Phospholipid asymmetry in the red blood cell (RBC) lipid bilayer is well maintained during the life of the cell, with phosphatidylserine (PS) virtually exclusively located in the inner monolayer. Loss of phospholipid asymmetry, and consequently exposure of PS, is thought to play an important role in red cell pathology. The anemia in the human thalassemias is caused by a combination of ineffective erythropoiesis (intramedullary hemolysis) and a decreased survival of adult RBCs in the peripheral blood. This premature destruction of the thalassemic RBC could in part be due to a loss of phospholipid asymmetry, because cells that expose PS are recognized and removed by macrophages. In addition, PS exposure can play a role in the hypercoagulable state reported to exist in severe β-thalassemia intermedia. We describe PS exposure in RBCs of 56 comparably anemic patients with different genetic backgrounds of the α- or β-thalassemia phenotype. The use of fluorescently labeled annexin V allowed us to determine loss of phospholipid asymmetry in individual cells. Our data indicate that in a number of thalassemic patients, subpopulations of red cells circulate that expose PS on their outer surface. The number of such cells can vary dramatically from patient to patient, from as low as that found in normal controls (less than 0.2%) up to 20%. Analysis by fluorescent microscopy of β-thalassemic RBCs indicates that PS on the outer leaflet is distributed either over the entire membrane or localized in areas possibly related to regions rich in membrane-bound α-globin chains. We hypothesize that these membrane sites in which iron carrying globin chains accumulate and cause oxidative damage, could be important in the loss of membrane lipid organization. In conclusion, we report the presence of PS-exposing subpopulations of thalassemic RBC that are most likely physiologically important, because they could provide a surface for enhancing hemostasis as recently reported, and because such exposure may mediate the rapid removal of these RBCs from the circulation, thereby contributing to the anemia.
THE ANEMIA IN THE human thalassemias is caused by a combination of ineffective erythropoiesis (intramedullary hemolysis) and hemolysis of adult RBCs in the peripheral blood. Although ineffective erythropoiesis might play a more important role in β-thalassemic variants, destruction of RBCs in the peripheral blood may be relatively more important in comparably severe α-thalassemia.1,2 Several studies have proposed possible mechanisms that lead to destruction of RBCs in the peripheral blood. Both α- and β-thalassemic RBCs have an altered morphology and exhibit a decreased deformability as measured by ektacytometry.3 This altered deformability is in part due to rigidity of their membranes and the state of hydration of these cells.3 Oxidative damage to the membrane skeleton might in part be responsible for this membrane rigidity, adversely affecting the deformability of the cell.4-6 The decreased deformability could impair passage of RBCs through the sinusoidal walls of reticuloendothelial organs,7 and consequently trigger the removal of these cells from the circulation.
In addition to these well-described mechanical abnormalities, β-thalassemic RBCs are phagocytosed by murine monocytes/macrophages at a rate 20 times normal8 and by human macrophages at a rate 2 to 3 times normal.9 Several mechanisms have been implicated in the enhanced recognition and phagocytosis of thalassemic RBCs. One hypothesis is that the lower sialic acid level of β-thalassemic RBCs leads to enhanced recognition and phagocytosis.8 Another observation is that RBCs from β-thalassemia intermedia patients show aggregated clusters of hemichrome and band 3, presumably as a result of oxidant injury. Immunoglobulin (perhaps autologous antibodies) and complement components localize at the membrane exoface over these clusters.10 Macrophages recognize immunoglobulin as well as complement components and act to remove these damaged cells.9 Loss of phospholipid (PL) asymmetry has also been implicated in the recognition of RBCs. In particular, phosphatidylserine (PS), which in normal cells is strictly confined to the inner half of the membrane phospholipid bilayer, is recognized as a first step before removal in vitro11 and in vivo.12 It has been proposed that PS exposure might play a role both in the removal of β-thalassemic RBCs and the hypercoagulable state thought to exist in severe β-thalassemia intermedia.13 β-thalassemic red cells induce increased thrombin formation in vitro when incubated with purified prothrombinase factors, indicating that a PS-containing surface is available.14 These studies, however, do not resolve whether PS exposure correlates with disease severity, or if it is similar in α- or β-thalassemic patients with comparable anemia. Moreover, because PS exposure could lead to RBC removal, it is of importance to know if loss of PL asymmetry occurs to some degree in all cells, or if subpopulations of cells exist that expose PS while the rest of the red cell population exhibits a normal phospholipid asymmetry.
We have recently developed a method to determine the exposure of PS in individual red cells, using fluorescently labeled annexin V (AV).15 This approach also allows the selection of these cells from the population for further analysis.15Identification and selection of RBCs using this method is advantageous, because the PS-exposing cells are selected by binding to AV in the presence of physiologic concentrations of calcium and can be isolated from the probe by simply lowering the calcium concentration.
The purpose of this study was to determine if there are differences in PS exposure on red cells in comparably anemic patients with the different genetic background of the α- or β-thalassemia phenotypes. Differences could suggest not only the mechanism for an imbalanced hemostatic system, but also a cause for early removal of these cells. Because individual cells can be studied, we were able to identify colocalization in RBCs of areas of PS exposure with areas of excess globin chain accumulation. Sites of globin chain accumulation carrying iron, heme, and hemichromes could be involved in oxidant attack on the membrane and thus result in membrane lipid organizational damage.
MATERIALS AND METHODS
Blood samples.
Blood was collected from patients and controls in Bangkok, Thailand, and in the San Francisco Bay Area at Children's Hospital, Oakland. Blood was withdrawn under protocols approved by the Institutional Review Boards of the relevant institutions (Ramathibodi Hospital, Siriraj Hospital, both in Bangkok, Thailand, and Children's Hospital Oakland, Oakland, CA) into anticoagulant citrate dextrose (ACD; Sigma, St Louis, MO) or citrate phosphate dextrose (CPD; Sigma) and shipped from Thailand to Oakland or Stanford, CA on ice along with control normal blood (shipment controls). Typically, samples arrived within 48 hours and were immediately processed. The RBC phenotypes studied included: Hemoglobin H (HbH), variants of Hemoglobin Constant Spring (HbCS), and Hemoglobin E/β thalassemia (HbE/β-thal). The patients from Bangkok were ethnic Thai adults between the ages of 20 and 40 and had not been transfused for at least 3 months before the samples were sent. The diagnoses were based on globin gene analysis as in our prior publication.16 The severity of anemia varied greatly in both the α- and β-thalassemics16 and some of the most severely affected patients had been splenectomized. The patients from Oakland were immigrants from Southeast Asia and China. All data reported were from samples of different patients.
Erythrocytes.
Erythrocyte suspensions were prepared from samples as indicated above or from fresh human venous blood collected in ACD. Erythrocytes were pelleted by centrifugation, washed twice with 0.9% NaCl and once with incubation buffer, and finally diluted in incubation buffer to the appropriate hematocrit. Either Hanks buffered salt solution, pH 7.4 (HBSS, Sigma, St Louis, MO), or 10 mmol/L Tris/HCl buffered saline, pH 7.4 (TBS), was used as buffer throughout the experiments; similar results were found with both buffers. Additional ingredients, all of reagent quality, such as CaCl2, N-ethyl maleimide (NEM, Sigma) and Phenylhydrazine (Sigma) were added as indicated.
NEM and calcium and ionophore treatment of RBCs.
To generate RBCs that expose PS on their outer surface, which can serve as a positive control, normal red cells were incubated with NEM and calcium ionophore as described before.15 NEM inhibits the aminophospholipid translocase by reacting with a sulfhydryl group necessary for its activity. RBCs at 30% hematocrit were incubated in buffer containing 10 mmol/L NEM (Sigma) for 30 minutes at room temperature and subsequently washed in buffer without NEM. Calcium and ionophore treatment will induce membrane lipid scrambling. RBCs at a 16% hematocrit were equilibrated in incubation buffer with 1 mmol/L calcium for 3 minutes at 37°C. Subsequently, calcium ionophore A23187 was added to the RBC suspension to a final concentration of 4 μmol/L. The suspension was incubated for 1 hour at 37°C, washed with 5 mmol/L EDTA and buffer containing 1% bovine serum albumin (BSA) to remove ionophore, and resuspended in buffer.
Phenylhydrazine treatment.
Phenylhydrazine was used to generate Heinz bodies as described before.5 RBCs at a 10% hematocrit were incubated in TBS with 0.2 mg/mL phenylhydrazine for 60 minutes at 37°C and subsequently washed five times in TBS. Microscopy showed at least one Heinz body in each red cell.
AV purification and fluorescein-5-isothiocyanate (FITC) labeling.
Recombinant AV was purified from an Escherichia coli expression system by phospholipid affinity chromatography. Purified AV was incubated in 50 mmol/L Borate buffer, pH 9.0, at 4°C for 16 hours in the dark at a final concentration of 1 mg AV/mL in the presence of 20 molar equivalents of FITC (Molecular Probes, Eugene, OR). Subsequently, unreacted FITC was removed by incubation with 1.0 mol/L Tris/HCl, pH 8.0, and filtration on a PD-10 Sephadex G-25 column (Pharmacia, Uppsala, Sweden). The heterogeneously labeled AV (AV-FITC) species were separated by fast protein liquid chromatography (FPLC) and the brightly labeled fractions were collected. The preparation used in our studies had an average of 3 FITC molecules per protein molecule. Alternatively, a commercially available AV-FITC was used (R&D Systems). Virtually identical results were obtained with both probes.
AV labeling of RBC.
RBCs were suspended in buffer to a final concentration of 4 × 106 cells/mL. Four μL of a 500 μM FITC-labeled AV solution was added to 0.5 mL of this suspension in the presence of 2 mmol/L Ca2+. The samples were incubated for 30 minutes at room temperature and subsequently resuspended to approximately 106 cells per 250 μL in buffer with 2 mmol/L calcium for flow cytometric and microscopic analysis as described before.15
Magnetic cell separation.
Magnetic beads (average size 15 nm), covered with an anti-FITC antibody, were supplied by Miltenyi Biotec Inc (Auburn, CA). The stock solution of beads was fivefold diluted in AV-labeling buffer containing 2 mmol/L calcium. Red cells labeled with FITC-AV were washed and 6 × 107 cells were taken up in 80 μL of the calcium containing labeling buffer. To the cell suspension, 20 μL of the diluted beads were added. After a 10 minute incubation at room temperature, the cells were separated in a magnetic separation setup (Minimac, Miltenyi Biotec Inc, Auburn, CA), following the standard protocol supplied by the manufacturer. The cells were eluted with HBSS without calcium.
Flow cytometry.
Samples were analyzed by flow cytometry as described before.15 The instrument was calibrated according standard protocols to achieve day-to-day reproducibility. The red cell population was defined by size in forward and side scatter plots. Events that correlated with intact RBC were analyzed for fluorescence intensity using the same standard settings on a calibrated flow cytometer at each measurement. Fluorescence intensities were expressed in logarithmic mode. Each sample was incubated in the absence or presence of FITC-AV. The control sample incubated without FITC-AV was used to set the region for positive fluorescence such that the fraction of cells with positive (auto-) fluorescence was lower than 0.2% of total. The population of cells labeled with FITC-AV above background was determined from the fraction of cells in this region in excess of that obtained with the (unlabeled) control.
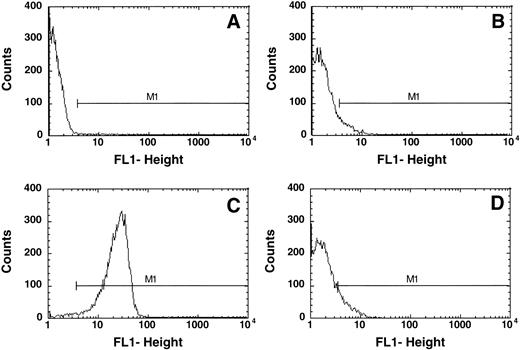
This approach was of particular importance because a number of thalassemic RBCs exhibited an increased autofluorescence as compared with normal control cells. An example is given in Fig 1. Normal control cells show very low fluorescence in FACS analysis in the absence of an added fluorophore (Fig 1A) and the marker M1 is set such that less than 0.2% of the cells are included in this region. Addition of FITC-AV resulted in a shift of 0.26 ± 0.2% (n = 42) cells into this region, indicating the presence of PS on the surface of these cells. Treatment of control RBCs with NEM followed by incubation with calcium and ionophore15 resulted in PS exposure, the binding of FITC-labeled AV to the cells and a shift of the cells into the positive region M1 (Fig 1C). In comparison with the control cells (Fig 1A), samples of thalassemic red cells exhibited an increased background fluorescence in the absence of FITC-AV (Fig 1B). Addition of FITC-AV led to a shift of a subpopulation of cells into the region marked by M1 (Fig 1D). This increase in the number of cells was defined as cells that expose PS as indicated in the result section.
Typical flow cytometric analysis of (A) normal RBCs and (B) thalassemic RBCs (HbE/β-thal (splx), incubated without AV-FITC; (C) normal RBCs incubated with 1 mmol/L calcium in the presence of 4 μmol/L ionophore A23187 labeled with AV-FITC, and (D) thal RBCs (HbE/β-thal (splx) labeled with AV-FITC. RBCs were selected by their light scattering properties and the number of cells with fluorescence above background was defined by gate M1.
Typical flow cytometric analysis of (A) normal RBCs and (B) thalassemic RBCs (HbE/β-thal (splx), incubated without AV-FITC; (C) normal RBCs incubated with 1 mmol/L calcium in the presence of 4 μmol/L ionophore A23187 labeled with AV-FITC, and (D) thal RBCs (HbE/β-thal (splx) labeled with AV-FITC. RBCs were selected by their light scattering properties and the number of cells with fluorescence above background was defined by gate M1.
Labeling of globin chains.
A monoclonal antibody directed against the unique 17-26 peptide sequence of human α-globin chains17 was used to label α-globin chains bound to membranes. This antibody does not label hemoglobin and has an apparent specificity for denatured α-globin chains, such as those found in Heinz bodies.17,18 A secondary Texas-Red–labeled goat antimurine immunoglobulin was used to show the localization of this antibody under fluorescent microscopy as described before.17 18
Fluorescence microscopy.
Confocal scanning optical microscopy (CSOM) was performed on AV-FITC–positive RBCs as described before.17 18 When required, CSOM analyses provided optical sections at 0.5 μm cuts through the AV-FITC positive RBCs. Alcian Blue coated glass was used to immobilize the cells during confocal scanning.
RBCs were incubated with AV-FITC and the AV-FITC positive cells were purified with anti-FITC magnetic beads as described above. The AV-FITC-magnetic bead complex was removed in buffer low in calcium. The RBCs were subsequently incubated in labeling buffer, and relabeled with AV-FITC. The population, enriched in AV-FITC labeled cells was studied by fluorescent microscopy.
In experiments where we wished to simultaneously identify PS exposure by AV-FITC and α-globin chains by a secondary Texas-Red–labeled antibody, we lightly fixed and permeabilized RBCs.17 In performing these experiments special care was taken that RBCs were labeled with AV-FITC before this treatment. Hence, RBCs labeled with AV-FITC (as above) were subsequently fixed, permeabilized, labeled with α-globin antibody followed by the secondary Texas-Red–labeled antibody, and analyzed by two-color immunofluorescence. Areas of colocalization of red and green fluorescence appear yellow.
To rule out cross-reactivity of the Texas-Red–labeled goat antimurine immunoglobulin with AV-FITC, we used normal red cells in which the phospholipid organization was scrambled by treatment with NEM, calcium, and ionophore. Although more than 95% of the cells were fluorescent after AV-FITC labeling (Fig 1C), neither the scrambled cells nor the AV-FITC–labeled cells showed any fluorescence in the Texas-Red channel after incubation with Texas-Red–labeled goat antimurine in the absence or presence of α-globin chain antibody (not shown). These data indicate that neither α-globin chain antibody nor the secondary Texas-Red–labeled goat antimurine immunoglobulin binds to PS or AV-FITC. This apparent lack of cross-reactivity suggests that the Texas-Red–labeled antibodies and the AV-FITC label recognize distinct sites in or adjacent to the membrane, albeit sometimes in the same region.
RESULTS
Flow cytometry.
Figure 1 indicates typical flow cytometric analyses of normal and thalassemic RBCs. In the absence of AV-FITC, a number of thalassemic RBCs (Fig 1B) showed an increased fluorescence as compared with normal control cells (Fig 1A), as shown here for a HbE/β-thalassemic, splenectomized sample. This shift varied significantly between the different samples. Although it was not observed in any of the HbH RBCs, it was very pronounced in a number of the β-thalassemic RBC samples. Hence, it was important to appropriately select a standard gate (M1) and correct for background fluorescence in each individual sample to obtain the correct fraction of AV-FITC–labeled cells as indicated in the Materials and Methods section.
Figure 1C shows control cells treated with NEM, calcium, and ionophore to scramble their phospholipid organization, and virtually all RBCs (95% ± 5%) are found in gate M1 indicating labeling with AV.
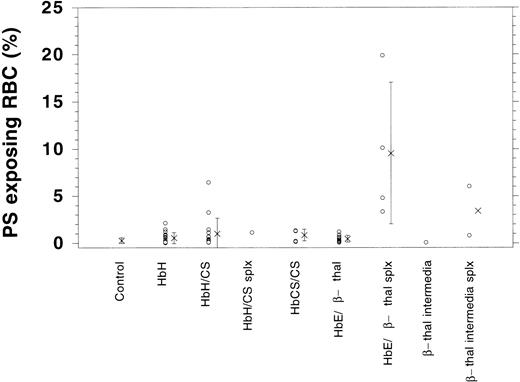
Although control cells show a very low increase in fluorescence when AV is added, the thalassemic RBCs shown (HbE/β-thalassemia, splenectomized) exhibit an increase in fluorescence on addition of AV-FITC (Fig 1D). In this case, 10.1% of cells increased their fluorescence into gate M1, indicating that these cells label with AV-FITC and expose PS. The fluorescence per thalassemic cell varies considerably, as indicated in Fig 1D. Instead of a distinct peak, as observed after the scrambling with calcium and ionophore (Fig 1C), merely a shift in the fluorescence of thalassemic cells is found. Although some cells exhibit a significant fluorescence, others increase their fluorescence only slightly above background. This wide range of fluorescence was observed in all thalassemic samples analyzed. We determined the fraction of cells in the population that appear in channel M1 and are positive for AV-FITC labeling as the result of PS exposure. These data are given in Table 1. Fresh control RBCs exhibit low numbers of cells in the population that label with AV-FITC (0.26 ± 0.2, n = 42). The shipment controls show no difference as compared with the fresh samples (range 0.23% to 0.42%). RBC membranes scrambled by treatment with NEM, calcium, and ionophore showed high levels of labeling (95% ± 5%). A good correlation was found between the labeling with AV-FITC and stimulation of prothrombinase activity as was reported before,15confirming the exposure of PS (not shown). Table 1 records results of 56 samples of α- and β-thalassemic RBCs analyzed by flow cytometry. A wide range in the number of cells in the population that exposed PS was observed. The size of the subpopulation that could be labeled with AV-FITC ranged from normal (0.2%) to significantly increased above normal (up to 20%), indicating a wide variety in the number of cells that expose PS. This wide range is further emphasized by the graphic presentation of the data in Fig 2. Although many thalassemic samples were found in the normal range, a number were significantly increased above normal. These data suggest that certain thalassemic mutations are more likely to result in PS exposure than others. More importantly, samples from patients that had undergone splenectomy seemed to show increased numbers of cells in the population that exposed PS, in particular in the HbE/β-thalassemic variants. These data indicate that in contrast with normal cells, thalassemic RBC might contain a substantial subpopulation of cells that expose PS, in particular in severely affected patients.
PS Exposure in Normal and Thalassamic RBC
| Mutation . | AV Binding (%) . | ±SD . | n . |
|---|---|---|---|
| Normal control | 0.26 | 0.20 | 42 |
| HbH | 0.53 | 0.60 | 17 |
| HbH/CS | 1.00 | 1.66 | 16 |
| HbH/CS, splenectomized | 1.11 | 1 | |
| HbCS/CS | 0.85 | 0.61 | 5 |
| HbE/β-thal | 0.45 | 0.37 | 10 |
| HbE/β-thal, splenectomized | 9.5 | 7.5 | 4 |
| β-thal intermedia | 0.07 | 1 | |
| β-thal intermedia splenectomized | 3.4 | 3.7 | 2 |
| All thalassemic cells | 1.42 | 1.01 | 56 |
| Mutation . | AV Binding (%) . | ±SD . | n . |
|---|---|---|---|
| Normal control | 0.26 | 0.20 | 42 |
| HbH | 0.53 | 0.60 | 17 |
| HbH/CS | 1.00 | 1.66 | 16 |
| HbH/CS, splenectomized | 1.11 | 1 | |
| HbCS/CS | 0.85 | 0.61 | 5 |
| HbE/β-thal | 0.45 | 0.37 | 10 |
| HbE/β-thal, splenectomized | 9.5 | 7.5 | 4 |
| β-thal intermedia | 0.07 | 1 | |
| β-thal intermedia splenectomized | 3.4 | 3.7 | 2 |
| All thalassemic cells | 1.42 | 1.01 | 56 |
AV-FITC labeling of normal controls (fresh and travel controls) and patient samples. Indicated is the percentage of cells in the population that were labeled with AV-FITC above background as indicated in Fig 1.
Graphic presentation of RBCs that expose PS as determined by AV-FITC labeling in normal controls and patient samples. Indicated is the percentage of cells in the population that were labeled with AV-FITC above background (o) as well as the average (x) and standard deviation.
Graphic presentation of RBCs that expose PS as determined by AV-FITC labeling in normal controls and patient samples. Indicated is the percentage of cells in the population that were labeled with AV-FITC above background (o) as well as the average (x) and standard deviation.
Image analysis.
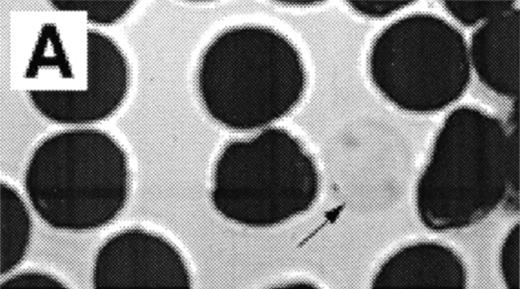
When normal RBC phospholipid asymmetry is scrambled by treatment with NEM, calcium, and ionophore they can be analyzed by fluorescent microscopy (Fig3A, B). Analysis by CSOM shows uniform labeling of the membrane by AV-FITC (Fig 3C) of these spherocytic cells. Because a simple wash in calcium-poor buffer removes all AV-FITC labeling from the cell, the data indicate that the AV-FITC complex is bound to PS on the outside of these cells, as was reported before.15
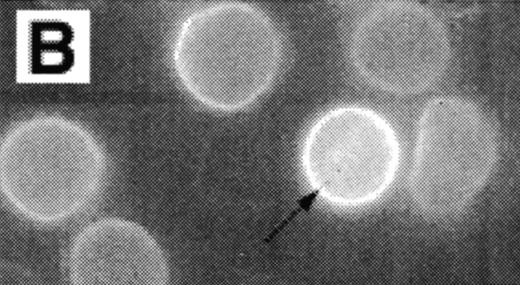
Analysis of RBCs incubated with FITC-labeled AV after treatment with calcium in the presence of 4 μmol/L ionophore A23187 by fluorescence microscopy. (A) A representative field of this population as observed in bright field and (B) in fluorescence; note the RBC ghost indicated by arrow. (C) A calcium ionophore scrambled spherocytic red cell analyzed by serial optical sections in confocal fluorescent microscopy.
Analysis of RBCs incubated with FITC-labeled AV after treatment with calcium in the presence of 4 μmol/L ionophore A23187 by fluorescence microscopy. (A) A representative field of this population as observed in bright field and (B) in fluorescence; note the RBC ghost indicated by arrow. (C) A calcium ionophore scrambled spherocytic red cell analyzed by serial optical sections in confocal fluorescent microscopy.
Because the number of positive cells in the thalassemic RBC samples was low, the population was enriched in AV-FITC–positive cells by magnetic bead separation as described before.15 After separation, the cells were washed with calcium-free buffer, which removed all of the AV-FITC/magnetic bead complex from the cells. These RBCs were reincubated in AV-FITC in the presence of calcium to visualize the distribution of PS on the membrane surface. These unfixed AV-FITC–labeled RBCs were then studied by CSOM, and optical sections taken at 0.5 μm cuts were made when indicated (Fig 4). When it was necessary to simultaneously analyze the distribution of PS on the red cell surface and α-globin deposits on the cytosolic site of the membrane, AV-FITC–labeled cells were lightly fixed, permeabilized, and incubated with the murine monoclonal anti–α-globin antibody, followed by Texas-Red–labeled goat antimurine immunoglobulin antibody.17 18
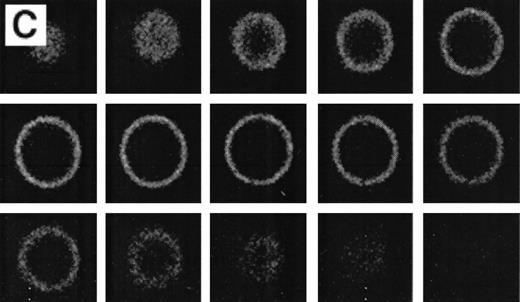
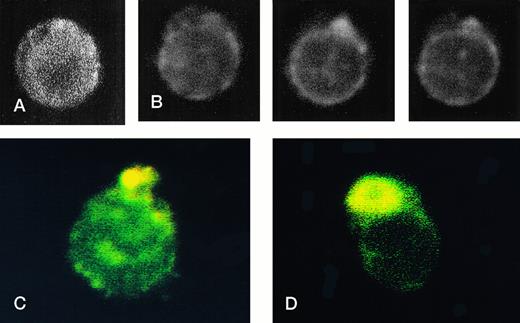
RBCs from a thalassemic patient (HbE/β-thalassemia, splenectomized) enriched from the population labeled with AV-FITC using magnetic beads coated with an FITC antibody (see the Materials and Methods section). (A, B) Typical cells labeled with AV-FITC in the native unfixed state and then analyzed by serial optical sections in confocal fluorescent microscopy. Panel A shows the equatorial section of a cell homogeneously labeled with AV-FITC. Panel B shows three equatorial sections of a cell heterogeneously labeled with AV-FITC. (C,D) RBCs initially labeled with AV-FITC in the unfixed state and then labeled with monoclonal anti–α-globin chain antibody, and secondary Texas-Red–labeled goat antimurine antibody. This double-labeled cell was then analyzed by fluorescent microscopy. The areas where Texas Red (red) and FITC (green) overlap are yellow.
RBCs from a thalassemic patient (HbE/β-thalassemia, splenectomized) enriched from the population labeled with AV-FITC using magnetic beads coated with an FITC antibody (see the Materials and Methods section). (A, B) Typical cells labeled with AV-FITC in the native unfixed state and then analyzed by serial optical sections in confocal fluorescent microscopy. Panel A shows the equatorial section of a cell homogeneously labeled with AV-FITC. Panel B shows three equatorial sections of a cell heterogeneously labeled with AV-FITC. (C,D) RBCs initially labeled with AV-FITC in the unfixed state and then labeled with monoclonal anti–α-globin chain antibody, and secondary Texas-Red–labeled goat antimurine antibody. This double-labeled cell was then analyzed by fluorescent microscopy. The areas where Texas Red (red) and FITC (green) overlap are yellow.
Figure 4 shows representative fields of RBCs from a splenectomized patient with HbE/β-thalassemia PS exposing RBCs, enriched by magnetic bead separation, relabeled with AV-FITC, and analyzed by CSOM, showed two patterns of fluorescence. Approximately half of the AV-FITC–labeled thalassemic RBCs show a smooth rim fluorescence over the entire membrane (Fig 4A), similar to normal cells with a scrambled membrane (Fig 3). However, in other RBCs the AV-FITC membrane fluorescence was more heterogeneously distributed. In addition to a rim fluorescence, sites with increased fluorescence were observed, indicating that PS was enriched in these areas (Fig 4B). Interestingly, these AV-FITC–labeled sites at the surface of the cell were localized to areas that seemed to bulge over an inclusion body as shown in the equatorial cut of such labeled cells shown in Fig 4B. Because this patient had severe β-thalassemia intermedia, it was logical to suppose that the inclusion was a membrane associated deposit of excess α-globin chains, which we tried to confirm using the monoclonal antibody to denatured α-globin chains shown by Texas-Red–labeled antimurine immunoglobulin.
The equatorial cut (midsection) of such labeled cells showed the enrichment of Texas Red in distinct areas, indicating that denatured α-globin chains were localized in domains adjacent to the membrane, confirming results reported before.18 When such RBCs were analyzed for both AV-FITC (green) and anti–α-globin/Texas-Red (red), fluorescence microscopy showed a bright-yellow fluorescence in dual-color analysis, indicating that both AV-FITC and the Texas-Red label were enriched in the same membrane regions (Fig 4B and C). In contrast, the cells that labeled homogeneously with AV-FITC did not label with Texas Red. These data strongly suggest a colocalization of denatured α-globin chains and PS in the same area.
To evaluate the effect of Heinz bodies on the (re)distribution of PS, we treated normal cells with phenylhydrazine and subsequently incubated them with AV-FITC. Although these cells contained an abundance of Heinz bodies,5 they did not expose their membrane PS as shown by the absence of AV-FITC–labeled cells in flow cytometry. These findings and the effect of other oxidants on the asymmetric distribution of phospholipids in the normal red cell are published elsewhere.19
DISCUSSION
The increased autofluorescence observed in samples from thalassemic patients suggests the presence of fluorescent products in the red cells, presumably as the result of increased levels of oxidative damage.19 Our data indicates the presence of PS on the outside of subpopulations of thalassemic RBCs. Rather than a uniform loss of phospholipid asymmetry in all cells, distinct subpopulations of RBCs that expose PS on their outer surface were found in moderately severe thalassemics, particularly splenectomized patients with HbE/β-thalassemia. In this study we could not identify a direct correlation between the severity of the anemia and the proportion of RBC-exposing PS. Similar results were reported for sickle cells,15, 20 and as was observed in sickle cell samples, the size of this subpopulation can vary considerably. Although a number of thalassemic samples were in the normal range, others exhibited a significant increase in the population that exposes PS. The lack of appropriate splenectomized normal controls and the relatively low numbers of comparable splenectomized and nonsplenectomized patients do not allow a conclusion on the statistical significance of splenectomy on the presence of PS-exposing cells.
Importantly, there is a caveat that relates to the events that produce the exposure of PS and the rapidity by which such RBCs are possibly removed from the circulation. We noted that splenectomized HbE/β-thal patients exhibited larger populations of AV-FITC–positive RBCs than nonsplenectomized HbE/β-thal patients. This increased proportion of RBCs with an altered phospholipid bilayer could reflect the increased severity of the disease (anemia) that leads to the splenectomy. Alternatively, removal of the spleen, a major macrophagic organ, would allow RBCs that expose PS to circulate longer whereas in the presence of a functioning spleen they would have been expeditiously removed. In other words, the AV-FITC–positive cells observed in the peripheral blood of patients might very well be a cohort of short-lived cells that appears as a subpopulation when the removal system is less than optimal.
The labeling with AV-FITC allows identification of individual cells that expose PS by fluorescent microscopy. AV-FITC binds to the outer surface of cells that have lost their normal phospholipid asymmetry as indicated by CSOM analysis. Those cells that have lost their membrane integrity (ghosts) are brightly labeled due to the fact that AV-FITC has access to the PS in the inner monolayer. AV-FITC can be removed from intact red cells by a simple wash in calcium-poor buffer, a further indication that the FITC-labeled 38 kD protein has only access to the outer surface of the cell. In order to increase the number of AV-FITC–positive cells in the thalassemic red cell population to make analysis by fluorescent microscopy more feasible, we used a technique that selects these cells by magnetic bead separation.15Analysis after renewed labeling of unfixed RBC with AV-FITC shows at least two types of PS exposure in thalassemic red cells. On the one hand, a similar labeling is found (Fig 4A) as with normal red cells scrambled with calcium and ionophore as shown in Fig 3C. In these cells, uniform rim fluorescence indicates a uniform distribution of PS on the outer surface. Other cells showed enhanced domains of PS on the surface of the thalassemic red cells (Fig 4B). The underlying mechanism for these hot spots is not clear. These data suggest that the normal maintained asymmetry of the PL bilayer can be disrupted locally. However, based on the rapid diffusion rates of phospholipids in the plane of the bilayer before AV-FITC labeling, one would not expect these domains in the case of unrestricted movement of PS on the surface of the cell. Hence, this would lead to the conclusion that PS movement is restricted in the plane of the bilayer of these cells confining PS to local areas that are identified by AV-FITC labeling. Although the concept of lipid domains as the result of restricted movement has been hypothesized, few reports have been able to indicate such regions.
Interestingly, in RBCs that had been labeled with AV-FITC in the native unfixed state and then lightly fixed and permeabilized, antibodies against α-globin chains colocalize with AV-FITC (Fig 4C and D), leading to similar pictures as found with AV-FITC labeling of intact unfixed cells (Fig 4B). These data suggest that in some cases PS is enriched on the outer surface in areas of α-globin chain accumulation. It was recently reported that endogenous red cell AV is found in regions where Heinz bodies attach to the plasma membrane,21 thereby suggesting that PS was enriched in these areas in the inner monolayer. Membrane skeletal proteins including spectrin22 and a band 4.1,23 24 have been shown to interact with PS. A change in the distribution or lipid/protein interaction of these proteins could be involved in the local accumulation of PS. However, the underlying mechanism is not known at present.
One possibility for the localization of PS in these regions could be found in the expected local oxidative damage of the membrane induced by the accumulation of heme containing α-globin chains.10The local generation of oxygen radical species could locally damage normal interactions in the membrane, as well as interactions of the bilayer with the cytoskeleton. Phenylhydrazine that oxidizes α-globin chains and produces a surrogate β-thalassemia lesion,5did not reproduce the PS exposing cells seen in β-thalassemic RBCs. Under the in vitro conditions chosen, Heinz bodies were formed, and α-globin aggregates were formed adjacent to the membrane. However, these conditions appeared not sufficient to reproduce the events that occur in vivo. In addition, other means of oxidative stress also failed to induce the exposure of PS as we report elsewhere.19These data suggest that oxidative damage will not necessarily lead to the loss of phospholipid asymmetry. It might very well be an important factor, but another at present unknown factor also seems to play an important role in the events that will lead to the exposure of PS, preferentially localized to areas that seemed to bulge over an inclusion body.
In conclusion, we have shown that in thalassemic patients, subpopulations of red cells circulate that expose PS on their outer surface; there is not an overall loss of phospholipid asymmetry in all cells. The number of such cells can vary dramatically from patient to patient. PS is found to be either distributed over the entire membrane or localized in areas seemingly related to α-globin–rich regions. The presence of these subpopulations of cells are physiologically important. They could form an increased red cell–derived PS surface responsible for the hypercoagulable state proposed to occur by some investigators in severe β-thalassemia. Furthermore, it can be speculated that these cells will be rapidly removed from the circulation, thereby contributing to the anemia.
Supported by the National Institutes of Health Grants No. DK32094, HL20985, HL55213, HL27059, and DK13682. Presented previously at the 36th meeting of the American Society of Hematology (Blood 84:259a,1994 [abstr, suppl 1]).
Address correspondence to Frans A. Kuypers, PhD, Children's Hospital Oakland Research Institute, 747 52nd Street, Oakland, CA 94609.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal