Abstract
Untreated patients with Hurler syndrome (MPSIH) experience progressive neurologic deterioration and early death. Allogeneic bone marrow transplantation (BMT) ameliorates or halts this course. The Storage Disease Collaborative Study Group was formed to evaluate the effectiveness and toxicity of BMT. Effectiveness was defined as engrafted survival with continuing cognitive development. Fifty-four patients deficient in leukocyte α-l-iduronidase enzyme activity (median age, 1.8 years; range, 0.4 to 7.9) received high-dose chemotherapy with or without irradiation and BMT from HLA-genotypically identical sibling (GIS) or HLA-haploidentical related (HIR) donors between September 16, 1983 and July 14, 1995; all children were included in this report. Thirty-nine of 54 patients (72%) engrafted following the first BMT. The probability of grade II to IV acute graft-versus-host disease (GVHD) at 100 days was 32% for GIS and 55% for HIR patients. The probability of extensive chronic GVHD was 0% for GIS and 24% for HIR patients. The actuarial probability of survival at 5 years was 64% for all patients, 75% for GIS patients, 53% for HIR patients, and 53% for patients with donor marrow engraftment. The baseline Mental Developmental Index (MDI) was examined both for children less than and greater than 24 months of age at BMT. Children transplanted before 24 months had a mean baseline MDI of 78, while those transplanted after 24 months had a mean baseline MDI of 63 (P = .0002). Both baseline and post-BMT neuropsychologic data were available for 26 of 30 engrafted survivors. Of 14 patients transplanted before 24 months of age, nine demonstrated developmental trajectories that were normal or somewhat slower than normal. In contrast, of 12 patients transplanted after 24 months of age, only three showed developmental trajectories that were normal or somewhat slower than normal (P = .01). For children with a baseline MDI greater than 70, there was a significant correlation between the MDI at follow-up study and leukocyte α-l-iduronidase enzyme activity (P = .02). Children were more likely to maintain normal cognitive development if they were fully engrafted following BMT from a donor with homozygous normal leukocyte α-l-iduronidase enzyme activity. Children who developed acute GVHD of grade II or worse had significantly poorer cognitive outcomes (P < .009). No difference in the post-BMT MDI was observed between patients whose preparative therapies did (n = 10; radiation dose, 300 to 1,400 cGy) or did not (n = 16) include radiation. We conclude that MPSIH patients, particularly those less than 24 months of age with a baseline MDI greater than 70, can achieve a favorable long-term outcome with continuing cognitive development and prolonged survival after successful BMT from a related donor with homozygous normal enzyme activity.
HURLER SYNDROME (MPSIH) is an autosomal recessive inborn error of metabolism due to a deficiency of α-l-iduronidase enzyme activity, which results in accumulation of heparan sulfate and dermatan sulfate substrates. Progressive hepatosplenomegaly, cardiac disease, severe skeletal abnormalities, hydrocephalus, and mental retardation result in substantial morbidity and early death, often by 5 years of age.1
Hematopoietic stem cell transplantation corrects enzymatic deficiencies in selected lysosomal and peroxisomal disorders.2 While hundreds of children with storage diseases, including nearly 200 patients with MPSIH, have received allogeneic bone marrow transplantation (BMT) worldwide, reports have described relatively small cohorts of patients.3-7 Recently, The Storage Disease Collaborative Study Group described its experience using unrelated donor (URD) BMT in MPSIH patients.8 We now report our experience with HLA-genotypically identical sibling (GIS) and HLA-haploidentical related (HIR) donor BMT in 54 MPSIH patients. The Storage Disease Collaborative Study Group's data on these and 40 URD BMT patients represent the largest such collective experience in MPSIH.
SUBJECTS AND METHODS
Patients, preparative regimens, and transplantation.
Children deficient in leukocyte α-l-iduronidase enzyme activity received GIS or HIR donor BMT with preparative regimens determined by the transplant centers. Patient characteristics are summarized in Table 1. Parental consent was obtained for all patients, and protocols were approved by the institutional review boards. Forty-six of these patients (23 GIS and 23 HIR) have been briefly described in a previous publication.5 Furthermore, 11 of these 23 GIS patients were also reported by Whitley et al.4
Patient Characteristics
| Characteristic . | GIS BMT . | HIR BMT . |
|---|---|---|
| Sex (F/M) | 18/10 | 12/14 |
| Median age at BMT, yr (range) | 1.8 (0.4-4.6) | 1.9 (0.7-7.9) |
| Bone marrow donor enzyme status (n) | ||
| Homozygous normal/heterozygous carrier | 15/13 | 3/23 |
| Bone marrow manipulation (n) | ||
| T-lymphocyte depletion | 0 | 14 |
| T10B9 | 11 | |
| Antibody + complement | 1 | |
| Soybean agglutinin + sheep RBC rosetting | 2 | |
| No T-lymphocyte depletion | 26 | 11 |
| Not assessable | 2 | 0 |
| Unknown | 0 | 1 |
| Degree of HLA matching (n) | ||
| 6 of 6 | 28 | 5 |
| 5 of 6 | 0 | 8 |
| 4 of 6 | 0 | 8 |
| 3 of 6 | 0 | 4 |
| Unknown | 0 | 1 |
| Preparative therapy (n) | ||
| BU/cy | 23 | 8 |
| BU/cy + TBI | 1 | 0 |
| Ara-C/cy + TBI | 0 | 12 |
| cy + TBI | 1 | 3 |
| BU/cy + TBI | 1 | 1 |
| Not assessable | 2 | 0 |
| VP-16/Ara-C/cy + TBI | 0 | 1 |
| Unknown | 0 | 1 |
| GVHD prophylaxis (n) | ||
| Cyclosporin/corticosteroid ± ATG | 11 | 6 |
| Cyclosporin/methotrexate | 8 | 3 |
| Anti–CD-5/corticosteroid | 0 | 10 |
| Methotrexate/corticosteroid ± ATG | 6 | 0 |
| ATG or ALG/corticosteroid | 0 | 2 |
| None | 1 | 1 |
| Other | 0 | 3 |
| Not assessable | 2 | 0 |
| Unknown | 0 | 1 |
| Median bone marrow cell dose (range)-150 | 3.6 (1.9-10.3) | 2.9 (0.6-5.1) |
| Characteristic . | GIS BMT . | HIR BMT . |
|---|---|---|
| Sex (F/M) | 18/10 | 12/14 |
| Median age at BMT, yr (range) | 1.8 (0.4-4.6) | 1.9 (0.7-7.9) |
| Bone marrow donor enzyme status (n) | ||
| Homozygous normal/heterozygous carrier | 15/13 | 3/23 |
| Bone marrow manipulation (n) | ||
| T-lymphocyte depletion | 0 | 14 |
| T10B9 | 11 | |
| Antibody + complement | 1 | |
| Soybean agglutinin + sheep RBC rosetting | 2 | |
| No T-lymphocyte depletion | 26 | 11 |
| Not assessable | 2 | 0 |
| Unknown | 0 | 1 |
| Degree of HLA matching (n) | ||
| 6 of 6 | 28 | 5 |
| 5 of 6 | 0 | 8 |
| 4 of 6 | 0 | 8 |
| 3 of 6 | 0 | 4 |
| Unknown | 0 | 1 |
| Preparative therapy (n) | ||
| BU/cy | 23 | 8 |
| BU/cy + TBI | 1 | 0 |
| Ara-C/cy + TBI | 0 | 12 |
| cy + TBI | 1 | 3 |
| BU/cy + TBI | 1 | 1 |
| Not assessable | 2 | 0 |
| VP-16/Ara-C/cy + TBI | 0 | 1 |
| Unknown | 0 | 1 |
| GVHD prophylaxis (n) | ||
| Cyclosporin/corticosteroid ± ATG | 11 | 6 |
| Cyclosporin/methotrexate | 8 | 3 |
| Anti–CD-5/corticosteroid | 0 | 10 |
| Methotrexate/corticosteroid ± ATG | 6 | 0 |
| ATG or ALG/corticosteroid | 0 | 2 |
| None | 1 | 1 |
| Other | 0 | 3 |
| Not assessable | 2 | 0 |
| Unknown | 0 | 1 |
| Median bone marrow cell dose (range)-150 | 3.6 (1.9-10.3) | 2.9 (0.6-5.1) |
Abbreviations: T10B9, monoclonal antibody T10B9.1-31A and rabbit serum complement; antibody + complement, anti-CD2 antibody and rabbit serum complement; soybean agglutinin + sheep RBC rosetting, soybean agglutination and sheep RBC rosetting; BU/cy, busulfan (range, 12 to 25 mg/kg in divided doses every 6 hours over 4 days)/cyclophosphamide (range, 200 to 240 mg/kg in 4 divided doses over 4 days) with or without antithymocyte globulin (ATG); TBI, total-body irradiation (20 patients, 300 to 1,400 cGy; single dose, 3 patients); Ara-C/cy + TBI, cytosine arabinoside (18 g/m2in 6 divided doses over 3 days)/cy (range, 90 to 100 mg/kg in 2 divided doses over 2 days) and TBI; cy + TBI,cy (120 mg/m2 in 2 divided doses over 2 days) and TBI; BU/cy + TBI, reduced-dose BU (320 mg/m2 in divided doses every 6 hours over 2 days)/reduced-dose cy (120 mg/kg in 2 divided doses over 2 days) and TBI; VP-16/Ara-C/cy + TBI, etoposide (500 mg/m2 in 2 divided doses over 2 days)/Ara-C (18 g/m2 in 6 divided doses over 3 days)/cy (90 mg/kg in 2 divided doses over 2 days) and TBI (TBI dosage range and patient number: 300 cGy, n = 1; 720 to 850 cGy, n = 3; 1,200 cGy, n = 3; 1,400 cGy, n = 11; unknown, n = 2); anti–CD-5/corticosteroid, anti-CD5 ricin A chain–conjugated immunotoxin and corticosteroid; ALG, antilymphocyte globulin.
× 108 nucleated cells/kg recipient body weight.
Prophylaxis and grading of graft-versus-host disease.
Bone marrow grafts were depleted of T lymphocytes (TLD) ex vivo by a variety of methods for 14 of 26 HIR BMTs (Table 1).9-13Various drugs were used for graft-versus-host disease (GVHD) prophylaxis (Table 1). The severity of acute GVHD was diagnosed and graded according to Glucksberg et al,14 and the severity of chronic GVHD was diagnosed and graded according to Shulman et al.15 Moderate to severe acute GVHD was defined as acute GVHD of at least grade II. Patients were considered assessable for acute GVHD if they survived 42 days and for chronic GVHD if they survived 100 days following BMT.
Engraftment.
If the patient survived 21 days, engraftment was assessable by leukocyte α-l-iduronidase enzyme activity, sex chromosome analysis, and/or semiquantitative restriction fragment length polymorphism (RFLP) evaluation of monocytes and/or neutrophils.16-18 Engraftment was deemed to be permanent at 1 year following GIS or HIR BMT. Complete donor chimerism was defined as greater than 90% donor enzyme activity and/or greater than 90% donor cells by RFLP analysis. Leukocyte α-l-iduronidase enzyme activity is 100% in a homozygous normal donor; it is approximately 50% of normal in a heterozygous carrier donor. Mixed donor-recipient chimerism was defined as 10% to 90% donor enzyme activity and/or 10% to 90% donor cells by RFLP analysis. Autologous recovery was defined as less than 10% donor enzyme activity and/or less than 10% donor cells by RFLP analysis.
Neuropsychologic testing.
A battery of standardized neuropsychological tests were used to assess the child's developmental or intellectual level, adaptive behavior, academic readiness or performance, neuropsychologic functioning in several domains (language, perception, memory, attention, and executive functions), and emotional and social functioning as described previously.8 Testing performed within 6 months prior to BMT served as a baseline; subsequent age-appropriate tests were administered annually after BMT. For this report, only the results of developmental and intelligence tests are reported. Baseline Mental Developmental Indices (MDIs) from the Bayley Scales of Infant Development were calculated from normative tables for all children.19 At follow-up study, the children were administered tests appropriate for their age (eg, Bayley Scales of Infant Development, Stanford Binet Intelligence Scale, and Wechsler Scales). Age-equivalent scores were used for monitoring developmental and intelligence status. These scores provided a mechanism for comparing results across developmental tests and information about whether a child was losing mental ability, plateauing in learning, gaining more slowly than normally expected, or progressing at an appropriate rate. Because some children had a MDI below the lower limit of the normative tables (MDI < 50), MDIs could not be used for longitudinal assessment.
Statistical analysis.
Survival curves were calculated by the method of Kaplan and Meier.20 Statistical comparisons of categoric data were performed by χ2 analysis. Correlation coefficients were assessed by Spearman's rank order. Nonparametric comparisons of neuropsychologic outcome according to severity of GVHD were performed using the Mann-Whitney U statistic. Vital status was ascertained for all patients on July 29, 1997.
RESULTS
From September 16, 1983 to July 14, 1995, 54 children with leukocyte α-l-iduronidase enzyme deficiency underwent GIS or HIR BMT at a median age of 1.8 years (range, 0.4 to 7.9) at 13 centers in North America: 7 centers (54%) each cared for 1 child, 4 centers (31%) cared for 3 to 5 children, and 2 centers (15%) cared for 12 or 21 children. Twenty-six patients received marrow from GIS donors, and 26 children received marrow from HIR donors. Two children who were to receive GIS donor BMT died during preparative therapy. The median follow-up period for all GIS BMT patients is 7.3 years. The median follow-up period for all HIR BMT patients is 4.6 years.
Transplant-related toxicity and GVHD.
Outcomes for MPSIH patients undergoing GIS and HIR BMT are presented in Table 2. Transplant-related complications included cardiopulmonary arrest, acute and chronic GVHD, infection, hemorrhage, organ failure, and pneumonitis. Of 28 patients scheduled to receive a GIS BMT, 2 died of cardiopulmonary arrest during preparative therapy. Moderate to severe acute GVHD developed in 8 of 25 patients (32%; 95% confidence interval [CI], 15% to 49%) following GIS BMT and in 12 of 22 patients (55%; 95% CI, 33% to 77%) following HIR BMT (P = .15). Acute GVHD could not be assessed because of early death for 1 GIS BMT patient and 4 HIR BMT patients. Moderate to severe acute GVHD occurred in 4 of 11 BMTs using cyclosporin and corticosteroid with or without ATG for GVHD prophylaxis and in 4 of 15 BMTs using other GVHD prophylaxis regimens following GIS BMT. Moderate to severe acute GVHD occurred in 5 of 10 BMTs using anti-CD5 ricin A chain–conjugated immunotoxin and corticosteroid for GVHD prophylaxis and in 7 of 16 BMTs using other GVHD prophylaxis regimens following HIR BMT. The probability of extensive chronic GVHD was 0% (95% CI, 0% to 14%) for GIS patients and 24% (95% CI, 4% to 44%) for HIR patients (P = .0003). The primary cause of death was cardiopulmonary arrest or failure (6 patients) and GVHD (6 patients).
Treatment Outcomes
| Parameter . | GIS BMT . | HIR BMT . |
|---|---|---|
| No. of patients | 28* | 26 |
| Engraftment | ||
| Complete/partial/autologous (n) | 15/7/4 | 16/1/9 |
| α-l-Iduronidase enzyme activity† | ||
| Median, range, n‡ | 21.0, 14.3-41.0, 14 | 14.4, 8.8-21.0, 6 |
| Other§ | ||
| Acute GVHD (n) | ||
| Grade 0 to I/II to IV | 17/8 | 10/12 |
| Not applicable or assessable | 3 | 4 |
| Chronic GVHD (n) | ||
| None/limited/extensive | 25/0/0 | 13/4/5 |
| Not applicable or assessable | 3 | 4 |
| Survival (n) | ||
| Alive/alive and engrafted | 21/21 | 14/9 |
| Follow-up of survivors (yr) | ||
| Median (range) | 8.7 (2.2-13.9) | 7.2 (2.0-10.2) |
| Cause of death (n) | ||
| Cardiac | 2 | 4 |
| GVHD | 1 | 5 |
| Sepsis | 1 | 1 |
| Hemorrhage | 1 | 0 |
| MPSIH | 1 | 0 |
| Organ failure | 0 | 1 |
| Pneumonitis | 1 | 0 |
| Unknown | 0 | 1 |
| Parameter . | GIS BMT . | HIR BMT . |
|---|---|---|
| No. of patients | 28* | 26 |
| Engraftment | ||
| Complete/partial/autologous (n) | 15/7/4 | 16/1/9 |
| α-l-Iduronidase enzyme activity† | ||
| Median, range, n‡ | 21.0, 14.3-41.0, 14 | 14.4, 8.8-21.0, 6 |
| Other§ | ||
| Acute GVHD (n) | ||
| Grade 0 to I/II to IV | 17/8 | 10/12 |
| Not applicable or assessable | 3 | 4 |
| Chronic GVHD (n) | ||
| None/limited/extensive | 25/0/0 | 13/4/5 |
| Not applicable or assessable | 3 | 4 |
| Survival (n) | ||
| Alive/alive and engrafted | 21/21 | 14/9 |
| Follow-up of survivors (yr) | ||
| Median (range) | 8.7 (2.2-13.9) | 7.2 (2.0-10.2) |
| Cause of death (n) | ||
| Cardiac | 2 | 4 |
| GVHD | 1 | 5 |
| Sepsis | 1 | 1 |
| Hemorrhage | 1 | 0 |
| MPSIH | 1 | 0 |
| Organ failure | 0 | 1 |
| Pneumonitis | 1 | 0 |
| Unknown | 0 | 1 |
*Two patients died during preparative therapy.
†Leukocyte α-l-iduronidase enzyme activity in engrafted survivors 0.7 to 10.9 years following BMT.
‡Normal: 39 ± 15 nmol/mg protein/h.
§GIS BMT, n = 1 for each of the following: 4.1 (normal, 11.7 to 20.6 nmol/mg protein/h); 9.7 (normal, 14.5 to 23.3 nmol/mg protein/h); 17.4 (normal, 15.1 to 50.9 nmol/mg protein/h); 1.9 (normal, 21.3 to 22.6 nmol/mg protein/h); 21.8 (normal, 21.8 to 39.4 nmol/mg protein/h); 23.2 (normal, 39.6 to 50.9 nmol/mg protein/h); HIR BMT, n = 1 for each of the following: 188.0 (normal, 615.4 pmol/mg protein/min); 527.1 (normal, 558.1 pmol/mg protein/min).
The rates for survival and engrafted survival were comparable for children who received GIS or HIR BMT during the periods 1983 to 1987, 1988 to 1990, and 1991 to 1995 (P = .70). On September 1, 1991, the 5-year National Institutes of Health, National Institute of Neurological Disorders and Stroke (NINDS)–supported clinical trial of the value of BMT for storage diseases started.
Engraftment.
Twenty-two of 26 GIS BMT patients engrafted. Fifteen patients experienced complete donor engraftment, 7 achieved mixed donor-recipient chimeric grafts, and 2 died of transplant-related causes following engraftment. Thus, 20 of 28 patients survived engrafted following the first GIS BMT.
Four patients failed to engraft following the initial GIS BMT. One patient died of sepsis; the remaining 3 received a second GIS BMT. One child is alive with engraftment 12.4 years following the second BMT. Thus, 21 of 28 GIS BMT patients are alive and engrafted, 20 following the first BMT and 1 following the second BMT. Durable complete chimerism was observed in 15 of these 21 survivors of GIS BMT, with mixed chimerism in 6.
Of 26 patients receiving a HIR BMT, 17 patients engrafted; 8 of these 17 died of transplant-related causes following engraftment. All but 1 of the 17 HIR BMT patients who engrafted had complete donor engraftment. Nine patients failed to engraft following the initial HIR BMT. One patient underwent a second HIR BMT and died unengrafted. Two patients died of transplant-related causes. One patient died of cardiopulmonary arrest 2.1 years from BMT. Five patients are alive but auto-engrafted. Thus, 9 of 26 HIR BMT patients are alive and engrafted. Durable complete chimerism was observed in 8 and mixed chimerism in one of 14 survivors of HIR BMT; five survivors had autologous marrow recovery.
Leukocyte α-l-iduronidase enzyme activity measured 0.7 to 10.9 years following BMT was available for 20 of 21 engrafted GIS BMT patients and 8 of 9 HIR BMT patients surviving 1 year. Six of 10 patients who received grafts from homozygous normal donors achieved a normal level of enzyme activity. Of the remaining 4 patients, three had enzyme levels consistent with carrier status and one had an enzyme level below 25% of normal. Fifteen of 18 patients who received grafts from heterozygous carrier donors achieved a carrier level of enzyme activity. The remaining 3 patients had enzyme levels in the lower limit of the carrier range.
Survival.
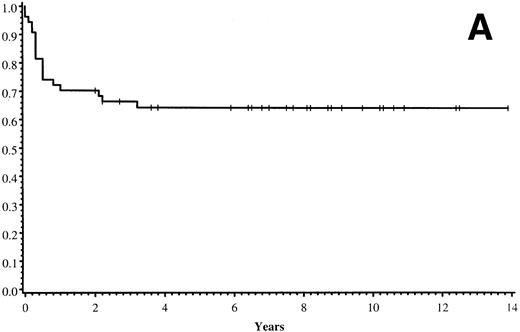
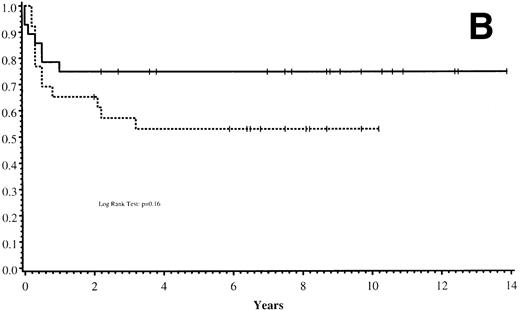
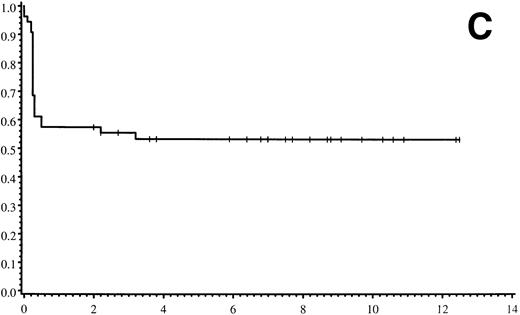
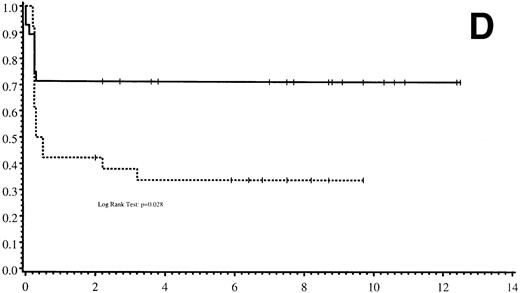
Thirty-five of 54 patients are alive 2.0 to 13.9 years post-BMT. The overall actuarial probability of survival at 5 years was 64% (95% CI, 51% to 77%) for all patients (Fig 1A).The overall actuarial probability of survival at 5 years was 75% (95% CI, 59% to 91%) for GIS BMT recipients and 53% (95% CI, 34% to 73%) for HIR BMT recipients (Fig 1B; P = .16). For patients with donor cell engraftment, the actuarial probability of survival at 5 years was 53% (95% CI, 40% to 67%; Fig 1C). The overall actuarial probability of survival at 5 years was 71% (95% CI, 55% to 88%) for GIS BMT recipients with donor cell engraftment and 34% (95% CI, 15% to 52%) for HIR BMT recipients with donor cell engraftment (Fig 1D;P = .03). Twenty-nine of 35 surviving patients engrafted after the first BMT. Four patients received a second BMT because of graft rejection or failure; one patient is an engrafted survivor.
(A) Overall actuarial probability of survival (—) of 54 MPSIH patients treated with related-donor BMT. (B) Overall actuarial probability of survival of MPSIH patients treated with GIS (—) or HIR (--) donor BMT. (C) Overall actuarial probability of survival (—) of MPSIH patients with donor cell engraftment following related-donor BMT. (D) Overall actuarial probability of survival of MPSIH patients with donor cell engraftment following GIS (—) or HIR (--) donor BMT.
(A) Overall actuarial probability of survival (—) of 54 MPSIH patients treated with related-donor BMT. (B) Overall actuarial probability of survival of MPSIH patients treated with GIS (—) or HIR (--) donor BMT. (C) Overall actuarial probability of survival (—) of MPSIH patients with donor cell engraftment following related-donor BMT. (D) Overall actuarial probability of survival of MPSIH patients with donor cell engraftment following GIS (—) or HIR (--) donor BMT.
Neuropsychologic function.
Twenty-one children who received a GIS BMT and 9 who received a HIR BMT are alive and engrafted. Baseline and follow-up neuropsychologic data are available for 18 of 21 GIS BMT and 8 of 9 HIR BMT children. Developmental trajectories derived from age-equivalent scores of serial neuropsychologic tests were calculated for each child. We found no statistical differences in the slope of the developmental trajectories between the GIS and HIR groups (mean, 0.50 and 0.40, respectively).
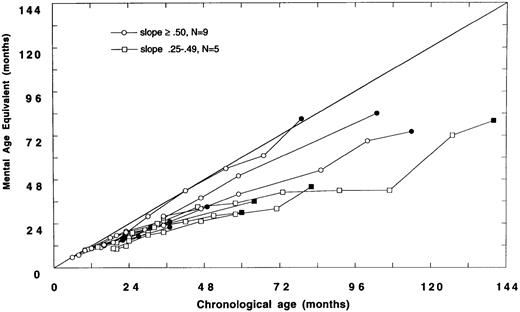
Data were analyzed to examine the trajectories of cognitive development in children transplanted before or after 24 months of age. Fourteen of 26 children with neuropsychologic data were transplanted before 24 months of age (Fig 2A). Of these 14, nine demonstrated relatively normal development (slope ≥ .50); five had slower than expected developmental gains (slope .25 to .49), and no child has plateaued (slope < .25).
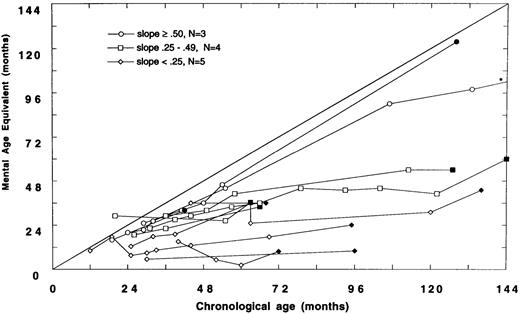
(A) Mental age-equivalent scores of MPSIH patients receiving related-donor BMT before 24 months of age (normal, —; slope ≥ .50, ○; slope .25 to .49, □; solid symbol denotes most recent neuropsychologic evaluation). (B) Mental age-equivalent scores of MPSIH patients receiving related-donor BMT after 24 months of age (normal, —; slope ≥ .50, ○; slope .25 to .49, □; slope < .25, ◊; solid symbol denotes most recent neuropsychologic evaluation). *Latest neuropsychologic evaluation at 159 months of age and a mental age-equivalent score of 108 months.
(A) Mental age-equivalent scores of MPSIH patients receiving related-donor BMT before 24 months of age (normal, —; slope ≥ .50, ○; slope .25 to .49, □; solid symbol denotes most recent neuropsychologic evaluation). (B) Mental age-equivalent scores of MPSIH patients receiving related-donor BMT after 24 months of age (normal, —; slope ≥ .50, ○; slope .25 to .49, □; slope < .25, ◊; solid symbol denotes most recent neuropsychologic evaluation). *Latest neuropsychologic evaluation at 159 months of age and a mental age-equivalent score of 108 months.
Of the 12 children with neuropsychologic data transplanted after 24 months of age, only three showed relatively normal developmental trajectories. Four children acquired skills more slowly than normal. Five children have plateaued (Fig 2B). The mean difference in the slopes of the developmental trajectories between children transplanted before and after 24 months was significant (mean, .58 and .34, respectively, t = 2.5, P = .02). A Spearman rank-order correlation of −.51, (P = .01) between the age at BMT and the slope of the line approximating the developmental trajectory was found.
The baseline MDI was examined for both children less than and greater than 24 months of age at the time of BMT. Children transplanted before 24 months had a mean baseline MDI of 78, while those transplanted after 24 months had a mean baseline MDI of 63. A Spearman rank-order correlation of .77 (P = .0002) between the baseline MDI and slope of the line approximating the developmental trajectory was found.
Further analysis to determine the effect of donor enzyme status on neuropsychologic outcome was limited to the 15 children who had a baseline MDI over 70. α-l-Iduronidase enzyme activity levels were normalized for comparison across laboratories. The correlation of the normalized α-l-iduronidase enzyme activity levels with the latest MDI scores for these 15 children was .59 (P = .02). No child whose ultimate enzyme activity level was low (ie, in the carrier range) due to either a heterozygous carrier donor or partial engraftment from a homozygous normal donor had normal mental functioning at follow-up study. Three children whose MDIs were normal (ie, >80) at follow-up study received BMTs from homozygous normal donors and fully engrafted. The enzyme activity levels in these three children at follow-up study were completely normal. There were seven children whose MDIs at follow-up evaluation were greater than 60 but less than 80; four had homozygous normal donors and three had heterozygous carrier donors. All of these children had an enzyme level at least 1.6 SD from normal. Finally, five children had follow-up MDIs of at least 48 but less than 60; two had homozygous normal donors, with one partially engrafted, and three had heterozygous carrier donors, with one fully engrafted, one partially engrafted, and one with very low enzyme activity (ie, <25% of normal).
The effect of acute GVHD on neuropsychologic function was examined. Acute GVHD was assigned a score according to severity: 1 for grade 0 or I and 2 for grades II to IV. Cognitive outcomes were significantly poorer (t = 2.81, P < .009) in children with the higher acute GVHD rating (mean MDI, 46 ± 12, n = 9) compared with children with the lower acute GVHD rating (mean MDI, 64 ± 19, n = 19). For children whose baseline MDI pre-BMT was over 70, only two patients had grade II or III acute GVHD (post-BMT MDI, 48 and 50). There were 14 patients with grade 0 or I acute GVHD (mean post-BMT MDI, 70 ± 15). The result of nonparametric Mann-Whitney Utesting was significant (P < .006).
No difference in post-BMT MDIs was observed between patients whose preparative therapies did (slope of developmental trajectory: median, .49; range, .00 to .69; n = 10; radiation dose range, 300 to 1,400 cGy) or did not (slope of developmental trajectory: median, .49; range, −.18 to 1.04; n = 16) include radiation.
DISCUSSION
This report represents the largest, most comprehensive related-donor BMT experience for MPSIH patients. Long-term adoptive enzyme replacement has occurred in patients successfully engrafted following BMT.3 Substrate reduction in the liver, tonsils, conjunctiva, and urine occurs due to enzyme replenishment.3,21-24 Central nervous system substrate reduction is measured in CSF and by magnetic resonance imaging and computed axial tomography.4,25 These imaging modalities demonstrate a reduction of glycosaminoglycans in the Virchow-Robin spaces and an arrest of the pathologic process leading toward hydrocephalus. Auditory improvements occur following successful BMT.26 Cardiac manifestations including heart failure, coronary artery narrowing, and tachyarryhthmias are eliminated 1 year after successful BMT.27,28 However, despite engraftment, skeletal deformities and some ophthalmologic abnormalities persist and often progress.29 30
Failure to achieve stable engraftment in MPSIH patients was a major obstacle following HIR BMT, as it was following URD BMT.8 A consensus regarding optimal myeloablative and immunosuppressive preparation for BMT in MPSIH was not established from this study. In the absence of a common protocol, the transplant centers determined the preparative regimen: 88% (23 of 26) of GIS BMTs and 31% (8 of 26) of HIR BMTs for MPSIH used busulfan and cyclophosphamide without irradiation ([BU/CY] busulfan 12 to 25 mg/kg in divided doses every 6 hours over 4 days/cyclophosphamide 200 to 240 mg/kg in four divided doses over 4 days with or without antithymocyte globulin; Table 1). Engraftment occurred in only 65% of patients surviving at least 1 year from the first HIR BMT. This differs from the 95% engraftment in patients surviving at least 1 year from the first GIS BMT for MPSIH in this study. While manipulation of marrow grafts with TLD has reduced the incidence and severity of GVHD,13,15,31-35 there has been an increase in rejection in patients with leukemia.36 37 However, the consortium experience has shown that TLD of the marrow apparently did not compromise either the rate of engraftment or survival at 1 year. In fact, survival at 1 year for patients receiving TLD marrow was 69%, and it was only 46% for those not receiving TLD marrow. Of note, busulfan clearance can be accelerated in children38,39; however, systematic evaluation of busulfan pharmacokinetics was not performed in this retrospective study. We speculate that insufficient myeloablative and/or immunosuppressive therapy was the principal cause of graft failure (ie, autologous recovery). The deposition of glycosaminoglycan in the hematopoietic microenvironment may also contribute to the high rate of autologous recovery.
Using the extensive array of BMT preparative and GVHD prophylactic therapies, BMT-related mortality was 18% for GIS BMT and 44% for HIR BMT in MPSIH patients. The overall actuarial survival at 5 years following BMT in these 54 patients was 64%: 75% for patients receiving GIS BMT and 53% for patients receiving HIR BMT. The two leading causes of death were cardiopulmonary arrest or failure and GVHD (six deaths each). These causes of death reflect the need to reduce GVHD severity and to closely monitor the underlying pathophysiology in MPSIH patients. The rates of survival and engrafted survival were comparable for children who received GIS or HIR BMT before or after September 1, 1991, the initiation of the 5-year NIH NINDS–supported clinical trial of the value of BMT for storage diseases.
There are 35 survivors of related-donor BMT for MPSIH. Of these, 30 are engrafted. Long-term enzyme determinations were available in 28. Six patients who received marrow from a donor with homozygous normal leukocyte α-l-iduronidase enzyme activity demonstrated normal levels of enzyme activity up to 10.4 years post-BMT, while 18 patients demonstrated carrier enzyme levels up to 10.9 years following BMT. These data support the notion that successful BMT is a means of achieving long-term adoptive enzyme therapy. The decrease in enzyme activity that can be observed following replacement by BMT suggests that alternate donors (eg, URD bone marrow or umbilical cord blood) with higher enzyme activity may be preferable to achieve optimal enzyme activity.
The impact of bone marrow cell dose in providing optimal enzyme replacement is also incompletely understood. While a bone marrow cell dose of at least 3.5 × 108 cells/kg was associated with a higher rate of engraftment and better long-term survival in MPSIH recipients of URD BMT,8 such an association was not observed in recipients of GIS and HIR donor BMT. Additional study is needed to determine whether the cell dose is a critical factor in achieving engraftment and long-term survival.
While neuropsychologic capabilities vary widely after matched-sibling BMT, selected patients have maintained their rate of learning with low-normal intelligence.40 Previous research has shown that children with MPSIH transplanted after 24 months of age demonstrate a different and less favorable trajectory of development than children transplanted before 24 months.8 These data formed the rationale for examining the cognitive development of children transplanted before or after 24 months of age. Hence, in addition to examining the long-term neuropsychologic outcome of children transplanted with either a GIS or HIR donor, an additional goal of this study was to analyze the developmental outcome of children transplanted before or after 24 months of age.
Baseline and follow-up neuropsychologic data were available for 18 survivors of GIS BMT and eight survivors of HIR BMT. There were no significant differences between GIS and HIR BMT groups with respect to follow-up neuropsychologic status up to 159 months post-BMT. However, we did find that the developmental outcome following BMT was strongly associated with the age at BMT. Children transplanted before 24 months of age showed a significantly better developmental trajectory compared with children transplanted after 24 months of age. Thus, the older the child was at BMT, the higher the likelihood of poor neuropsychologic outcome. As reflected in the Figures, many of the children transplanted after 24 months of age (Fig 2B) received BMT earlier in the consortium's experience and hence have longer follow-up periods than children transplanted before 24 months of age (Fig 2A).
During the 12 years that the 54 children with MPSIH described in this report were transplanted, there was a growing awareness of the factors that could affect outcomes. On September 1, 1991, the Storage Disease Collaborative Study Group received a 5-year grant from the NIH NINDS to conduct the study, “Control Study of Value of BMT for Storage Diseases.” In the eligibility criteria, it was recommended that MPSIH patients be less than 24 months of age with a MDI of 75 or greater when preparation for BMT was initiated. There has been a trend toward transplanting children with MPSIH at younger ages. After 1991, the median age at BMT for GIS and HIR MPSIH patients decreased from 1.9 to 1.3 years, while the median age at BMT for URD MPSIH patients decreased slightly from 1.8 to 1.7 years.
We found a significant association between the age at BMT and the baseline MDI. A correlation of −.82 between the age and MDI in untransplanted children has been reported previously.40Children transplanted before 24 months of age had a mean baseline MDI that was significantly higher than in children transplanted after 24 months of age. In addition, there was a significant association between the baseline MDI and long-term developmental outcome. As expected, children transplanted with a MDI less than 70 experienced more compromise in neuropsychologic functioning following BMT than children transplanted with a baseline MDI above 70. Thus, if there was significant developmental delay pre-BMT, there was an increased likelihood of poor neuropsychologic outcome post-BMT. These results suggested that the optimal neuropsychologic outcome following BMT for MPSIH would occur when the child was less than 24 months of age and had a baseline MDI greater than 70. While the two variables age at BMT and baseline MDI were highly correlated, there were children with an MDI greater than 70 despite an age over 24 months, as well as children with a MDI less than 70 at an age less than 24 months. These cases emphasize the variability in disease progression and its importance in clinical decision-making.
We analyzed the effect of donor enzyme level on neuropsychologic outcome in the 15 children who had a baseline MDI over 70. The correlation of the normalized α-l-iduronidase enzyme activity level with the latest MDI score for these 15 children was significant. However, it was difficult to assess this effect because of differences in our patients pre-BMT. Specifically, a significant correlation between the enzyme status (ie, homozygous normal vheterozygous carrier) of the donor and the baseline MDI was found. Children with homozygous normal donors had a significantly higher baseline MDI and were also significantly younger at the time of BMT. Due to the small sample size, we were unable to assess the contribution of these various factors independently. However, we did observe a significant correlation between the donor enzyme status and the MDI at follow-up study in children whose baseline MDI was over 70. We also noted that children who retained fully normal intelligence (defined as MDI >80) were all completely engrafted from a donor with homozygous normal enzyme activity and therefore demonstrated normal α-l-iduronidase enzyme activity levels at follow-up study. This observation should be considered when selecting a potential donor. Achieving a normal α-l-iduronidase enzyme activity level following BMT contributes favorably to the ultimate neuropsychologic function of the child. Such an enzyme activity level is achievable only if the donor has a homozygous normal enzyme activity and engraftment is complete.
We also analyzed the effect of GVHD on neuropsychologic outcome in the 15 children who had a baseline MDI over 70. The development of moderate to severe acute GVHD (ie, grade II to IV) was correlated with a poorer ultimate neuropsychologic outcome. However, because the incidence of acute GVHD was higher in children undergoing HIR BMT and since almost all of these children received marrow from heterozygous carrier donors, the relationship between acute GVHD and cognitive outcome could be confounded by the lower (ie, carrier) enzyme status of the donor.
There were 20 children who received a radiation-containing preparative regimen; pre- and post-BMT neuropsychologic data are available for 10 survivors (total-body irradiation dose, 300 to 1,400 cGy). There were 31 children whose preparative regimen did not include radiation; pre- and post-BMT neuropsychologic data are available for 16 survivors. The median slopes of the neuropsychologic developmental trajectories for the 10 children who received a radiation-containing preparative regimen and the 16 children whose preparative regimen did not include radiation were identical (ie, .49). We conclude that radiation-containing preparative regimens do not adversely affect the long-term neuropsychologic development of MPSIH children undergoing BMT.
Only by pooling international patient resources can The Storage Disease Collaborative Study Group make substantial progress. We anticipate that an international study could accrue 75 to 100 MPSIH patients per year and thus afford the Group the opportunity to conduct randomized phase III clinical trials. Such studies would address questions such as the impact of radiation on the ultimate neuropsychologic outcome in these patients and how to optimize engraftment and decrease GVHD in patients with MPSIH. At a recent consortium meeting, members unanimously supported the concept of an international randomized trial designed to evaluate preparative therapies and long-term neuropsychologic outcomes in transplanted MPSIH patients.
Stable, life-long normalization of α-l-iduronidase enzyme activity can be achieved by BMT in MPSIH patients. While research continues in the areas of gene transfer and genetically engineered enzyme therapy, BMT currently remains the only proven therapeutic modality for many lysosomal and peroxisomal storage diseases, including MPSIH.
ACKNOWLEDGMENT
The contributors to this study are as follows: principal author, Charles Peters; statistician, James Anderson; bone marrow transplanters, Charles Peters, P. Jean Henslee-Downey, Martin R. Klemperer, Morton J. Cowan, E. Fred Saunders, Pedro A. deAlarcon, Clare Twist, James B. Nachman, Gregory A. Hale, Richard E. Harris, Marta K. Rozans, Joanne Kurtzberg, Guy H. Grayson, Thomas E. Williams, Carl Lenarsky, John E. Wagner, and William Krivit; neuropsychologists, Elsa G. Shapiro, Michael Balthazor, Valerie A. Cool, and Mary Crittenden; geneticists, Joe T.R. Clarke, Seymour Packman, and Emmanuel Shapira; neurologist, Lawrence A. Lockman; and radiation therapist, Kathryn Dusenbery. The following centers participated in The Storage Disease Collaborative Study Group, cared for patients, and contributed to this report: the Departments of Pediatrics (C.P., W.K., and J.E.W.), Neurology (E.G.S., M.B., and L.A.L.), and Therapeutic Radiology (K.D.), University of Minnesota School of Medicine; Department of Preventive and Societal Medicine, University of Nebraska (J.A.); University of Kentucky School of Medicine (P.J.H.-D. and G.A.H.); University of South Florida (M.R.K.); University of California, San Francisco (M.J.C., M.C., and S.P.); University of Toronto (E.F.S. and J.T.R.C.); University of Iowa College of Medicine (P.A.D. and V.A.C.); Harvard University Medical School (C.T.); University of Chicago (J.B.N.); University of Cincinnati (R.E.H.); Tulane University (M.K.R. and E.S.); Duke University (J.K.); University of Texas, San Antonio (G.H.G. and T.E.W.); and University of Southern California (C.L.).
The Storage Disease Collaborative Study Group recognizes the excellent clinical care provided to these patients by the nurses, physician assistants, pediatric housestaff, and fellows. The authors also thank Robert Zajac, principal data coordinator, the BMT center data managers, secretarial staff, and Dr W. Dobyns for a critical review of the manuscript.
Supported in part by National Institutes of Health Grant No. NS29099.
Address reprint requests to Charles Peters, MD, The Storage Disease Collaborative Study Group, University of Minnesota, Department of Pediatrics, Division of Blood and Bone Marrow Transplant, Box 477, Room D-548, Mayo Memorial Building, 420 Delaware St SE, Minneapolis, MN 55455.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal