Recently, a novel gene of the major histocompatibility complex (MHC) class I family, HFE (HLA-H), has been found to be mutated in a large proportion of hereditary hemochromatosis (HH) patients. Further support for a causative role of HFE in this disease comes from the observation that β2-microglobulin knockout (β2m−/−) mice, that fail to express MHC class I products, develop iron overload. We have now used this animal model of HH to examine the capacity to adapt iron absorption in response to altered iron metabolism in the absence of β2m-dependent molecule(s). Mucosal uptake, mucosal transfer and retention of iron were measured in control and β2m−/−mice with altered iron metabolism. Mucosal uptake of Fe(III), but not of Fe(II), by the mutant mice was significantly higher when compared with B6 control mice. Mucosal transfer in the β2m−/−mice was higher, independent of the iron form tested. No significant differences were found in iron absorption between control and β2m−/− mice when anemia was induced either by repetitive bleeding or by hemolysis through phenylhydrazine treatment. However, iron absorption in mice made anemic by dietary deprivation of iron was significantly higher in the mutant mice. Furthermore, the β2m−/− mice manifested an impaired capacity to downmodulate iron absorption when dietary or parenterally iron-loaded. The expression of the defect in iron absorption in the β2m−/− mice is quantitative, with iron absorption being excessively high for the size of body iron stores. The higher iron absorption capacity in the β2m−/− mice may involve the initial step of ferric mucosal uptake and the subsequent step of mucosal transfer of iron to the plasma.
IRON HOMEOSTASIS is maintained primarily by controlling intestinal absorption.1 Several factors are known to affect iron absorption, including body iron stores (store-regulator2) and the rate of erythropoiesis (erythroid-regulator3,4). At present, it is not known whether these two main regulators of iron absorption operate through the same mechanism(s) in the intestinal mucosa, although there is some evidence that they may act independently. Inborn abnormalities of the iron regulators occur, as for example in the iron overload syndrome hereditary hemochromatosis (HH). HH is an autosomal recessive disease, characterized by a defect in regulation of iron absorption, an increase of transferrin saturation, and progressive iron deposition predominantly in parenchymal cells of several organs.5Recently, a novel gene of the major histocompatibility complex (MHC) class I family, HFE (HLA-H), has been found to be mutated in a large proportion of HH patients.6 Previously, we characterized iron metabolism in β2-microglobulin knockout (β2m−/−) mice that fail to express MHC class I products.7,8 Transferrin saturation in the β2m−/− mice is abnormally high and pathologic iron depositions occur predominantly in liver parenchymal cells.8,9 We have now used this animal model of HH to examine the role of β2m-dependent molecules in the regulation of iron absorption. Iron absorption is a multistep process, consisting of the initial mucosal iron uptake from the lumen of the gut and the subsequent transfer of iron into the plasma. In mice, reduction of Fe(III) to Fe(II) is a prerequisite for iron uptake by the intestine.10 Recently, a persistent increase in the reduction and uptake of iron by the intestine in HH patients has been reported,11 whereas other studies indicate that the mucosal transfer of iron is also increased.12 Our previous studies on the absorption of a reduced form of iron [Fe(II)] in β2m−/− mice showed that the mutant mice fail to limit the transfer of iron from mucosal cells into the plasma, whereas the mucosal uptake is similar to normal B6 mice.8Thus, the reducing step in this study was not investigated. In view of this, we examined iron uptake, transfer, and retention in β2m−/− and B6 control mice with altered iron metabolism to determine (1) the absorption of both Fe(III) and Fe(II); (2) the organ distribution of the newly absorbed, radiolabeled iron; (3) whether iron absorption in β2m−/− mice is differently affected by erythroid demand versus altered iron stores; and (4) the step of iron absorption at which the defect is located.
The results show that, in β2m−/− mice, mucosal uptake and retention of iron delivered as Fe(III) is higher than in control mice, while no differences in mucosal uptake are detectable from a test dose containing Fe(II). Mucosal transfer when testing Fe(III) or Fe(II) is similar and significantly increased. Upregulation of iron absorption in response to dietary iron deficiency is excessively high in β2m−/− mice, but increased to the same extent as control mice when erythroid demand increases. Furthermore, β2m−/− mice have a limited capacity to downregulate iron absorption in response to increased iron stores. Taken together, these results show that the expression of the defect in iron absorption in the β2m−/− mice is quantitative, with iron absorption being excessively high for the size of body iron stores.
MATERIALS AND METHODS
Animals and Treatments
C57BL/6 (B6) female mice aged 6 to 8 weeks were purchased from the IFFA Credo (Brussels, Belgium), and used as controls. The β2m−/− mice13 were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA) and further bred in our animal facility. The mutant mice had been back-crossed 11 times onto the C57BL/6. Control animals received a commercial diet (RMH-B; Hope Farms, Woerden, The Netherlands). Dietary iron deficiency was induced by placing mice on a purified diet with low iron content14 and demineralized water for a period of 2 weeks. Dietary iron-loading was obtained by placing mice on an iron-enriched diet containing 2.5% (wt/wt) carbonyl iron (Sigma Immunochemicals, St Louis, MO) for 2 weeks. Before iron absorption testing, all animals received the same control diet for 2 days to avoid any direct influence of the diet on intestinal iron absorption. To induce parenteral iron-loading, 5 mg of iron-dextran (Sigma Immunochemicals) was injected subcutaneously and the mice were left for 2 weeks to allow for iron redistribution. Hemolytic anemia was induced through phenylhydrazine (PHZ; Sigma Immunochemicals) by subcutaneous injection of 60 mg/kg of body weight on 2 consecutive days, and iron absorption was measured at day 4. To induce anemia through phlebotomy, a total of approximately 1 mL of blood was extracted over a period of 2 weeks by retro-orbital puncture.
For all animal experiments, written consent was obtained from the local Animal Experiments Committee of Utrecht University (Utrecht, The Netherlands).
Hematologic Measurements
Heparinized blood was obtained by orbital puncture under diethylether anesthesia. Red blood cell count (RBC), hemoglobin (Hb), hematocrit (HCT), and mean corpuscular volume (MCV) were determined using a Cell-Dyn 1600 counter (Sequoia-Turner Corp, Mountain View, CA).
Measurement of Liver Iron Concentration
Liver samples were weighed wet and then dried overnight at 106°C and weighed again. The dried samples were ashed in an oven at 500°C for 17 hours and then fully solubilized in 6 mol/L HCl; the final solution was adjusted with demineralized water to a final HCl concentration of 1.2 mol/L. Iron concentration of the samples was determined by flame atomic absorption spectrometry (Varian SpectrAA 250 Plus; Varian, Mulgrave, Victoria, Australia).
Gastrointestinal Iron Absorption
Measurement of iron absorption was performed as previously described.15 Briefly, in a series of experiments,59Fe(III) citrate was added to Fe(II) as ferrous sulphate solution with a 20-fold molar excess of L-ascorbic acid to reduce the Fe(III). 51CrCl3 was added as a nonabsorbable indicator. The total amount of Fe(II) per test dose was 5 μg per mouse and had a final volume of 0.3 mL. When absorption of Fe(III) was tested, ferric-citrate (Sigma Immunochemicals) was added to obtain a total of 5 μg per mouse, with a 20-fold molar excess of sodium citrate dihydrate (Sigma Immunochemicals) to maintain mononuclear ferric-citrate complexes and to prevent precipitation. Each mouse received approximately 50 kBq of 59Fe and 200 kBq of51Cr.
The test dose was orally applied with the use of an olive-tipped oroesophageal needle. Total body radioactivity was measured with a whole-body gamma counter (Automatic Scanner DS4/4S; Tracelab Ltd, Weybridge, Surrey, UK) with separate detection windows for59Fe and 51Cr peaks. The values were corrected for radioisotope decay, contribution of 59Fe to the51Cr peak, and day-to-day fluctuations of the scanner with the use of a radium source. Mucosal uptake of iron (MU) was calculated from the activity of 59Fe and 51Cr administered (measured immediately after test dose administration and considered as 100%) and the activity of 59Fe (F1) and 51Cr (C1) found within the body 22 hours later, using the following equation: MU = 100 × (F1 − C1)/(100 − C1)%. F1 and C1 were expressed as the percentage of the amount of 59Fe and 51Cr administered, respectively. 59Fe retention (IR) was determined by whole-body counting 4 days after the administration of the test dose. The mucosal transfer fraction of iron (MT) was determined as the ratio IR/MU.
Tissue Distribution of Gastrointestinally Absorbed Iron
To determine the tissue distribution of 59Fe after gastrointestinal administration of Fe(II), animals were killed at day 6, and whole organs were then removed and washed in physiologic salt. The radioactivity of whole organs was measured in a gamma counter (Packard Instruments, Downers Grove, IL).
Statistical Analysis
Results are presented as the mean ± SD or, when indicated, as the mean ± SEM. The Student's t-test was used for comparison between the control and knockout mouse groups. The level of significance was pre-set at P < .05.
RESULTS
Iron Absorption of Fe(II) Versus Fe(III)
Reduction of Fe(III) to Fe(II) seems to be a prerequisite for iron uptake by the intestine in both mice and humans.10,11 This enzymatic step is bypassed if iron reaches the microvillous membrane as Fe(II), eg, when excess ascorbic acid or orange juice is added to the radioactive iron test dose.12 16 We therefore compared Fe(III) and Fe(II) iron absorption steps in control B6 and β2m−/− mice. As shown in Table 1, mucosal uptake of Fe(III) was significantly lower than Fe(II) in control mice, but not in the β2m−/− mice. Mucosal transfer of iron, representing transport across the basolateral membrane into the plasma, was higher in β2m−/− mice than in control mice, regardless of the iron oxidation state. Accordingly, ultimate iron retention was significantly higher in the β2m−/− mice compared with control mice when iron was administered in the ferric form.
Absorption of Ferrous and Ferric Iron
| Strain . | n . | Form of Iron . | Mucosal Uptake (%) . | Mucosal Transfer (IR/MU) . | Iron Retention (%) . |
|---|---|---|---|---|---|
| B6 | 8 | Fe2+ | 22 ± 4 | 0.73 ± 0.06 | 16 ± 2 |
| 8 | Fe3+ | 16 ± 3-150 | 0.61 ± 0.05-150 | 10 ± 2-151 | |
| β2m−/− | 8 | Fe2+ | 20 ± 2 | 0.89 ± 0.03-152 | 17 ± 2 |
| 8 | Fe3+ | 21 ± 3-153 | 0.80 ± 0.05-150,-152 | 16 ± 2-152 |
| Strain . | n . | Form of Iron . | Mucosal Uptake (%) . | Mucosal Transfer (IR/MU) . | Iron Retention (%) . |
|---|---|---|---|---|---|
| B6 | 8 | Fe2+ | 22 ± 4 | 0.73 ± 0.06 | 16 ± 2 |
| 8 | Fe3+ | 16 ± 3-150 | 0.61 ± 0.05-150 | 10 ± 2-151 | |
| β2m−/− | 8 | Fe2+ | 20 ± 2 | 0.89 ± 0.03-152 | 17 ± 2 |
| 8 | Fe3+ | 21 ± 3-153 | 0.80 ± 0.05-150,-152 | 16 ± 2-152 |
Abbreviation: n, number of animals.
P < .05, comparison between form of iron.
P < .0001, comparison between form of iron.
P < .0001, comparison between strains with the same form of iron.
P < .05, comparison between strains with the same form of iron.
Tissue Distribution of Gastrointestinally Absorbed Iron
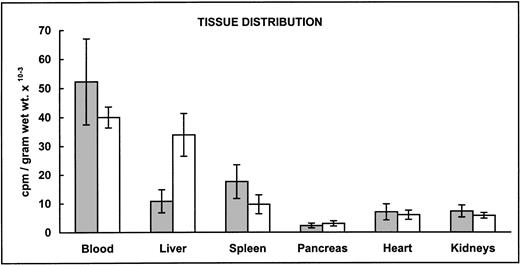
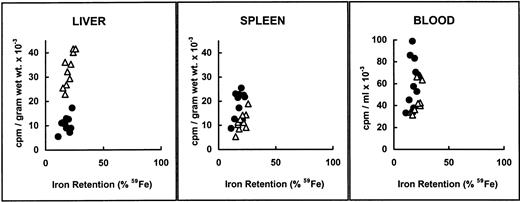
We next examined organ distribution of newly absorbed 59Fe administered as Fe(II). We chose to test iron absorption of ferrous iron to achieve similar values of iron retention in both mice strains and therefore compare tissue iron distribution resulting from a similar amount of radioactive iron. As shown by the radioactivity detected in different organs, higher amounts of iron were deposited in the liver of β2m−/− mice, with somewhat lesser amounts being found in spleen and blood (Fig 1A). Iron retention, here defined as the percentage of 59Fe found in the body 6 days after the administration of an oral radioactive test dose, was plotted against the recovered radioactivity from liver, spleen, and blood (Fig 1B). As expected, iron retention from the ferrous test dose was not significantly different between the two mouse strains. The differences reside in the quantity distributed in the liver.
Distribution of gastointestinally absorbed59Fe. (A) Control B6 (▧) and β2m−/−(□) mice received a radioactive test dose solution of ferrous iron. At day 6, the mice were exsanguinated and their organs were dissected. The amount of radioactivity in selected organs was measured in a gamma counter. The data are presented as the mean specific activity (counts per minute per gram of wet weight of tissue) ± SD (n = 12; combined data from 2 experiments). Mean values in livers and spleens from β2m−/− mice were significantly different from those of B6 mice (Student's t-test): P < .05. (B) Radioactivity recovered from livers, spleens, and blood versus iron retention in control B6 (•) and β2m−/− (▵) mice from the same experiments. Iron retention was determined at day 6 in a whole-body counter. Individual values for each mouse are shown.
Distribution of gastointestinally absorbed59Fe. (A) Control B6 (▧) and β2m−/−(□) mice received a radioactive test dose solution of ferrous iron. At day 6, the mice were exsanguinated and their organs were dissected. The amount of radioactivity in selected organs was measured in a gamma counter. The data are presented as the mean specific activity (counts per minute per gram of wet weight of tissue) ± SD (n = 12; combined data from 2 experiments). Mean values in livers and spleens from β2m−/− mice were significantly different from those of B6 mice (Student's t-test): P < .05. (B) Radioactivity recovered from livers, spleens, and blood versus iron retention in control B6 (•) and β2m−/− (▵) mice from the same experiments. Iron retention was determined at day 6 in a whole-body counter. Individual values for each mouse are shown.
Iron Absorption in Mice With Altered Iron Metabolism
Effect of modifying the erythroid regulator.
In the next set of experiments, we examined the capacity of β2m−/− to regulate iron absorption in response to increased erythropoiesis demand in the presence of unaltered or decreased iron stores. For this purpose, two models were used to stimulate the erythroid regulator. In one group, the mice were phlebotomized, and in another group, hemolytic anemia was induced by PHZ treatment. Thus, we could evaluate iron absorption changes in mice with increased erythropoiesis demand in two different situations: with depleted iron stores (phlebotomy treatment) and with unchanged iron stores (PHZ treatment). Hematological values measured at day 0, ie, when the test dose was administered, and at day 4, ie, when ultimate iron retention was measured, are given in Table 2. PHZ treatment resulted in a more severe reduction of RBCs in B6 (44%) when compared with β2m−/− mice (23%). MCV was significantly increased at day 4 in both mice strains, reflecting a young cell population as a result of increased erythropoiesis. Less pronounced changes were observed after phlebotomy treatment in respect to RBC counts, Hb, and MCV. Recovery of RBC and Hb values was observed in both mouse strains at day 4 (Table 2). These results show that both treatments were effective in increasing erythropoiesis demand at the time the radiolabeled iron was administrated.
Erythroid Parameters
| Strain . | Treatment . | RBC (×1012/L) . | Hb (mmol/L) . | MCV (fL) . | |||
|---|---|---|---|---|---|---|---|
| Day 0* . | Day 4† . | Day 0 . | Day 4 . | Day 0 . | Day 4 . | ||
| B6 (n = 6) | Untreated‡ | 9.1 ± 0.4 | 9.7 ± 0.5 | 45 ± 1 | |||
| Phenylhydrazine | 5.1 ± 0.61-153 | 7.9 ± 0.51-153 | 8.3 ± 1.4 | 8.5 ± 0.81-153 | 44 ± 1 | 56 ± 11-153 | |
| Phlebotomy | 8.5 ± 0.21-153 | 8.9 ± 0.2 | 8.7 ± 0.21-153 | 9.4 ± 0.2 | 46 ± 1 | 46 ± 0.11-153 | |
| Low dietary iron | 9.0 ± 0.8 | 9.2 ± 0.4 | 8.6 ± 0.51-153 | 9.7 ± 0.4 | 44 ± 1 | 45 ± 1 | |
| β2m−/− (n = 6) | Untreated‡ | 9.6 ± 0.21-155 | 10.8 ± 0.41-155 | 47 ± 11-155 | |||
| Phenylhydrazine | 7.4 ± 0.41-153,1-155 | 8.1 ± 0.31-153 | 11.4 ± 0.81-155 | 9.7 ± 0.31-153,1-155 | 46 ± 11-155 | 62 ± 11-153,1-155 | |
| Phlebotomy | 9.5 ± 0.31-155 | 9.6 ± 0.21-155 | 10.2 ± 0.41-153,1-155 | 10.8 ± 0.31-155 | 47 ± 11-155 | 48 ± 11-153,1-155 | |
| Low dietary iron | 9.0 ± 0.31-153 | 9.5 ± 0.1 | 9.4 ± 0.31-153,1-155 | 10.4 ± 0.41-155 | 46 ± 0.11-155 | 47 ± 11-155 | |
| Strain . | Treatment . | RBC (×1012/L) . | Hb (mmol/L) . | MCV (fL) . | |||
|---|---|---|---|---|---|---|---|
| Day 0* . | Day 4† . | Day 0 . | Day 4 . | Day 0 . | Day 4 . | ||
| B6 (n = 6) | Untreated‡ | 9.1 ± 0.4 | 9.7 ± 0.5 | 45 ± 1 | |||
| Phenylhydrazine | 5.1 ± 0.61-153 | 7.9 ± 0.51-153 | 8.3 ± 1.4 | 8.5 ± 0.81-153 | 44 ± 1 | 56 ± 11-153 | |
| Phlebotomy | 8.5 ± 0.21-153 | 8.9 ± 0.2 | 8.7 ± 0.21-153 | 9.4 ± 0.2 | 46 ± 1 | 46 ± 0.11-153 | |
| Low dietary iron | 9.0 ± 0.8 | 9.2 ± 0.4 | 8.6 ± 0.51-153 | 9.7 ± 0.4 | 44 ± 1 | 45 ± 1 | |
| β2m−/− (n = 6) | Untreated‡ | 9.6 ± 0.21-155 | 10.8 ± 0.41-155 | 47 ± 11-155 | |||
| Phenylhydrazine | 7.4 ± 0.41-153,1-155 | 8.1 ± 0.31-153 | 11.4 ± 0.81-155 | 9.7 ± 0.31-153,1-155 | 46 ± 11-155 | 62 ± 11-153,1-155 | |
| Phlebotomy | 9.5 ± 0.31-155 | 9.6 ± 0.21-155 | 10.2 ± 0.41-153,1-155 | 10.8 ± 0.31-155 | 47 ± 11-155 | 48 ± 11-153,1-155 | |
| Low dietary iron | 9.0 ± 0.31-153 | 9.5 ± 0.1 | 9.4 ± 0.31-153,1-155 | 10.4 ± 0.41-155 | 46 ± 0.11-155 | 47 ± 11-155 | |
Abbreviation: n, number of animals per group.
Day 0 refers to the day the test dose was applied.
Day 4 refers to the day iron retention was measured.
Plotted data from day 0 and day 4.
P < .05, comparison between untreated and treated mice.
P < .05, comparison between strains with the same treatment.
Liver iron concentration in untreated β2m−/−mice was significantly higher than in untreated B6 mice (P < .001; Table 3). PHZ-treated B6 mice showed a significant increase in liver iron concentration (P < .0001). In contrast, PHZ-treated β2m−/− mice significantly decreased liver iron concentration (P< .01; Table 3). This indicates that β2m−/− mice are able to rapidly mobilize the excess liver iron in response to increased demand for erythropoiesis. Indeed, when a higher dose of PHZ was used (120 mg/kg body weight), all B6 control mice died, whereas all β2m−/− mice survived (data not shown). After repetitive bleeding, no statistically significant changes in liver iron concentration were observed between treated and untreated mouse groups (Table 3).
Iron Concentration in Livers
| Treatment . | μg Fe/g Dry Weight . | |
|---|---|---|
| B6 . | β2m−/− . | |
| Untreated | 220 ± 15 | 437 ± 108* |
| PHZ | 294 ± 12† | 251 ± 58† |
| Phlebotomy | 204 ± 39 | 326 ± 82* |
| Low dietary iron | 164 ± 20† | 424 ± 75* |
| Iron-dextran | 935 ± 351† | 1,498 ± 222† |
| High dietary iron | 637 ± 114† | 893 ± 190† |
| Treatment . | μg Fe/g Dry Weight . | |
|---|---|---|
| B6 . | β2m−/− . | |
| Untreated | 220 ± 15 | 437 ± 108* |
| PHZ | 294 ± 12† | 251 ± 58† |
| Phlebotomy | 204 ± 39 | 326 ± 82* |
| Low dietary iron | 164 ± 20† | 424 ± 75* |
| Iron-dextran | 935 ± 351† | 1,498 ± 222† |
| High dietary iron | 637 ± 114† | 893 ± 190† |
*P < .05, comparison between strains with the same treatment.
P < .05, comparison between untreated and treated mice.
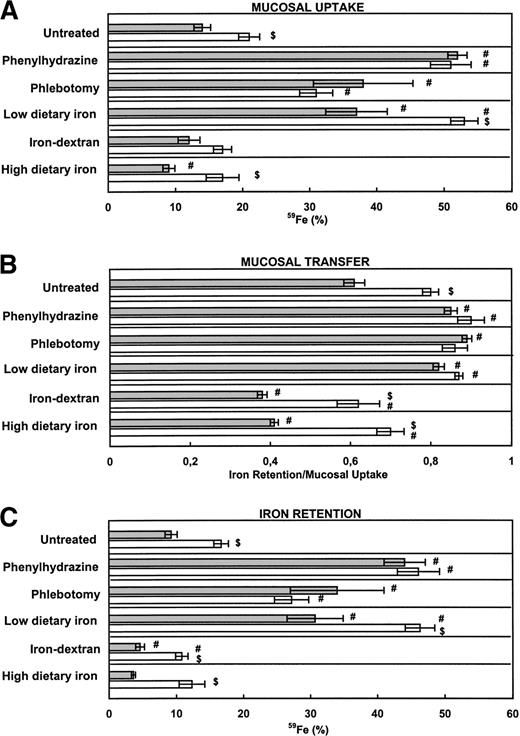
As shown in Fig 2, mucosal uptake, mucosal transfer, and retention of Fe(III) was again significantly higher in untreated β2m−/− mice than in untreated B6 mice. Phlebotomy treatment induced an increase in mucosal uptake, mucosal transfer, and retention of iron in B6 mice that was not significantly different from the mutant mice. Interestingly, PHZ treatment, which does not alter the size of total body iron stores, induced an even greater increase in mucosal iron uptake and retention, which was similar in both mice strains (Fig 2). This finding supports the notion that the erythroid regulator surpasses the store regulator in acute hemolysis in a way that is independent of β2m-associated molecule(s).
Iron absorption in mice with altered iron metabolism. (A) Mucosal uptake and (B) mucosal transfer of iron. (C) Iron retention. B6 (▧) and β2m−/− (□) mice were divided into 6 groups (n = 6 per group) and treated as described in Materials and Methods. Hemolysis was obtained by phenylhydrazine injection of 60 mg/kg body weight. Phlebotomy resulted in 1 mL blood loss from each mouse. The iron-deficient diet contained 6 mg Fe/Kg and the iron-enriched diet contained 2.5% wt/wt carbonyl iron. All other groups were maintained on a standard diet containing 164 mg Fe/kg. Parenteral loading was obtained by injecting 5 mg of iron-dextran. Mucosal uptake was measured at day 1 after the administration of a test dose of ferric iron, and iron retention was measured at day 4. Mucosal transfer of iron was calculated from the ratio IR/MU. The data are presented as the mean ± SEM. ($) Student's t-test for comparison of β2m−/− mice with B6 mice, same treatment (P < .05). (#) Student's t-test for comparison between untreated (control) and treated mice (P < .05).
Iron absorption in mice with altered iron metabolism. (A) Mucosal uptake and (B) mucosal transfer of iron. (C) Iron retention. B6 (▧) and β2m−/− (□) mice were divided into 6 groups (n = 6 per group) and treated as described in Materials and Methods. Hemolysis was obtained by phenylhydrazine injection of 60 mg/kg body weight. Phlebotomy resulted in 1 mL blood loss from each mouse. The iron-deficient diet contained 6 mg Fe/Kg and the iron-enriched diet contained 2.5% wt/wt carbonyl iron. All other groups were maintained on a standard diet containing 164 mg Fe/kg. Parenteral loading was obtained by injecting 5 mg of iron-dextran. Mucosal uptake was measured at day 1 after the administration of a test dose of ferric iron, and iron retention was measured at day 4. Mucosal transfer of iron was calculated from the ratio IR/MU. The data are presented as the mean ± SEM. ($) Student's t-test for comparison of β2m−/− mice with B6 mice, same treatment (P < .05). (#) Student's t-test for comparison between untreated (control) and treated mice (P < .05).
Effect of modifying the store regulator.
To manipulate the store regulator, animals were fed an iron-deficient or an iron-supplemented diet. A third group was parenterally iron-loaded, by injection of iron-dextran. Thus, iron absorption was evaluated in mice with insufficient erythropoiesis due to limited dietary iron and in mice with increased iron stores predominantly in parenchymal cells (dietary iron) versus reticulo-endothelial (RE) cells (parenteral iron-dextran). This distinction in cellular iron stores is important, because in HH patients excess iron is deposited in parenchymal cells, whereas little iron is found in RE cells until the later stages of the disease.17,18 Because intestinal iron absorption is inversely related to RE iron stores, an iron-handling defect of RE cells has been suggested to be responsible for both excess iron deposition in parenchymal cells and lack of feedback regulation of duodenal iron uptake in HH.19
Feeding of an iron-deficient diet to growing animals resulted in a lower liver iron concentration in B6 mice compared with the B6-untreated group (P < .0001), but remarkably not in β2m−/− mice (Table 3). A modest but significant decrease of Hb values at day 0 (Table 2) and increased mucosal uptake and transfer of iron, resulting in an increased iron retention (Fig 2), was observed in both mouse strains placed on an iron-deficient diet. Paradoxically, despite the fact that β2m−/− mice have increased amounts of iron stores (Table 3), these alterations in iron absorption steps were significantly more pronounced in these mice (Fig 2). However, taking into account the differences already seen in untreated groups, it should be noted that both mouse strains responded with a twofold to threefold increase in iron retention.
Iron-dextran treatment resulted in a more severe iron overload of the liver when compared with a 2-week iron-enriched regime (Table 3). Iron-dextran complexes are recovered from the plasma through phagocytosis by cells of the RE system, leading to the appearance of heavy iron-granules in Kupffer cells in both mouse strains (data not shown). No significant changes in mucosal uptake of iron were observed in these groups when compared with untreated groups (Fig 2A). Thus, the decrease observed in iron retention is a consequence of downmodulation of the mucosal transfer step (Fig 2B and C). Importantly, mucosal transfer and the ultimate iron retention in iron-dextran treated β2m−/− mice was significantly higher than in the corresponding B6 mouse group, approaching the levels observed in nontreated B6 mice.
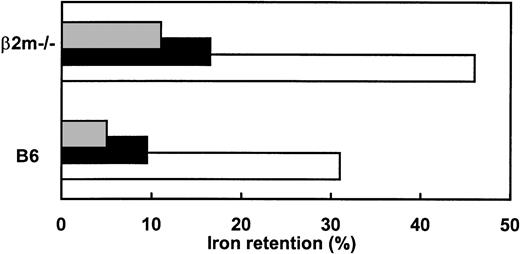
As previously reported,8 dietary iron loading resulted in visible iron deposits in Kupffer and parenchymal cells of B6 mice and predominantly in parenchymal cells of β2m−/−mice (data not shown). Compared with parenteral iron loading, the major difference in iron absorption values after dietary iron loading was an additional decrease in mucosal uptake observed in B6 mice, but not in β2m−/− mice (Fig 2A). Again, mucosal transfer and the ultimate iron retention, although slightly decreased in dietary iron-loaded β2m−/− mice, was at the level seen in untreated B6 mice (Fig 2B and C). These results clearly show that β2m−/− mice retain some ability to regulate iron absorption in response to altered iron stores. However, the ultimate iron retention is invariably higher than in the corresponding B6 mouse group (Fig 3). Furthermore, no significant differences were found between iron absorption in dietary and parenterally iron-loaded β2m−/− mice (Fig 2). Thus, despite increased iron deposition in Kupffer cells, achieved by iron-dextran injections in β2m−/−, no further feedback regulation of the levels of iron absorption was observed. This is in agreement with previous observations that reconstitution of β2m−/− mice with normal hematopoietic cells redistributes the iron from parenchymal to Kupffer cells, but does not correct the mucosal defect.8
Adaptive response of iron absorption in B6 and β2m−/− mice to dietary manipulations. Animals received a radioactive test dose solution of ferric iron and iron retention was measured at day 4. Data represent mean values for iron retention in mice fed an iron-enriched (▧), iron-deficient (□), or standard diet (▪).
Adaptive response of iron absorption in B6 and β2m−/− mice to dietary manipulations. Animals received a radioactive test dose solution of ferric iron and iron retention was measured at day 4. Data represent mean values for iron retention in mice fed an iron-enriched (▧), iron-deficient (□), or standard diet (▪).
DISCUSSION
It was expected that further elucidation of the mechanism(s) of iron absorption would be possible as soon as the gene responsible for HH, an HLA-A–linked disease of iron metabolism, could be identified. Recently, a new candidate gene for this disease has been found.6 The new gene encodes an MHC class I-like protein, and two mutations were found in more than 85% of HH patients. One of the mutations, present in 83% of the HH patients, results in a cysteine to tyrosine substitution at amino acid 282 (C282Y) and is thought to interfere with the binding of the HLA-H heavy chain to β2m.20 Further support for a causative role of HLA-H in this disease comes from the previous observation that β2m−/− mice develop iron overload.7-9 These mutant mice are unable to express MHC class I products, including the mouse homolog of HLA-H (HFE) gene, recently identified as MR2.21
Iron Absorption of Fe(II) Versus Fe(III)
Reduction of Fe(III) to Fe(II) has been shown to be a prerequisite for iron uptake by the intestine both in mice and humans.10,11,22,23 A significant increase in the duodenal mucosal Fe(III) reducing activity in HH patients compared with normal controls has been reported recently.11 Mucosal uptake of ferric iron by the β2m−/− mice was significantly higher when compared with B6 mice, whereas no differences were detected in mucosal uptake of ferrous iron. Our results comparing ferric iron absorption in B6 and β2m−/− mice are compatible with a higher reducing capacity of the intestinal mucosa in mice lacking functional β2m and β2m-dependent molecules. In addition, a higher mucosal transfer capacity independent of the iron form administrated has been confirmed.
Tissue Distribution of Gastrointestinally Absorbed Iron
Six days after the oral administration of radiolabeled iron, increased amounts of 59Fe were found in the livers of β2m−/− mice compared with B6 mice. Taking into account that β2m−/− mice have a higher transferrin saturation and reduced amounts of apo-transferrin in plasma,8 these results indicate that gastrointestinally absorbed iron is released from mucosal cells independently of the availability of transferrin in plasma and is deposited in the liver. This is in agreement with previous findings in hypotransferrinemic mice, characterized by a heritable reduction in circulating transferrin, leading to anemia but also to increased storage of iron in the liver.24 In these mice, the gastrointestinally absorbed iron not bound to transferrin is deposited in the liver, suggesting the existence of an uptake system for non–transferrin-bound iron.25
Iron Absorption in Mice With Altered Iron Metabolism
Iron homeostasis is maintained essentially by controlling intestinal iron absorption.1 Several factors have been identified that affect iron absorption, including body iron stores,2 the rate of erythropoiesis,3 hypoxia,26 and inflammation.27
We have used our animal model of HH to examine the capacity to adapt iron absorption in response to altered iron metabolism in the absence of β2m-dependent molecule(s). We wanted to differentiate between the erythroid regulator and the store regulator. Therefore, we stimulated differentially these iron regulators. The results showed that, as far as the erythroid regulator is concerned, no major differences in iron absorption between B6 control mice and β2m−/−mice were found. These treatments more severely affected B6 mice than β2m−/− mice, probably due to the increased iron stores already present in the β2m−/−mice. Both steps of iron absorption, ie, mucosal uptake and mucosal transfer, seem to increase in response to PHZ and to phlebotomy treatment. This supports the notion that the erythroid regulator surpasses the store regulator in acute hemolysis in a way that is independent of β2m-associated molecule(s).
Feeding an iron-deficient diet also increases both mucosal uptake and mucosal transfer of iron, with the opposite occurring when feeding an iron-enriched diet. The changes in mucosal uptake observed in these models could partially be caused by the altered iron content of the diet, although the mice were fed with normal diet 2 days before testing to normalize the intestinal lumen iron content. By comparison, injection of iron-dextran, while resulting in a higher iron overload of the liver, does not induce significant changes in the initial step of mucosal uptake. Rather, the resulting lower iron retention seems to reflect a lower transfer of iron from the mucosal cell into the blood. This means that these two processes, ie, mucosal uptake and mucosal transfer of iron, can be separately regulated. Further support for this view appears from studies performed in other genetically defective mice strains.28,29 One of these is sex-linked anemia (sla),in which the defect is expressed as reduced transfer of iron from mucosal cells to the blood.28,30 The second abnormality reported in mice is hereditary microcytic anemia (mk).29 In contrast to sla, mk mice display a markedly reduced uptake of iron across the microvillous membrane but no reduction in the subsequent transfer across the basolateral membrane. Recently, the mk has been identified as Nramp2,31 identical to a divalent cation-transporter that transports Fe(II).32 The data from sla andmk mice suggest that at least two independent iron-transporting complexes are involved in the handling of iron within mucosal cells. The present results indicate that in β2m−/−mice both steps of mucosal uptake and mucosal transfer are quantitatively affected. Indeed, the results concerning the store regulator clearly show that in β2m−/− mice, although being able to respond by increasing or decreasing iron absorption, the ultimate iron retention is invariably higher than seen in B6 mice (Fig 3). Importantly, in HH patients, iron absorption is also regulated to some extent. In fact, iron absorption is found to be within the normal range in many patients at presentation. With reduction of iron stores by phlebotomy, absorption increased to high levels and only became suppressed as iron stores reaccumulated.33 Thus, it can be said that HH is associated with a quantitative defect of intestinal function, in which net absorption of iron appears to be “inappropriate for the size of the storage pool of iron.”33-36
Because of the similarities in iron metabolism seen in HH and β2m−/− mice,8 this suggests that heterodimers composed of α-heavy chain and β2m (HFE in humans6 and MR2 in mice21) rather than α-heavy chain or β2m subunit per se may influence iron absorption. A possible interpretation could be that HFE/MR2 product may negatively interfere with the expression of Fe(II)-transporter(s) (eg, Nramp2) and/or with the expression of ferrireductase complexes presumably present at or near the microvillous membrane.
In conclusion, this study shows that the defect in the regulation of iron absorption in β2m−/− mice is quantitative rather than qualitative, sharing similarities with what has been observed in HH patients. The expression of the defect may involve the initial step of ferric mucosal uptake and the subsequent step of mucosal transfer of iron to the plasma.
ACKNOWLEDGMENT
The authors are grateful to Toon Hesp, Jan Smits, and Else Dorrestein for taking care of the animals; to Drs K. Wienk, F. Arosa, and G. Porto for valuable discussions; and to Dr N. Barker for reviewing the manuscript.
Supported by a grant from Junta Nacional de Investigação Cientı́fica e Tecnológica-PRAXIS XXI (BD/2866/94).
Address reprint requests to Manuela Santos, Department of Immunology, University Hospital Utrecht, Room F03.821, Heidelberglaan 100, 3584 CX Utrecht, The Netherlands.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal