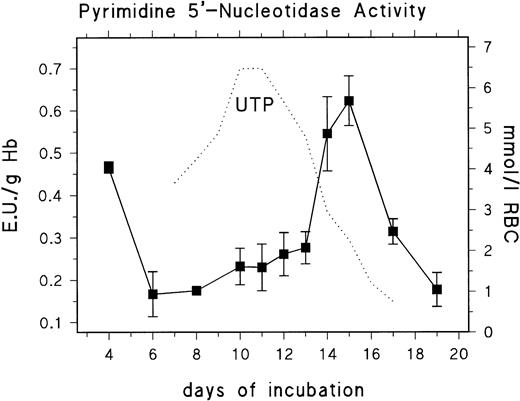
We analyzed the developmental profile of PNP and P-5′-N activity to find if the changes in nucleotide pattern correlated with altered enzyme activities. We found no significant activities of PNP when analyzing RBCs from day 11 to day 15. The activity was below the limit of detection (<0.42 mU/g Hb). This finding explains the results of Mathew et al,18 who reported that extracellular uridine was not a suitable substrate for the energy metabolism of RBCs from 14-day-old chick embryo. In contrast to this, the P-5′-N activity showed substantial changes of activity throughout development (Fig 2). Peak activities (0.47 U/g Hb; 0.013 standard deviation [SD]) were found early in development (day 4), when the circulating blood consists predominantly of polychromatic primitive erythroblasts, whereas, at day 6, when the blood contains primarily mature primitive RBCs, the activity was significantly reduced (0.17 U/g Hb; 0.053 SD). Between day 6 and day 13, the activity of P-5′-N showed only a small gradual increase to 0.27 U/g Hb by day 13, but increased rapidly to 0.54 to 0.62 U/g Hb at days 14 to 15, followed by a rapid decrease to 0.1 U/g Hb at day 19. The rapid decrease of RBC UTP concentration is closely coordinated to the increase of P-5′-N activity (Fig 2). The same can be inferred for CTP (Table 1).
Changes of erythrocyte P-5′-N activity during chick embryonic development (day 4 to day 19). Data are the mean values and SD from three to six experiments at each point. The dotted line gives parallel changes of RBC UTP concentration (data taken from Fig 1).
Changes of erythrocyte P-5′-N activity during chick embryonic development (day 4 to day 19). Data are the mean values and SD from three to six experiments at each point. The dotted line gives parallel changes of RBC UTP concentration (data taken from Fig 1).
Effect of β-adrenergic and adenosine receptor agonists on pyrimidine 5′-nucleotidase activity.
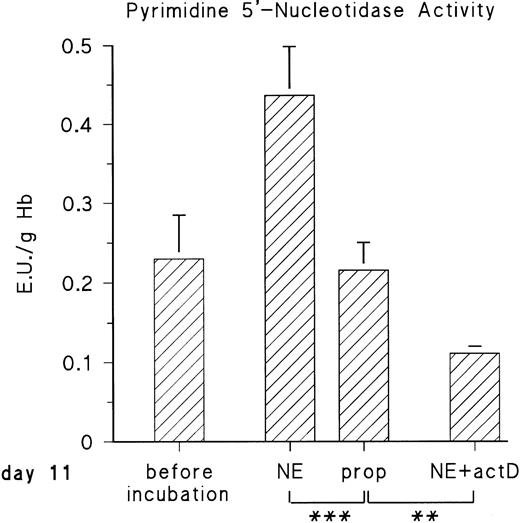
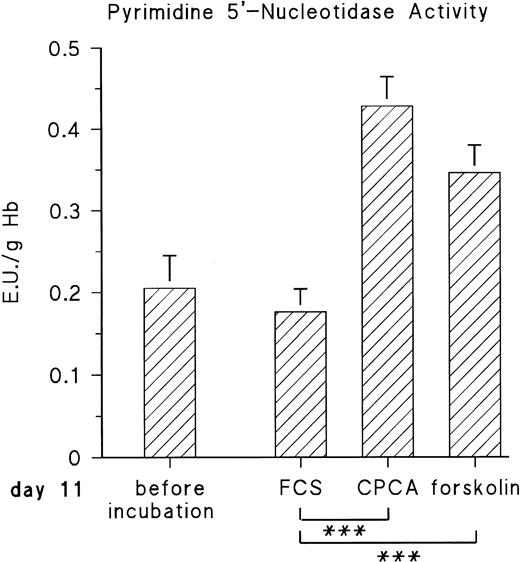
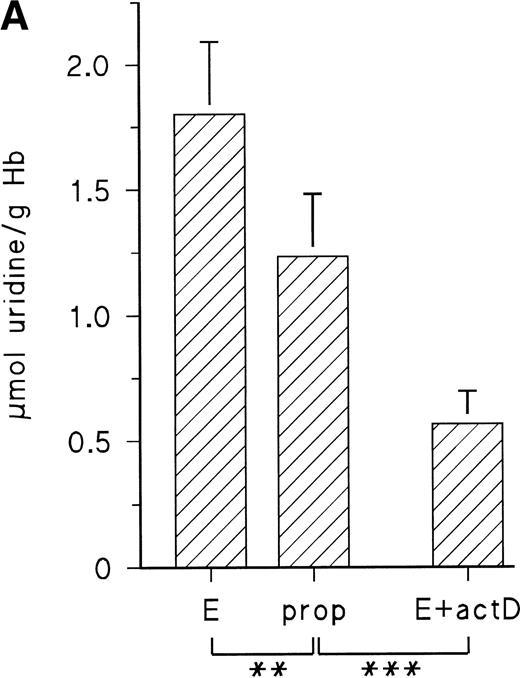
The observed increase of P-5′-N activity is correlated with the increase of NE concentration in the blood of the chick embryo.11 Because we could previously show that adrenergic agonists stimulate the synthesis of several, not yet identified, embryonic RBC proteins via β-adrenergic receptor activation, we tested if they influence P-5′-N activity. To this end, embryonic RBCs from day 11 were incubated in vitro for 16 hours (for details, see the Materials and Methods) in the absence and presence of NE or E (both agonists are equally effective on the β-adrenergic receptor of embryonic chick RBCs).11 As shown in Fig 3, the addition of 10 μmol/L NE doubled the P-5′-N activity during a 16-hour incubation period. This increase is completely blocked by the β-adrenergic antagonist propranolol. Chick embryonic RBCs also possess an adenosine A2-receptor coupled to adenylyl cyclase.12 The adenosine receptor agonist CPCA also increases the P-5′-N activity of isolated RBCs of 11-day-old chick embryos (Fig 4). Direct stimulation of adenylyl cyclase with 100 μmol/L forskolin leads to a less prominent activation of P-5′-N than β-adrenergic or adenosine receptor activation, corroborating previous results showing only moderate stimulation of adenylyl cyclase by forskolin.12
Adrenergic stimulation of RBC P-5′-N activity. RBCs (cytokrit 4%) from day 11 were incubated for 16 hours at 37°C in the absence and presence of 10 μmol/L NE, 10 μmol/L propranolol (prop), and 35 μmol/L actinomycin D (actD). Data are given as the mean value and SD from five experiments in each case. (***P< .001, **P < .01).
Adrenergic stimulation of RBC P-5′-N activity. RBCs (cytokrit 4%) from day 11 were incubated for 16 hours at 37°C in the absence and presence of 10 μmol/L NE, 10 μmol/L propranolol (prop), and 35 μmol/L actinomycin D (actD). Data are given as the mean value and SD from five experiments in each case. (***P< .001, **P < .01).
Effect of adenosine A2-receptor activation and direct stimulation of adenylyl cyclase with forskolin on P-5′-N activity. RBC cells from day-11 chick embryos were incubated for 16 hours with either 10 μmol/L CPCA or 100 μmol/L forskolin, and P-5′-N activity was determined for control and stimulated samples at the end of the incubation period. Data are the mean values and SD from five experiments. The asterisk indicates statistically significant difference tested by the Student'st-test for paired samples (***P < .001).
Effect of adenosine A2-receptor activation and direct stimulation of adenylyl cyclase with forskolin on P-5′-N activity. RBC cells from day-11 chick embryos were incubated for 16 hours with either 10 μmol/L CPCA or 100 μmol/L forskolin, and P-5′-N activity was determined for control and stimulated samples at the end of the incubation period. Data are the mean values and SD from five experiments. The asterisk indicates statistically significant difference tested by the Student'st-test for paired samples (***P < .001).
The cAMP-dependent increase of protein synthesis in embryonic RBCs requires transcriptional activation, because actinomycin D abolishes the effect of adenylyl cyclase stimulation by adrenergic or adenosine A2-receptor agonists.11 12 We therefore tested the influence of actinomycin D on adrenergic induction of P-5′-N. Actinomycin D not only inhibited the adrenergic stimulation of P-5′-N (Fig 3), but in its presence the P-5′-N activity falls significantly below that of the controls. This suggests that the enzyme, as well as its RNA, has a considerable turnover in the embryonic RBCs.
Effect of β-adrenergic stimulation and actinomycin D on uridine release from embryonic RBCs and UTP concentration in RBCs.
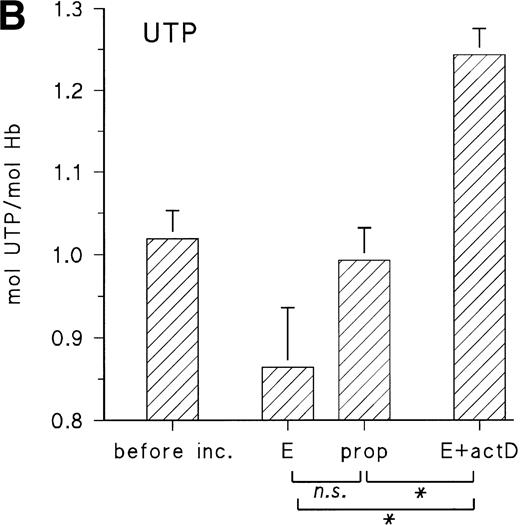
Figure 5A shows the uridine release from embryonic RBCs from day 11 during 16 hours of incubation with 10 μmol/L epinephrine in the absence and presence of propranolol (10 μmol/L) and actinomycin D (35 μmol/L). When cells are stimulated with epinephrine, they increase the uridine release by about 40% compared with the control with propranolol, whereas, in the presence of actinomycin D, uridine release is less than 50% of the control value. There are corresponding changes of the RBC UTP concentration, which is decreased in the presence of epinephrine and increased in the presence of actinomycin D (Fig 5B). This suggests that in vivo the embryonic RBC is a substantial source for provision of pyrimidine nucleosides and that the activity of P-5′-N is the limiting factor for uridine release.
(A) Uridine release from RBCs of day-11 chick embryos during 16 hours with 10 μmol/L E, 10 μmol/L propranolol (prop), and 35 μmol/L actinomycin D (actD). Incubation conditions were the same as in Fig 3. Data are given as the mean and SD from five experiments in each case (**P < .01; ***P < .001). (B) Changes of RBC UTP concentration during in vitro incubation with E, propranolol (prop), and actD. Agonist concentrations and incubation conditions as in (A). Data are the mean values and SD from three experiments in each case (*P < .05; n.s., not significant; P = .055).
(A) Uridine release from RBCs of day-11 chick embryos during 16 hours with 10 μmol/L E, 10 μmol/L propranolol (prop), and 35 μmol/L actinomycin D (actD). Incubation conditions were the same as in Fig 3. Data are given as the mean and SD from five experiments in each case (**P < .01; ***P < .001). (B) Changes of RBC UTP concentration during in vitro incubation with E, propranolol (prop), and actD. Agonist concentrations and incubation conditions as in (A). Data are the mean values and SD from three experiments in each case (*P < .05; n.s., not significant; P = .055).
Effect of CPCA and NE on cAMP production of embryonic RBCs harvested from 11- to 17-day-old chick embryos.
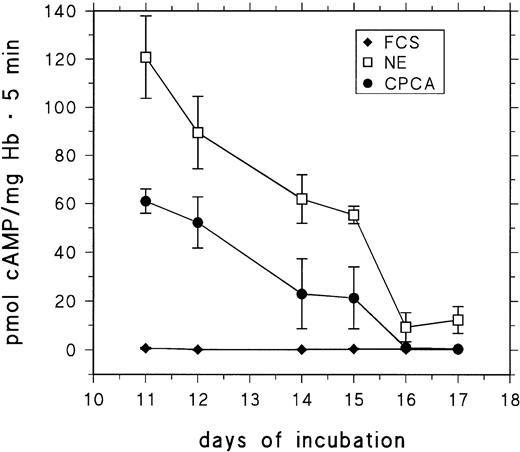
RBCs from day-11 to day-17 embryos were stimulated for 5 minutes with saturating concentrations of CPCA and NE. cAMP production was assessed and compared with control cells. The results are presented in Fig 6. The response to both NE and (particularly) CPCA is drastically decreased in RBCs from embryos older than 15 days.
Basal and stimulated cAMP-production of RBCs from 11- to 17-day-old chick embryos. RBCs were stimulated for 5 minutes at 37°C (see the Materials and Methods) with either 10 μmol/L CPCA or 10 μmol/L NE. Data are the mean values and SD from at least five determinations in each case.
Basal and stimulated cAMP-production of RBCs from 11- to 17-day-old chick embryos. RBCs were stimulated for 5 minutes at 37°C (see the Materials and Methods) with either 10 μmol/L CPCA or 10 μmol/L NE. Data are the mean values and SD from at least five determinations in each case.
DISCUSSION
P-5′-N synthesis of embryonic RBCs is stimulated by cAMP.
The developmental profile of the P-5′-N activity of chick embryonic RBCs shows a transient peak between days 13 and 15 of development and a high activity in immature primitive embryonic RBCs from day 4.
The following results support the conclusion that the increased P-5′-N activity of RBCs from day 4 and day 13 to day 15 is due to cAMP-dependent stimulation of P-5′-N synthesis. (1) Under in vitro conditions, forskolin, adenosine receptor agonist CPCA, and NE as well as E stimulate P-5′-N in embryonic chicken RBCs from day 11. (2) RBCs from day 11 to day 15 respond to CPCA and adrenergic agonists with a large increase of cAMP production. (3) During normal development, plasma catecholamine (NE) levels increase significantly after day 12.11 (4) Primitive RBCs from day 4 of incubation have an intrinsic cAMP level that is much higher than that of RBCs from day 6 (Dragon et al, manuscript in preparation).
From these data one can infer that, during normal development, the increase of P-5′-N activity at the end of the second week of incubation is largely due to the increased level of plasma catecholamines and subsequent activation of β-adrenergic receptors. In addition, external adenosine might also contribute to activation of P-5′-N synthesis by binding to A2a-receptors.
We have previously demonstrated that the synthesis of CAII of embryonic RBCs is also controlled by cAMP.12 However, in contrast to CAII, the P-5′-N of immature definitive RBCs seems to be submitted to a rapid turnover. Thus, in vitro incubation with actinomycin D lowered the P-5′-N activity to less than 50% of the initial value after 16 hours of incubation. This observation also explains partly the rapid decrease of P-5′-N after day 15, because the transcriptional activity of circulating RBCs is shut down during this period.9 A second factor that might contribute to the decrease of P-5′N activity is the observation that, in circulating RBCs from embryos older than 15 days, cAMP production by catecholamines and adenosine receptor agonist is greatly diminished.
The peak activities for P-5′-N reported in the present study (0.5 to 0.63 U/g Hb) are close to the activities reported for fetal human RBCs from 17 to 23 weeks of gestation with 0.48 U/g Hb.6
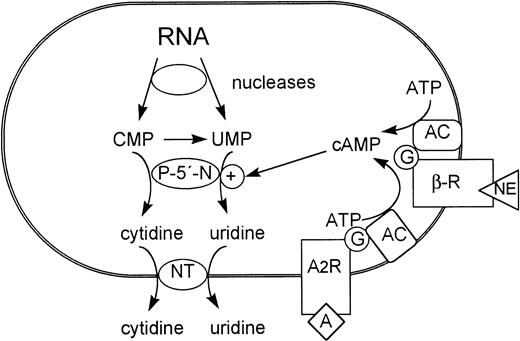
Both β-adrenergic and adenosine receptors have been described for immature adult mammalian RBCs (see Rapoport1), although the physiologic function of the receptors in RBC development is not known. Given the homology of major aspects of erythroid development in avian and mammalian species, our data suggest that activation of adenylyl cyclase via coupled receptors might partially control P-5′-N synthesis in differentiating mammalian RBCs during fetal as well as adult development and thus be of importance for the final steps of RNA metabolization (Fig 7).
Role of cAMP-inducible P-5′-N in the metabolism of terminally differentiating erythrocytes. NT, nucleoside transporter; AC, adenylyl cyclase; β-R, β-adrenergic receptor; A, adenosine; A2R, adenosine receptor.
Role of cAMP-inducible P-5′-N in the metabolism of terminally differentiating erythrocytes. NT, nucleoside transporter; AC, adenylyl cyclase; β-R, β-adrenergic receptor; A, adenosine; A2R, adenosine receptor.
Immature embryonic RBCs accumulate large amounts of pyrimidine trinucleotides.
The developmental pattern for the embryonic chick RBC UTP/CTP concentration shows that, in the second week of incubation, part of the UMP/CMP liberated from RNA degradation (cellular RNA decreases by more than 50% between days 6 and 10; Dragon et al, unpublished observation) is retained in the cell and phosphorylated to give UTP/CTP. In consequence, embryonic RBCs (from day 7 to day 12) contain about 8 to 9 mmol/L RBC pyrimidine trinucleotide in addition to about 10 to 13 mmol/L ATP. To our knowledge, the pyrimidine trinucleotide concentration is the highest reported for a cell so far.19
Obviously, RNA degradation can also contribute to the ATP pool of the erythrocyte and influence the developmental profile of the ATP concentration in embryonic RBCs. However, analysis of the ATP metabolism is complicated by the fact that embryonic RBCs have the capacity to convert significant amounts of extracellular adenosine to ATP by subsequent phosphorylation.8,20 That this latter process may be involved in the physiologic regulation of the ATP concentration is also indicated by the finding that, under in vitro conditions, early embryonic RBCs can maintain their high ATP concentration only in the presence of an extracellular source for adenosine.8 Thus, we are presently unable to evaluate the importance of adenosine salvage and RNA degradation at a quantitative level for establishing the high ATP concentration found early in development. We also do not know to which extent a decreasing activity of either metabolic pathway contributes to the rapid decrease of the ATP concentration after day 13. Further insight will be provided by investigating the developmental profile of various enzymes involved in the adenosine salvage and purine nucleotide degradation.
The fact that pyrimidine nucleotides are present in concentrations equimolar or above those of Hb raises the question of whether they can act as an allosteric effector of Hb, in competition with or in addition to ATP. This possibility is currently under investigation.
In addition, the embryonic RBC is a potential source for UTP release to the extracellular space of the embryo. This is of interest because a nucleotide receptor specific for UTP has recently been identified on cardiac endothelial cells21 and in the human placenta22 and very little is known about potential cellular sources for extracellular UTP, which in most adult blood cells amounts to much less than 0.5 mmol/L.19
Our data also show that the circulating embryonic RBC is a source for pyrimidine nucleosides. Because the embryonic RBCs cannot use pyrimidine nucleosides for their energy metabolism, due to absence of PNP, all of the pyrimidine nucleoside produced by the action of P-5′-N is released from the embryonic RBCs to the extracellular space. It follows that, at least in the avian embryo, circulating embryonic RBCs are a substantial source for provision of pyrimidine nucleoside to other embryonic tissues, particularly in the last part of development, when RBC UTP (CTP) decreases rapidly, thereby reducing the energy requirement for de novo synthesis of nucleoside at a time when the chick embryo faces increasing limitations for oxygen uptake.23 The same could be true for purine nucleosides, because embryonic RBCs contain only a small activity of purine nucleoside phosphorylase.8
For uridine, there are three metabolic sinks in the embryo. (1) It can enter de novo RNA synthesis in proliferating tissue24 and serve as precursor for thymidine nucleotides. (2) It can be degraded to β-alanine and serve as precursor for synthesis of carnosine in skeletal muscle; indeed, analysis of embryonic myoblasts shows increased uptake of β-alanine in the last stages of development.25,26 (3) It can enter the UDP-glucose pool required for glycogen synthesis in liver and yolk sac.27Taken together, the data indicate that the nucleated embryonic RBCs offer a variety of services to the embryo that are not connected to its respiratory function. This conclusion has also been made in a recent investigation of nucleated human embryonic RBCs, in which the investigators showed substantial enzymatic capacity for detoxification of endogenous and xenobiotic compounds.28
Our results can be applied to erythropoiesis in adults. In the adult organism, immature erythroid cells are segregated to the bone marrow. Upregulation of P-5′-N during the late phases of erythroid differentiation associated with RNA degradation and consequent release of pyrimidine nucleoside in erythroid foci of the bone marrow would provide an effective and energy-saving way to transfer pyrimidine nucleotide precursors to proliferating erythroid cells at earlier stages of differentiation.
Supported by DFG Ba691/5.
Address reprint requests to Stefanie Dragon, PhD, Physiologisches Institut, Universität Regensburg, 93040 Regensburg, Germany.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal