Fanconi anemia (FA) is a pleiotropic inherited disease that causes bone marrow failure in children. However, the specific involvement of FA genes in hematopoiesis and their relation to bone marrow (BM) failure is still unclear. The increased sensitivity of FA cells to DNA cross-linking agents such as mitomycin C (MMC) and diepoxybutane (DEB), including the induction of chromosomal aberrations and delay in the G2 phase of the cell cycle, have suggested a role for the FA genes in DNA repair, cell cycle regulation, and apoptosis. We previously reported the cloning of the FA group C gene (FAC) and the generation of a Fac mouse model. Surprisingly, the Fac −/− mice did not show any of the hematologic defects found in FA patients. To better understand the relationship of FA gene functions to BM failure, we have analyzed the in vivo effect of an FA-specific DNA damaging agent in Fac −/− mice. The mice were found to be highly sensitive to DNA cross-linking agents; acute exposure to MMC produced a marked BM hypoplasia and degeneration of proliferative tissues and caused death within a few days of treatment. However, sequential, nonlethal doses of MMC caused a progressive decrease in all peripheral blood parameters of Fac −/− mice. This treatment targeted specifically the BM compartment, with no effect on other proliferative tissues. The progressive pancytopenia resulted from a reduction in the number of early and committed hematopoietic progenitors. These results indicate that the FA genes are involved in the physiologic response of hematopoietic progenitor cells to DNA damage.
FANCONI ANEMIA (FA) is a recessive inherited bone marrow (BM) failure syndrome affecting children and is characterized by progressive aplastic anemia and a variety of congenital malformations (reviewed in Young and Alter1). The hematologic abnormalities are detected at a median age of 7 years, with thrombocytopenia generally preceding the onset of anemia. Pancytopenia was found to be associated with a decrease in BM cellularity. FA patients are also at a higher risk of cancer, in particular acute myeloid leukemia (AML).
At least 8 complementation groups have been identified, designated FA-A through FA-H,2 that may correspond to specific gene defects involving either the same biochemical pathway or alternative biological functions. Although two genes have been cloned (groups A and C), the basic cellular defect(s) in FA is not known.3-5 It has been postulated that the FA genes may be implicated in DNA repair due to the increased sensitivity of FA cells to DNA cross-linking agents, such as mitomycin C (MMC) and diepoxybutane (DEB), which induce chromosomal aberrations and abnormal delay in the G2 phase of the cell cycle. Studies on different cell lines have suggested abnormalities in other cellular processes, such as oxygen metabolism, growth factor homeostasis, cell cycle regulation, differentiation, and apoptosis.6-8 No specific function has been attributed to either of the two cloned genes. The most studied gene, FAC, encodes for a protein that is highly hydrophobic and localized to the cytoplasm,9 which precludes, for now, any direct role in DNA repair. No homologies have been found to known genes, proteins, or domains that could serve as clues to its biological role. In addition, comparison between human, mouse, rat, and bovine FAC sequences did not show any clearly delineated functional domains.10
To study the relationship of FA genes to BM failure, two mouse models of FA complementation group C have been generated by disruption of the mouse Fac gene.11,12 Although spleen cells obtained from mutant mice showed sensitivity to DNA cross-linking agents,Fac −/− mice did not exhibit any changes in blood parameters or in the amounts of hematopoietic progenitors, consistent with the hematologic phenotype seen in FA patients. The only phenotype observed was compromised gametogenesis similar to that seen in FA patients,1 although this phenotype is also observed in other mutant mice for genes involved in DNA repair.13-15 To further understand the physiological function of the FAC gene and its relation to the clinical phenotype seen in patients, we have exposed the Fac −/− mice to a specific DNA-damaging agent. Treatment of Fac −/− mice with low doses of MMC results in depletion of the early and committed hematopoietic progenitors leading to progressive pancytopenia.
MATERIALS AND METHODS
Mice and MMC injections.
Five- to 6-month-old Fac mice11 from different genetic backgrounds, consisting of 50% 129/R1 contribution associated with either 50% Balb/c, C57BL/6J, or 129/ag, were injected intraperitoneally with varying doses of MMC (Boehringer Mannheim, Canada, Laval, Quebec, Canada) diluted in saline solution. Control mice received saline injections of equivalent volumes. MMC-treated mice received either one single injection ranging from 0.01 to 1 mg/kg or weekly injections of 0.3 mg/kg. Mice body weight ranged from 25 to 40 g. The animal experiments were approved by the Animal Care Committee of the Hospital for Sick Children.
Histopathological analysis.
Mice were euthanized and tissues were immediately collected and placed in 10% neutral-buffered formalin. Fixed tissues were embedded in paraffin, sectioned at 4 μm, and stained with hematoxilin-eosin using standard methods.
Hematologic analysis.
Complete peripheral blood counts, including erythrocytes, leukocytes, and platelets, were obtained with an automated counting apparatus. Heparinized blood was collected in an eppendorf tube from the mouse tail tip. To determine leukocyte and platelet counts, peripheral blood was depleted of red blood cells by treatment using Zapisoton solution from Coulter Electronics (Miami, FL), as described by the manufacturer. For hematopoietic committed progenitor cell assays (colony-forming cells [CFC]), BM and spleen cells were collected either from femurs or spleen, seeded in complete methylcellulose medium according to the manufacturer (Stem Cell Technology, Vancouver, British Columbia, Canada), and incubated for 7 to 10 days at 37°C, 5% CO2. To determine the number of granulocytes (G), macrophages (M), or GM colonies, BM cells were plated at 2 to 2.5 × 104 cells/mL and spleen cells at 1 to 4 × 105 cells/mL. For the determination of erythroid (E) colonies (burst-forming unit-erythroid [BFU-E]) or mixed GME-megakaryocyte colonies, BM cells were plated at 1 × 105 cells/mL and spleen cells at 4 to 8 × 105 cells/mL. The total number of colonies was counted and depicted here as CFC. For in vitro sensitivity to MMC, various concentrations of MMC, ranging from 2 to 10 nmol/L, were added to the methylcellulose cultures at plating. BM cells from Fac−/− mice were plated at increasing concentrations with increasing MMC doses to obtain countable numbers of colonies. For long-term BM cultures (LTBMC), BM cells collected from femurs were plated as previously described,16 with some minor modifications.17 Cells were cultured at 33°C for 5 weeks. One half of media was replaced weekly, and at the end of the culture period, nonadherent fraction and adherent layer cells (ALC) were tested for their capacity to initiate colony formation (CFC). Nonadherent cells were seeded in methylcellulose at 2 to 10 × 105 cells/mL. ALCs were harvested by trypsinization, depleted of stromal cells by attachment to plastic culture dishes for 20 minutes at 37°C, and seeded in methylcellulose at 4 to 8 × 104 cell/dish for 7 to 10 days. All cultures were performed in triplicates. CFC numbers are expressed as the total number of CFC from the nonadherent and adherent fractions.
Statistical analysis.
Results are expressed in mean ± SEM. Estimation of the significance of the difference between means was performed using the Student's paired t-test.
RESULTS
Low-dose MMC induced pancytopenia in Fac −/− mice.
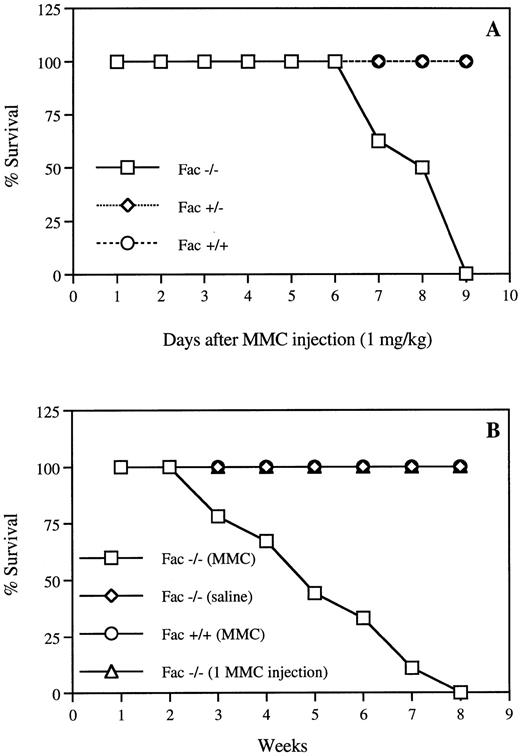
To test the in vivo sensitivity of Fac −/− mice to the cross-linking agent MMC, we first measured the effect of various doses of drug, ranging from 0.03 to 1 mg/kg, on hematopoiesis.Fac −/− mice showed hypersensitivity to single doses of 1 mg/kg, which induced death within 10 days (Fig 1A). Before death, peripheral white blood cell counts were reduced by 90%, hematocrit by 50%, and body weight by 30% in Fac −/− injected mice (data not shown). The estimated LD50 for Fac −/− mice was between 0.5 and 0.6 mg/kg, 10 times lower than for wild-type mice (data not shown). No effect was observed on either Fac +/+ or Fac +/− mice (Fig 1A). To better reflect more physiologic challenges that human patients may encounter in the environment, we exposed the Fac −/− mice to sequential, nonlethal doses of MMC. We found that repeated injections of 0.3 mg/kg of MMC caused death in the Fac −/− mice within 3 to 8 weeks, although a single injection had no lethal effect (Fig 1B). Weekly injections of 0.3 mg/kg MMC caused a progressive decrease in all peripheral blood parameters, with a marked decrease in platelet counts (Fig 2). Mice used in these experiments died between 6 and 8 weeks of treatments. Death followed shortly after a 50% decrease in red blood cell counts.
Hypersensitivity of Fac −/− mice to MMC. (A) Survival curves of Fac +/+, +/−, and −/− mice from different genetic backgrounds after one injection of 1 mg/kg MMC. (B) Survival curves of Fac +/+ and −/− mice from different genetic backgrounds after weekly injections of 0.3 mg/kg MMC. Controls include Fac −/− mice that received weekly saline injections or one single injection of 0.3 mg/kg MMC. Survival is expressed as the percentage of the number of live mice subjected to MMC treatment (n = 17). All control animals survived for up to several months before being killed for histological analysis.
Hypersensitivity of Fac −/− mice to MMC. (A) Survival curves of Fac +/+, +/−, and −/− mice from different genetic backgrounds after one injection of 1 mg/kg MMC. (B) Survival curves of Fac +/+ and −/− mice from different genetic backgrounds after weekly injections of 0.3 mg/kg MMC. Controls include Fac −/− mice that received weekly saline injections or one single injection of 0.3 mg/kg MMC. Survival is expressed as the percentage of the number of live mice subjected to MMC treatment (n = 17). All control animals survived for up to several months before being killed for histological analysis.
Peripheral blood parameters of Fac −/− andFac +/+ mice. (A) Red blood cells (RBC), (B) white blood cells (WBC), and (C) platelets. Measurements were made in mice from a 50%/50% 129ag/129R1 background treated with repeated MMC injections (0.3 mg/kg). Results are expressed as the mean number of cells measured in 4 mice. Mice used in this experiment died between 6 and 8 weeks of treatment. All control mice survived for more than 12 weeks before being killed for histological analysis. Fac −/− mice that received a single MMC injection or Fac +/− mice gave similar results as control Fac +/+ mice. Each point represents the mean ± SEM of four separate determinations. The absence of SEM bars represents values too low to appear in the graph.
Peripheral blood parameters of Fac −/− andFac +/+ mice. (A) Red blood cells (RBC), (B) white blood cells (WBC), and (C) platelets. Measurements were made in mice from a 50%/50% 129ag/129R1 background treated with repeated MMC injections (0.3 mg/kg). Results are expressed as the mean number of cells measured in 4 mice. Mice used in this experiment died between 6 and 8 weeks of treatment. All control mice survived for more than 12 weeks before being killed for histological analysis. Fac −/− mice that received a single MMC injection or Fac +/− mice gave similar results as control Fac +/+ mice. Each point represents the mean ± SEM of four separate determinations. The absence of SEM bars represents values too low to appear in the graph.
MMC is highly toxic to proliferative tissues of Fac−/− mice.
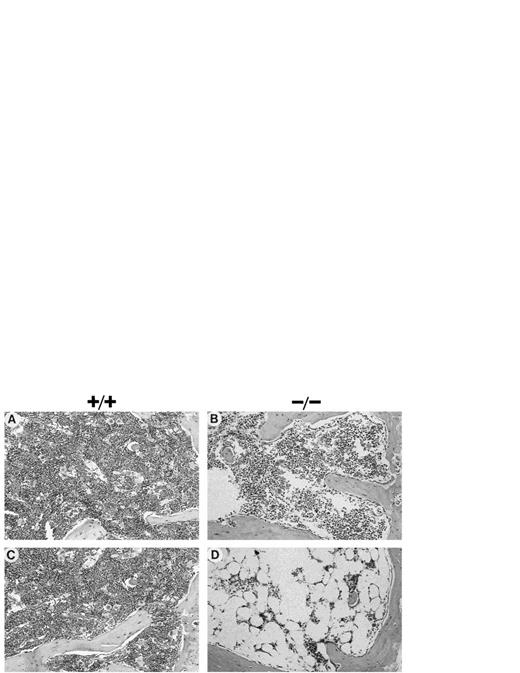
Histological analysis of Fac −/− BM tissue sections showed that a single MMC dose of 0.3 mg/kg caused a slight reduction in BM cellularity (Fig 3B), although repeated injections or chronic exposure to MMC (once per week for 5 weeks) markedly depleted all BM cell populations of Fac−/− mice (Fig 3D). Cellularity was estimated to be less than 10% and megakaryocytes were completely absent in Fac−/− sections examined, which correlate with the initial progressive decrease observed in platelet counts. BM sections fromFac +/+ (Fig 3A and C) and Fac +/− (data not shown) mice that received single or repeated injections of 0.3 mg/kg of MMC showed no remarkable effects. Repeated exposure to 0.3 mg/kg of MMC had no significant effect on other tissues of all MMC-injectedmice. Moderate atrophy of the gastric glandular mucosa was observed in some tissue sections from Fac −/− mice, although this was not significant enough to contribute to the death of the animals.
Histological appearance of tissues from mice after chronic exposure to MMC. Hematoxylin-eosin stained sections of BM (×400) from mice (50%/50% C57Bl/6/129R1 genetic background) after chronic exposure to MMC; 1 (A and B) or 5 (C and D) weekly injections of 0.3 mg/kg MMC. (A and C) Fac +/+; (B and D) Fac−/−.
Histological appearance of tissues from mice after chronic exposure to MMC. Hematoxylin-eosin stained sections of BM (×400) from mice (50%/50% C57Bl/6/129R1 genetic background) after chronic exposure to MMC; 1 (A and B) or 5 (C and D) weekly injections of 0.3 mg/kg MMC. (A and C) Fac +/+; (B and D) Fac−/−.
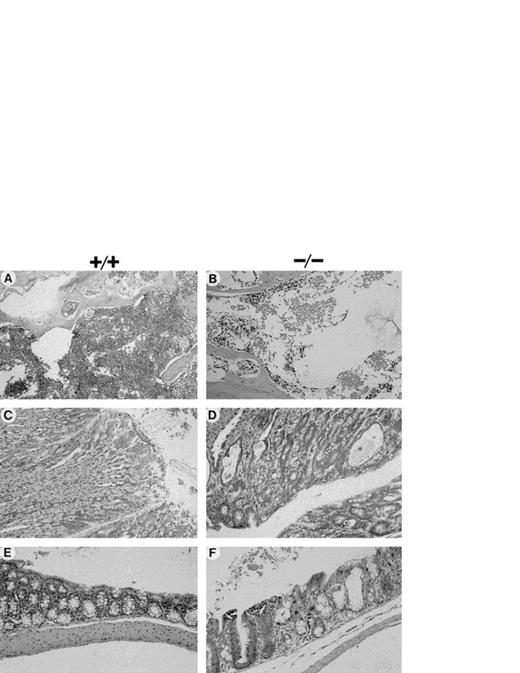
In contrast, analysis of tissue sections from Fac−/− mice after acute exposure to MMC (one single injection of 1 mg/kg) showed that death resulted from massive damage to highly proliferative tissues, including BM, gastric lining, and mucosa of the small and large intestine (Fig 4B, D, and F). The BM sections from Fac −/− mice showed a marked reduction in cellularity, with depletion of all cell types and almost complete absence of megakaryocytes (Fig 4B). This dramatic reduction in BM cellularity after acute exposure to MMC resembled chronic long-term exposure to low doses of MMC. No changes in BM cellularity were observed in either Fac +/+ (Fig 4A) andFac +/− injected mice (data not shown). In other proliferative tissues, the gastric and intestinal lining ofFac −/− mice injected with high doses of MMC showed marked degeneration and flattening of epithelial cells associated with edema and mononuclear inflammatory cell infiltration in the subjacent lamina propria (Fig 4D and F). Hepatocellular swelling and degeneration and splenic lympholysis was also observed in Fac−/− mice (data not shown). However, at high doses of MMC, no effect was observed on the stomach, intestine, or any other tissues of Fac +/+ (Fig 4C and E) and Fac +/− mice (data not shown). No apparent damage was detected in the brain, heart, lung, or kidney of any MMC-injected mice (data not shown).
Histological appearance of tissues from mice after acute exposure to MMC. Hematoxylin-eosin stained sections of mouse tissues (50%/50% C57Bl/6/129R1 genetic background) after acute exposure to 1.0 mg/kg MMC. (A and B) BM (×400); (C and D) glandular stomach (×200); (E and F) large intestine (×200). (A, C, and E) Fac+/+; (B, D, and F) Fac −/−.
Histological appearance of tissues from mice after acute exposure to MMC. Hematoxylin-eosin stained sections of mouse tissues (50%/50% C57Bl/6/129R1 genetic background) after acute exposure to 1.0 mg/kg MMC. (A and B) BM (×400); (C and D) glandular stomach (×200); (E and F) large intestine (×200). (A, C, and E) Fac+/+; (B, D, and F) Fac −/−.
MMC treatment reduces both clonogenic and primitive hematopoietic progenitors.
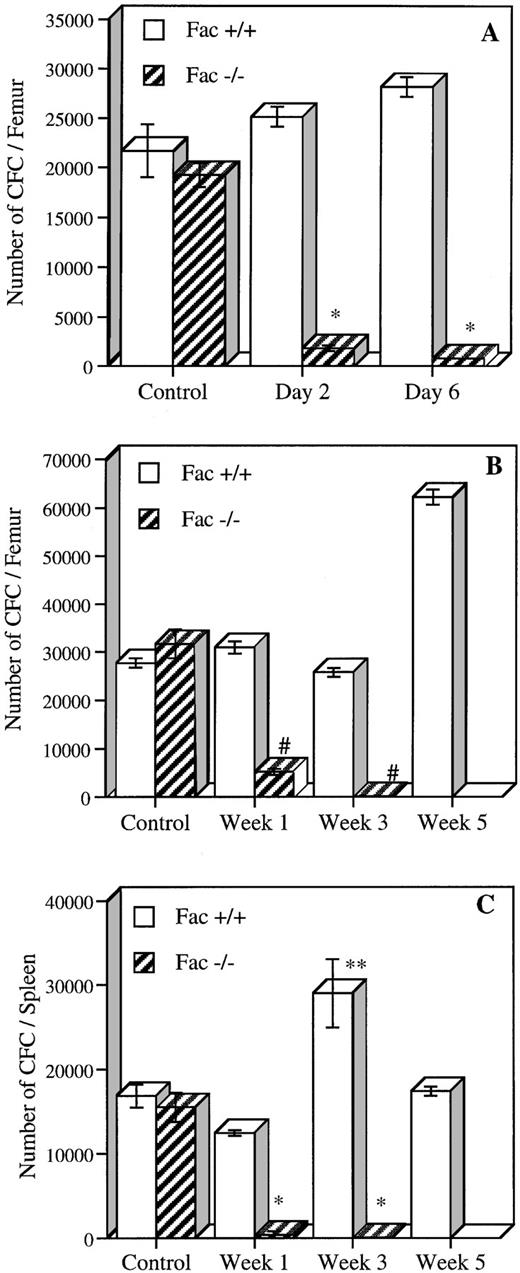
To determine if MMC was acting on early subsets of hematopoietic cells, we measured the number of early and committed progenitors present in Fac +/+, +/−, and −/− mice after in vivo exposure to either acute or chronic MMC treatment (Fig 5). For acute exposure, the number of BM progenitors was determined 2 and 6 days after a single MMC injection of 0.75 mg/kg. Fac −/− mice had a 90% to 95% reduction in the number of CFC at days 2 and 6, as compared with saline-injected Fac −/− mice (Fig 5A). A slight increase in CFC, although not significant (15% and 30% at days 2 and 6, respectively), was observed in Fac +/+ injected mice. BM from Fac +/− mice showed a significantly lower level of CFC at day 2 (27% of Fac +/+ control) but showed normal levels at day 6 (data not shown). Similar results were obtained from spleen CFC from Fac −/− mice, which had a decrease in progenitor contents at both time points (data not shown). The number of committed progenitors was also measured in mice after chronic MMC treatment. CFC from both BM and spleen were evaluated at 1, 3, or 5 weeks after weekly exposure to low doses of MMC (0.3 mg/kg; Fig 5B and C). BM CFC levels from low-dose injected Fac −/− mice decreased to 16% after the first week and to less than 1% after 3 weeks. CFC in spleen were markedly reduced to less than 3% after the first week of treatment and to less than 1% after 3 weeks. Chronic exposure to MMC showed a stimulatory effect on the number of committed progenitors from Fac +/+ mice, in which a twofold increase in CFC levels was observed in both BM and spleen after 5 and 3 weeks, respectively. This response is typical of the overshoot in progenitors seen in mice treated with other chemotherapeutic agents.18 19
In vivo MMC sensitivity of CFC from BM and spleen cells of Fac +/+ and Fac −/− mice. (A) The number of CFC established 2 and 6 days after a single MMC injection (0.75 mg/kg). (B and C) The number of CFC in BM (B) and spleen (C) after 1, 3, and 5 weekly injections of MMC (0.3 mg/kg). Controls represent saline-injected mice. Data represent the mean ± SEM of triplicate determinations from 2 mice. Significant differences between controls and MMC-injected mice (*P < .01; **P < .05; #P < .005; ##P < .0005). The absence of SEM bars represents values too low to appear in the graph.
In vivo MMC sensitivity of CFC from BM and spleen cells of Fac +/+ and Fac −/− mice. (A) The number of CFC established 2 and 6 days after a single MMC injection (0.75 mg/kg). (B and C) The number of CFC in BM (B) and spleen (C) after 1, 3, and 5 weekly injections of MMC (0.3 mg/kg). Controls represent saline-injected mice. Data represent the mean ± SEM of triplicate determinations from 2 mice. Significant differences between controls and MMC-injected mice (*P < .01; **P < .05; #P < .005; ##P < .0005). The absence of SEM bars represents values too low to appear in the graph.
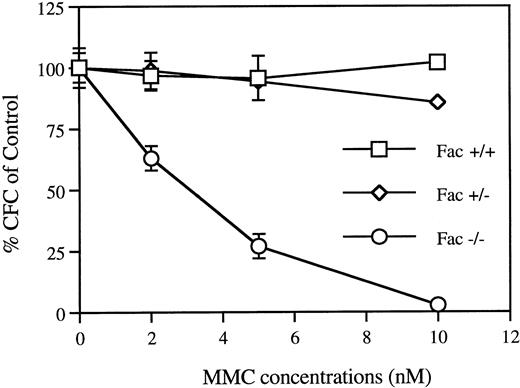
We also measured the in vitro sensitivity of BM clonogenic progenitors to increasing concentrations of MMC (Fig6). Clonogenic cells from Fac −/− mice were highly sensitive to MMC treatment, showing a dose-dependent decrease in colony formation. This indicates a direct action of MMC on committed hematopoietic progenitor cells.
In vitro MMC sensitivity of BM CFC cells from Fac−/−, +/−, and +/+ mice. BM cells were seeded in the presence of increasing concentration of MMC and cultured for 7 to 8 days. Curves represent the percentage of CFC relative to untreated cells. Each point represents the mean ± SEM of two separate experiments each performed in triplicate.
In vitro MMC sensitivity of BM CFC cells from Fac−/−, +/−, and +/+ mice. BM cells were seeded in the presence of increasing concentration of MMC and cultured for 7 to 8 days. Curves represent the percentage of CFC relative to untreated cells. Each point represents the mean ± SEM of two separate experiments each performed in triplicate.
To measure the effect of MMC on more primitive hematopoietic cells or long-term culture-initiating cells (LTC-IC), we established 5-week BM cultures (LTBMC) of BM cells from acute and chronic MMC-exposed mice (Fig 7). Five-week LTBMC enables the detection of cells capable of producing CFC for at least 5 weeks and is a reliable indicator of primitive cells.23 24 Acute exposure to MMC reduced the number of Fac −/− LTC-IC cells by more than 90% in cultures from both time points (Fig7A). LTC-IC from Fac+/+ showed a fivefold increase in CFC at day 2, which returned to control levels at day 6. We also estimated LTC-IC after repeated exposure to low doses of MMC (Fig 7B). The number of LTC-IC in both Fac−/− and Fac +/+ LTBMC cultures decreased to 33% of control level after one MMC injection. Growth was completely abolished in Fac −/− LTBMC initiated after 3 weekly injections of MMC. The total number of cells from these cultures were plated, but no CFC were observed. Fac+/+ mice showed a progressive increase in LTC-IC from LTBMC after 3 and 5 weekly injections of MMC. Similar levels of CFC formation toFac +/+ were found in Fac +/− LTC-IC cells (data not shown). These results are in agreement with the histological studies and demonstrate that death after repeated exposure to MMC is caused by progressive loss of early (LTC-IC) and committed progenitors causing BM failure.
In vivo MMC sensitivity of BM LTC-IC cells fromFac +/+ and Fac −/− mice. (A) The number of CFC from 5-week LTBMC established 2 and 6 days after 1 single MMC (0.75 mg/kg) injection. (B) The number of CFC from 5-week LTBMC established 1, 3, and 5 weeks after repeated MMC injection of 0.3 mg/kg. Controls represent saline-injected mice. Data represent the mean ± SEM of triplicate determinations from 2 mice. Significant differences between controls and MMC-injected mice (*P < .01; **P < .05; #P < .005; ##P < .0005). The absence of SEM bars represents values too low to appear in the graph.
In vivo MMC sensitivity of BM LTC-IC cells fromFac +/+ and Fac −/− mice. (A) The number of CFC from 5-week LTBMC established 2 and 6 days after 1 single MMC (0.75 mg/kg) injection. (B) The number of CFC from 5-week LTBMC established 1, 3, and 5 weeks after repeated MMC injection of 0.3 mg/kg. Controls represent saline-injected mice. Data represent the mean ± SEM of triplicate determinations from 2 mice. Significant differences between controls and MMC-injected mice (*P < .01; **P < .05; #P < .005; ##P < .0005). The absence of SEM bars represents values too low to appear in the graph.
DISCUSSION
We, and others, have generated mouse models for Fanconi anemia to better understand the pathophysiology of this BM failure syndrome.11,12 Although Fac −/− mice have no FAC protein present in their cells, these animals do not naturally develop any of the hematologic phenotypes characteristic of the disease. Absence of a hematologic phenotype in Fac−/− mice may be attributed to the lack of a stressful insult and/or response to DNA damage in the target tissue (BM). Because mouse models generated by the disruption of DNA repair genes only manifest phenotypes similar to those of the human disease after the induction of disease-specific DNA damage,20-22 we analyzed the in vivo effect of an FA-specific DNA-damaging agent, MMC. Under these conditions, Fac −/− mice developed a phenotype remarkably similar to that seen in FA patients.
Fac −/− mice were found to be highly sensitive to DNA cross-links and showed a marked decrease in survival to MMC doses 10 times lower than in normal mice. Acute exposure to MMC not only produced a marked BM hypoplasia in Fac −/− mice but also degeneration of the liver, spleen, and small and large intestines, resulting in death within a few days after treatment. These results correlate with the increased toxicity found in FA patients after exposure to the cross-linking agent cyclophosphamide used in conditioning regimens before BM transplantation.23-25
The increased sensitivity of Fac −/− mice to MMC is similar to the one observed in MGMT-deficient mice afterN-methyl-N-nitrosourea treatment or in ATM-deficient mice after γ-irradiation and correlates with toxicity of unrepaired disease-specific DNA lesions in proliferative tissues.13,15 26 However, chronic MMC treatment or progressive accumulation of lower levels of DNA lesions seemed to specifically target the hematopoietic system of Fac−/− mice, affecting early (LTC-IC) and committed (CFC) progenitors, suggesting that FAC specifically regulates regeneration of hematopoietic progenitors after DNA damage. These results also imply that other proliferative tissues such as gastrointestinal tissue can either tolerate a certain level of damage or rapidly regenerate progenitors, whereas the BM compartment cannot. These observations also support the notion that Fac −/− mice lack stressful insults in clean animal facilities.
In vivo exposure of Fac −/− mice to low doses of MMC induced a dramatic pancytopenia, the result of BM failure.Fac −/− mice died shortly after a decrease in all peripheral blood cells. This phenotype resembles that observed in FA patients in that thrombocytopenia or pancytopenia is often associated with decreased BM cellularity and is the first indicator of BM failure. In MMC-treated Fac −/− mice, BM failure was associated with decreased BM cellularity and, more specifically, by a reduction in the number of committed (CFC) and more primitive (LTC-IC) progenitor cells. Loss of in vitro growth potential of progenitors is also observed in FA patients and was found to correlate with poor clinical status.27 Also, the increased in vitro MMC sensitivity observed in CFC from Fac−/− mice is remarkably similar to that seen in FA patients. These results suggest that primitive hematopoietic FA cells are highly sensitive to DNA cross-linking agents and that their loss results in BM failure. Although FA patients are not in close contact to MMC, other agents present in the environment that can mimic cross-linking activity may induce DNA lesions in their BM cells, contributing to BM failure.
Injury to other BM components, such as the surrounding stroma, and/or disregulation of growth factor homeostasis may also influence the decrease and regeneration of hematopoietic cells after damage. FA patients were shown to have abnormal levels of tumor necrosis factor α (TNFα) and interleukin-6 in their serum fractions, which may, at high levels, be toxic to the BM cell populations.28-30 We did not observe any increase in TNFα levels in Fac −/− mice after chronic exposure to MMC or other stimuli such as lipopolysaccharides (data not shown), suggesting that changes in growth factor levels may occur locally or be a secondary effect to immune responses from infections to which theseFac −/− mice have not been subjected. MGMT −/− and ATM −/− mice have an increased sensitivity to bacterial infection after DNA damage, supporting the notion that impairment of the BM cell compartment in FA patients may contribute to an increased risk of infection triggering immune responses and thus high TNFα levels.13,15 26 This again supports the view that mice in animal colonies lack natural exposure to stressful agents from the environment that would contribute to the phenotype.
MMC is a well-known DNA cross-linking agent that may generate, although to a lesser extent, DNA damage through oxygen radical formation. Thus, damage to the BM compartment may also result from abnormal oxygen metabolism and increased production of reactive oxygen species (ROS).31 In vitro and ex vivo studies of FA cells have demonstrated overproduction of ROS and increased sensitivity to oxygen, as well as an increase in ROS-induced DNA lesions, particularly 8-hydroxy-2′-deoxyguanosine.37-39 However, this may be a secondary feature to the basic defect, because FA-C cells transfected with the FAC gene failed to complement their oxygen-induced growth inhibition.32 Regardless of whether abnormal oxygen metabolism is the fundamental defect in FA, direct or indirect excess production of oxidative DNA damage may contribute to the decrease in hematopoietic progenitors leading to BM failure in FA patients as in Fac −/− mice.
FA patients were found to have an increased risk of cancer, particularly acute myelogenous leukemia (AML), but we did not observe any tumor formation in untreated or MMC-treatedFac −/− mice. However, because mice subjected to acute or chronic MMC treatments died either from toxicity after acute treatment or BM failure after chronic treatment, tumor formation in these mice could only have been detected if they had survived the treatment.
Differences in types or abundance of species-specific genes may influence the FAC-mediated pathway and interfere or compensate for its absence. In preliminary experiments, we have observed differences in survival after chronic MMC treatment in Fac −/− mice from different strains, suggesting the involvement of modifier genes in the development of the FA-specific phenotype. Because the genetic background is known to modify the expression of mutant phenotypes,33 variation in the severity of the hematologic phenotype may be observed in different inbred strains of Fac−/− mice.
FA is considered a DNA repair related disease and a chromosomal instability syndrome due to the high cellular sensitivity to DNA cross-linking agents and occurrence of spontaneous or induced chromosomal breakage. The specific function of FAC has yet to be defined, but studies on in vitro systems and its cytoplasmic localization have suggested a role in either cell cycle regulation or apoptosis rather than in DNA repair.7,9 Also, studies on cross-link removal in FA cells have also demonstrated contradictory results as to a defect in DNA repair.34-36 Studies on mice deficient for nucleotide excision repair genes have shown that phenotypes corresponding to the human disease occur only after DNA damage induction,20-22 whereas mice defective for a putative signal transduction protein, ATM, show spontaneous phenotypes even in the absence of DNA damage.13,15 Furthermore, inactivation of antiapoptotic genes, such as Bcl-2 orBcl-x, leads to short-lived animals or a lethal phenotype.37 38 In comparison to those data, our in vivo studies would indicate that FAC has a more direct role in DNA repair than in either signal transduction or apoptosis. To reconcile previous findings with our in vivo results, FAC may act as a sensor of DNA damage and function as an inducer of repair; thus, lack of repair affects both cell cycle progression and possibly induces apoptosis. Experiments are under way to determine if apoptosis contributes to the decrease in Fac −/− BM progenitors after DNA damage.
The development of a Fac mouse model with a hematologic phenotype similar to that seen in FA patients should be useful for further studies of the genesis of BM failure and to test novel therapies for FA. This Fac mouse model should also help characterize the role of FA genes in the response to DNA damage and their relationship to BM function.
Supported by grants from the National Cancer Institute of Canada with funds from the Canadian Cancer Society (J.E.D. and M.B.), the Fanconi Anemia Research Fund (Eugene, OR; M.B.), the Bayer Canadian Red Cross Society Research and Development Fund (M.B.), the Medical Research Council of Canada (J.E.D.), the Canadian Genetic Diseases Network of the National Centers of Excellence (J.E.D.), a Research Scientist award from the NCIC and an MRC scientist award (J.E.D.), and the Sunnybrook Trust for Medical Research (C.M.).
Address reprint requests to Madeleine Carreau, PhD, Department of Genetics, Research Institute, Hospital for Sick Children, 555 University Ave, Toronto, Ontario, Canada M5G 1X8.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal