Abstract
Relapsing experimental autoimmune encephalomyelitis (R-EAE) is an immune-mediated demyelinating central nervous system (CNS) disease. Myeloablation and syngeneic bone marrow transplantation (SBMT), when performed at the peak of acute disease (day 14), prevented glial scarring and ameliorated the disease severity. In contrast, when syngeneic BMT was performed late in chronic phase (day 78), significant glial scarring remained and the clinical severity did not differ significantly from that of the controls. After SBMT in either the acute or chronic phase of disease, the posttransplant immune system remained responsive to myelin epitopes as determined by in vitro proliferation and interferon-γ (IFN-γ) production. However, in mice undergoing SBMT, in vivo delayed-type hypersensitivity (DTH) responses were significantly decreased while IFN-γ RNA levels and inflammatory infiltrates within the CNS were slightly improved. We conclude that failure of SBMT to improve the clinical disease when performed in chronic phase may be due to preexisting glial scarring. We also conclude that in the absence of glial scarring and irreversible neuronal injury, in vivo DTH responses and histology are better predictors of clinical improvement than in vitro proliferation or IFN-γ cytokine production.
BONE MARROW hematopoietic stem cells expand and differentiate not only into platelets, red blood cells, and neutrophils but also into cells that mediate immunity and tolerance such as lymphocytes and macrophages. Therefore, hematopoietic stem cell transplantation has been suggested as a therapy for immune-mediated diseases.1-3 This approach is based on the assumption that ablation of the immune system followed by reconstitution of the hematopoietic and immune compartments from the infused bone marrow will restore unresponsiveness to self-epitopes. To test this hypothesis, we evaluated the effects of bone marrow transplantation (BMT) in relapsing experimental autoimmune encephalomyelitis (R-EAE), an immune-mediated demyelinating disease with clinical signs and histopathology similar to multiple sclerosis (MS).4 5
R-EAE has an initial relapsing-remitting clinical course similar to certain types of MS. The disease can be adoptively transferred to naive animals using myelin-specific CD4+ T cells. The immunologic mechanisms involved in mediating remission and relapse are not completely understood. Alterations in cytokine patterns, changes in Th1 and Th2 T-cell subsets, upregulation of apoptotic receptors (eg, Fas or tumor necrosis factor receptor) in activated cells, and/or an idiotype network of suppressor cells may contribute to the dynamic changes in the clinical course.6-11
It has been demonstrated that as the disease progresses, the immune system responds to a greater number of myelin epitopes.12-17 For example, R-EAE may be initiated in SJL/J mice by immunization with the immunodominant epitope from proteolipid protein (PLP) 139-151. During the subsequent relapse, the immune system not only responds to the disease-initiating epitope (PLP 139-151) but also to relapse-associated epitopes such as PLP 178-191. This phenomenon of epitope or determinate spreading provides an opportunity to document the efficacy of various therapies for the treatment of ongoing R-EAE by quantifying immune responsiveness to unique encephalitogenic epitopes of myelin.
MATERIALS AND METHODS
Animals.
Six-week-old female SJL/J mice were obtained from Harlan Laboratories (Madison, WI). They were maintained on standard mouse chow and water ad libitum in a conventional animal facility. Neomycin sulfate (0.7 mmol/L), tetracycline (0.1 mmol/L), and trimethoprim/sulfamethoxazole (0.4 mmol/L) were added to the drinking water for 2 weeks after BMT to prevent infections.
Induction of R-EAE.
PLP 139-151 was provided by Dr David Klapper (University of North Carolina, Chapel Hill) and emulsified in complete Freund adjuvant (4 mg/mL Mycobacterium butricum in incomplete Freund adjuvant) at a concentration of 0.88 mg/mL. Female mice were injected subcutaneously with a total of 0.1 mL over three different sites on the back. Ten days after immunization, draining lymph nodes were removed and lymphocytes were cultured ex vivo in Dulbecco's modified Eagle's medium (DMEM) for 4 days with the disease-initiating peptide PLP 139-151 (50 μg/mL). On day zero, 3 × 106 PLP 139-151 blasts were injected intraperitoneally into naive syngeneic 6-week-old female SJL/J mice.
Scoring of R-EAE.
R-EAE was scored clinically according to the neurologic deficit as follows: 0, asymptomatic; 1, tail weakness; 2, unsteady gait when walking across the grill on the cage roof; 3, severe hindlimb weakness; 4, total hindlimb paralysis; and 5, death. A relapse is defined as an increase in the score of at least 1 point recorded on at least two consecutive evaluations. All clinical scoring was performed blinded by the same observer.
Treatment.
By definition, day 0 was the day of adoptive transfer of PLP 139-151 lymphoblasts into SJL/J mice. At two time points, the peak of acute disease (day 14) or chronic phase (day 74), SJL/J mice were divided into the following groups: control, bone marrow control (BMC), and treatment groups. Control mice received no further therapy. BMC mice received an intravenous injection of 107 syngeneic nucleated marrow cells from naive same-sex mice. The treatment groups underwent myeloablation and bone marrow rescue using 107syngeneic nucleated marrow cells from naive same-sex mice. Myeloablation consisted of one of three conditioning regimens: (1) TBI, total-body irradiation at a total dose of 1,100 cGy in two fractions of 550 cGy 6 hours apart 1 day before marrow infusion; (2) MP/TBI, methylprednisolone 50 mg/kg intraperitoneally for 3 days on the day before, the day of, and the day after the TBI given at a total dose of 1,100 cGy 6 hours apart as two 550-cGy doses on the day before marrow infusion; or (3) Cy/TBI, cyclophosphamide 60 mg/kg intraperitoneally the day before starting TBI at a total dose of 1,200 cGy divided into fractions of 200 cGy twice per day for 3 consecutive days before marrow infusion.
Histology.
Ten to 12 1-μm thick, Epon-embedded sections stained with toluidine blue were examined from each spinal cord and scored as follows: ±, mild inflammation without demyelination; +, inflammation with focal demyelination; ++, inflammation with multiple foci of demyelination; and +++, marked inflammation with bilateral converging areas of demyelination. Glial scarring was characterized according to the majority of sections involved as follows: absent; mild, focal glial bands in the margin of the cord; moderate, glial bands covering more than 50% of the cord's margin; or severe, glial scarring covering the entire thickness of the anterior column. Mice that had histologic examination of the spinal cord were not used for any other assays. Mice selected for histologic examination had clinical scores that represented the mean for their group. The neuropathologist performing histologic scoring was blinded as to animal treatment and clinical score.
Myelin-specific T-cell responses.
3H-thymidine (3H-TdR) uptake of splenocytes was performed after in vitro culture with either PLP 139-151 or PLP 178-191. Splenocytes from three mice in each group were washed and resuspended in DMEM + 5% fetal bovine serum and plated at 5 × 105/well in 96-well, flat-bottom microtiter plates with the indicated concentrations of peptide. Cultures were incubated at 37°C in 7.5% CO2, pulsed at 72 hours with 3H-TdR, and harvested 18 hours later. Incorporation was quantified by liquid scintillation counting. Supernatants from splenocyte cultures were harvested at 48 hours for assessment of interferon-γ (IFN-γ) levels in response to activation with 50 mmol/L PLP 139-151 or PLP 178-191. Cytokine levels were determined by enzyme-linked immunosorbent assay (ELISA) (Endogen, Cambridge, MA). 3H-TdR assays and ELISAs were repeated in triplicate.
Peptide-specific delayed-type hypersensitivity (DTH) responses were quantified using a 24-hour in vivo ear-swelling assay. Three mice from each group were challenged with 10 μg of the indicated PLP peptide in 0.01 mL saline. Twenty-four hours after challenge, the increase in ear swelling was quantified with an engineer's micrometer (Mitutoyo model 7326; Schlesinger Tools, Brooklyn, NY). Results were corrected for prechallenge ear thickness. The mice selected for DTH responses were not used for any other assay.
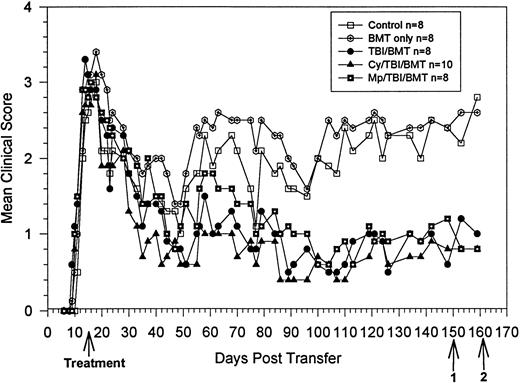
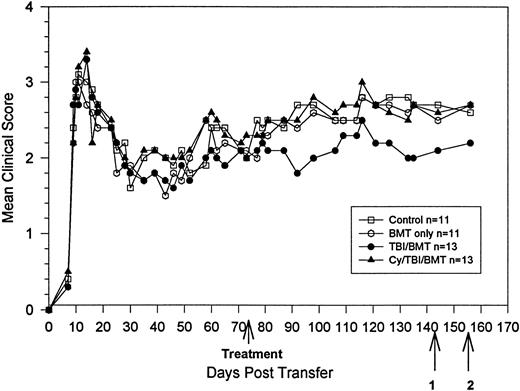
BMT at peak of acute disease. Clinical course is reported in terms of the mean clinical score, which is the total score for animals in a group divided by the number of animals in the group. Statistical analysis by the Student t-test on day 140 showed significant clinical improvement in the treatment groups, withP = .009 for TBI, P = .004 for Cy/TBI, andP = .01 for MP/TBI. Bold arrow indicates time of treatment. Arrow 1 indicates killing of 3 animals from each group for proliferation and ELISA assays. Arrow 2 indicates killing of 1 animal from each group for histology and use of 3 animals from each group for DTH.
BMT at peak of acute disease. Clinical course is reported in terms of the mean clinical score, which is the total score for animals in a group divided by the number of animals in the group. Statistical analysis by the Student t-test on day 140 showed significant clinical improvement in the treatment groups, withP = .009 for TBI, P = .004 for Cy/TBI, andP = .01 for MP/TBI. Bold arrow indicates time of treatment. Arrow 1 indicates killing of 3 animals from each group for proliferation and ELISA assays. Arrow 2 indicates killing of 1 animal from each group for histology and use of 3 animals from each group for DTH.
Central nervous system IFN-γ RNA.
Analysis of mRNA levels in the central nervous system (CNS) was performed by homogenizing phosphate-buffered saline–perfused spinal cord from three mice in each group in guanidium isothiocyanate and isolating total RNA by CsCl gradient. First-strand cDNA synthesis was performed using 2 μL (0.5 μg/μL) RNA in 10.5 μL diethyl pyrocarbonate (DEPC)-treated water, 1 μL oligo-(dT) primer, and 6.5 μL of a master mix (4 μL 5× reaction buffer containing 1 μL dNTP (10 mmol/L each), 0.5 μL RNase inhibitor, and 1.0 μL M-MLV reverse transcriptase [RT]). Polymerase chain reaction (PCR) primers for IFN-γ and HPRT, a housekeeping gene, encompass 234 and 352 base pairs of wild-type cDNA, respectively. First-strand cDNA was amplified 35 cycles (Perkin Elmer Thermocycler, Norwalk, CT). The reaction mixture and cycling conditions were described previously.18
RESULTS
Syngeneic BMT at peak of acute disease: day 14 after adoptive transfer of disease-initiating lymphocytes.
At the peak of acute disease, the mice were divided into five groups of eight to 10 animals each (Fig 1). There were three treatment groups: TBI, MP/TBI, or Cy/TBI. Beginning after day 60, all treatment groups showed similar clinical improvement, reaching their lowest clinical scores at about day 100, and thereafter, the mean clinical scores remained stable. All three treatment groups (mean score <1) had significantly fewer neurologic deficits compared with the control and BMC groups (mean score >2.0). Clinical results were also analyzed according to the relapse rate. The total number of relapses in the Cy/TBI and MP/TBI treatment groups were decreased compared with the control groups (Table1). However, the differences were not statistically significant.
BMT at Peak of Acute Disease: Relapse Rate
| Group . | No. of Mice Relapsing/ Total No. of Mice . | Total No. of Relapses/ Total No. of Mice . |
|---|---|---|
| Control | 8/8 | 19/8 |
| Bone marrow only | 8/8 P = .34 | 16/8 P = .63 |
| TBI/BMT | 6/8 P = .23 | 15/8 P = .50 |
| Cy/TBI/BMT | 5/7-150P = .20 | 7/7 P = .10 |
| MP/TBI/BMT | 7/8 P = .50 | 12/8 P = .21 |
| Group . | No. of Mice Relapsing/ Total No. of Mice . | Total No. of Relapses/ Total No. of Mice . |
|---|---|---|
| Control | 8/8 | 19/8 |
| Bone marrow only | 8/8 P = .34 | 16/8 P = .63 |
| TBI/BMT | 6/8 P = .23 | 15/8 P = .50 |
| Cy/TBI/BMT | 5/7-150P = .20 | 7/7 P = .10 |
| MP/TBI/BMT | 7/8 P = .50 | 12/8 P = .21 |
Relapse rate shown according to the number of animals that relapsed in each group (statistics by Fisher's exact test for 2 × 2 contingency tables) and the ratio for the total number of relapses to the number of mice per group (statistics by Studentt test).
Three animals died within days of conditioning and are not included in analysis.
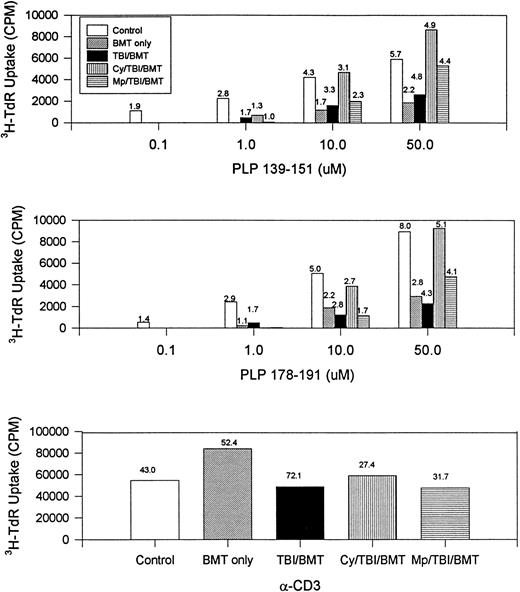
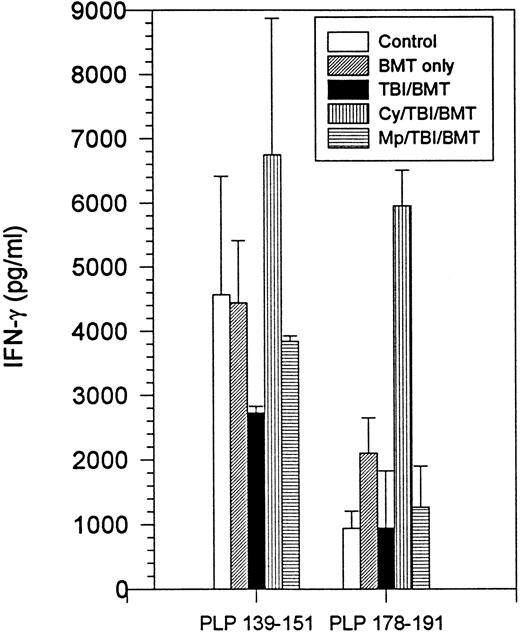
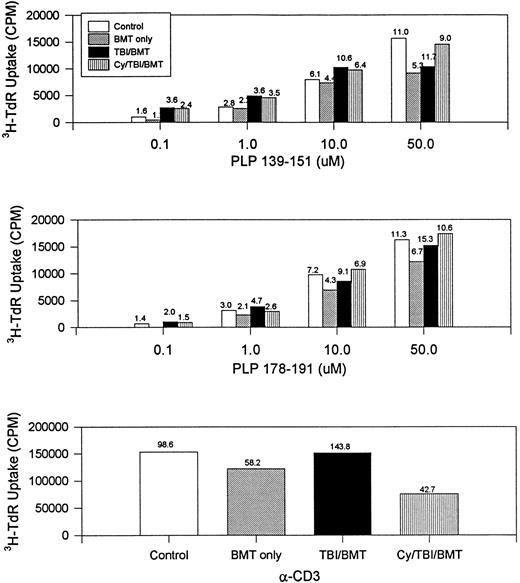
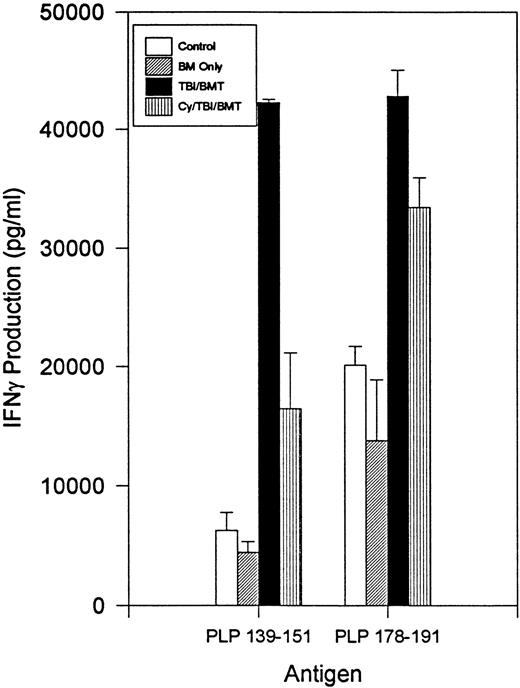
The immune responsiveness to disease-initiating and relapse-associated epitopes persisted as measured by the proliferation of splenocytes to increasing concentrations of PLP 139-151 and PLP 178-191 peptides (Fig2). Since R-EAE is known to be a Th1-mediated disease, we also evaluated IFN-γ production by splenocytes. When exposed to disease-initiating or relapse-associated PLP epitopes, splenocytes from all SBMT treatment groups produced IFN-γ to at least the same extent as the controls (Fig 3).
BMT at peak of acute disease. Day 150 splenocyte proliferative response to increasing concentrations of disease-initiating (PLP 139-151) and relapse-associated (PLP 178-191) epitopes of PLP. The number above each bar is the stimulation index (ratio of CPM with antigen divided by CPM without antigen). Splenocytes from naive mice did not respond to PLP 139-151 or PLP 178-191. CPM, counts per minute; α-CD3, stimulation by the antibody to the T-cell receptor.
BMT at peak of acute disease. Day 150 splenocyte proliferative response to increasing concentrations of disease-initiating (PLP 139-151) and relapse-associated (PLP 178-191) epitopes of PLP. The number above each bar is the stimulation index (ratio of CPM with antigen divided by CPM without antigen). Splenocytes from naive mice did not respond to PLP 139-151 or PLP 178-191. CPM, counts per minute; α-CD3, stimulation by the antibody to the T-cell receptor.
BMT at peak of acute disease. Day 150 splenocyte IFN-γ production following incubation with PLP 139-151 or PLP 178-191. Splenocytes from naive mice had no detectable IFN-γ production.
BMT at peak of acute disease. Day 150 splenocyte IFN-γ production following incubation with PLP 139-151 or PLP 178-191. Splenocytes from naive mice had no detectable IFN-γ production.
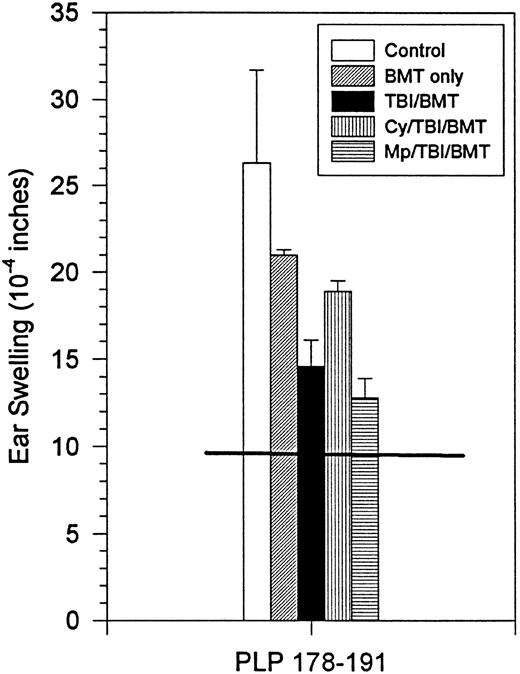
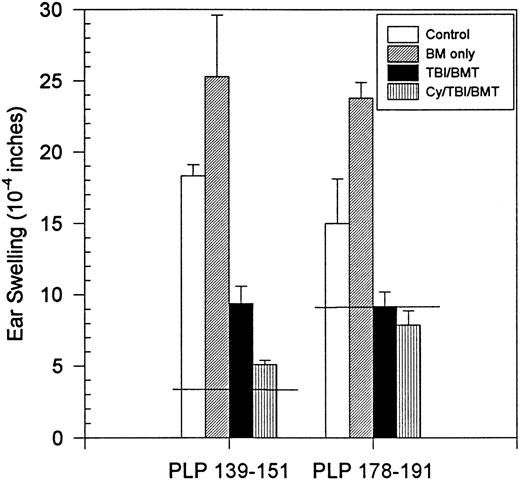
Although the clinical course did not correlate with in vitro proliferation or IFN-γ production, clinical improvement did correlate with DTH responses and histology. There was a decrease in DTH responses to PLP 178-191 in all treatment groups (Fig4). In two treatment groups (TBI,P = .05; MP/TBI, P = .017), DTH responses were significantly diminished as compared with responses in the control group.
BMT at peak of acute disease. Day 160 in vivo DTH reaction to PLP 178-191. Horizontal line shows the mean ear swelling in 3 naive mice.
BMT at peak of acute disease. Day 160 in vivo DTH reaction to PLP 178-191. Horizontal line shows the mean ear swelling in 3 naive mice.
Histologic examination of the spinal cord showed moderate inflammation with multiple foci of demyelination in all sections from control and BMC groups (Table 2). In contrast, the Cy/TBI and MP/TBI treatment groups displayed reduced CNS pathology with little or no evidence of focal inflammation or demyelination in most spinal cord sections. The TBI treatment group also showed a modest decrease in CNS pathology versus the control groups, but not to the extent of the other treatments (Table 2). Importantly, glial scarring was absent in all treatment groups.
BMT at Peak of Acute Disease: Histology
| Group . | Score . | Glial Scarring . |
|---|---|---|
| Control | 2+ (10/10) | Severe |
| Bone marrow only | 2+ (10/10) | Moderate |
| TBI/BMT | 1+ (10/10) | Absent |
| Cy/TBI/BMT | − (2/10), ± (4/10), 1+ (4/10) | Absent |
| MP/TBI/BMT | − (4/10), ± (1/10), 1+ (5/10) | Absent |
| Group . | Score . | Glial Scarring . |
|---|---|---|
| Control | 2+ (10/10) | Severe |
| Bone marrow only | 2+ (10/10) | Moderate |
| TBI/BMT | 1+ (10/10) | Absent |
| Cy/TBI/BMT | − (2/10), ± (4/10), 1+ (4/10) | Absent |
| MP/TBI/BMT | − (4/10), ± (1/10), 1+ (5/10) | Absent |
Histologic score shown for 10 spinal cord sections from 1 animal in each group at day 160.
SBMT during the chronic phase of disease: day 74 after adoptive transfer of disease-initiating lymphocytes.
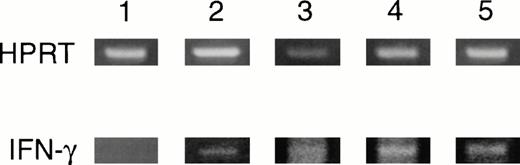
On day 74 after adoptive transfer of disease-initiating PLP 139-151 blasts, mice were divided into four groups (control, BMC, TBI, and Cy/TBI) with 11 to 13 animals in each group. When immune ablation and BMT were performed during the chronic phase of disease, no significant clinical difference in the disease course, severity, or relapse rate was seen between the control, BMC, and treatment groups regardless of the myeloablative regimen (Fig 5 and Table3). Proliferative responses and IFN-γ production to either disease-initiating PLP 139-151 or relapse-associated PLP 178-191 were not statistically diminished in animals undergoing SBMT compared with controls (Figs 6 and7).Despite a lack of improvement in neurologic deficits or proliferative responses, in vivo DTHs to PLP 139-151 and PLP 178-191 were significantly less in mice that underwent myeloablation and SBMT (P < .05) compared with the control groups (Fig8). There was also a slight improvement in the histology of the spinal cord between the control and treatment groups (Table 4). RT-PCR of the CNS revealed detectable IFN-γ mRNA levels in both the control and treatment groups. However, semiquantitative densitometry showed slightly decreased ratios of IFN-γ to HPRT RNA in the TBI (0.31) and Cy/TBI (0.24) treatment groups compared with the control (0.37) or BMC (0.59) groups (Fig 9). Therefore, mRNA levels in the CNS seem to correlate with the in vivo DTH responses.
BMT in chronic phase. Clinical course is reported in terms of the mean clinical score. There is no statistically significant difference between treated and untreated mice. Bold arrow indicates time of treatment. Arrow 1 indicates killing of 3 animals from each group for proliferation and ELISA assays and use of 3 animals from each group for DTH. Arrow 2 indicates killing of 1 animal from each group for histology and 3 from each group for RT-PCR of the spinal cord.
BMT in chronic phase. Clinical course is reported in terms of the mean clinical score. There is no statistically significant difference between treated and untreated mice. Bold arrow indicates time of treatment. Arrow 1 indicates killing of 3 animals from each group for proliferation and ELISA assays and use of 3 animals from each group for DTH. Arrow 2 indicates killing of 1 animal from each group for histology and 3 from each group for RT-PCR of the spinal cord.
BMT in Chronic Phase: Relapse Rate
| Group . | No. of Mice Relapsing/ Total No. of Mice . | Total No. of Relapses/ Total No. of Mice . |
|---|---|---|
| Control | 6/11 | 8/11 |
| Bone marrow only | 8/11 P = .33 | 10/11 P = .51 |
| TBI/BMT | 7/13 P = .50 | 7/13 P = .49 |
| Cy/TBI/BMT | 6/13 P = .35 | 9/13 P = .92 |
| Group . | No. of Mice Relapsing/ Total No. of Mice . | Total No. of Relapses/ Total No. of Mice . |
|---|---|---|
| Control | 6/11 | 8/11 |
| Bone marrow only | 8/11 P = .33 | 10/11 P = .51 |
| TBI/BMT | 7/13 P = .50 | 7/13 P = .49 |
| Cy/TBI/BMT | 6/13 P = .35 | 9/13 P = .92 |
Relapse rate shown according to the number of animals that relapsed in each group (statistics by Fisher's exact test for 2 × 2 contingency tables) and the ratio for the total number of relapses to the number of mice per group (statistics by Studentt test).
BMT in chronic phase. Day 142 splenocyte proliferative response to increasing concentrations of disease-initiating (PLP 139-151) and relapse-associated (PLP 178-191) epitopes of PLP. The number above each bar is the stimulation index (ratio of CPM with antigen divided by CPM without antigen). Splenocytes from naive mice did not respond to PLP 139-151 or PLP 178-191. CPM, counts per minute; α-CD3, stimulation by the antibody to the T-cell receptor.
BMT in chronic phase. Day 142 splenocyte proliferative response to increasing concentrations of disease-initiating (PLP 139-151) and relapse-associated (PLP 178-191) epitopes of PLP. The number above each bar is the stimulation index (ratio of CPM with antigen divided by CPM without antigen). Splenocytes from naive mice did not respond to PLP 139-151 or PLP 178-191. CPM, counts per minute; α-CD3, stimulation by the antibody to the T-cell receptor.
BMT in chronic phase. Day 142 splenocyte IFN-γ production following incubation with PLP 139-151 or PLP 178-191. Splenocytes from naive mice had no detectable IFN-γ production.
BMT in chronic phase. Day 142 splenocyte IFN-γ production following incubation with PLP 139-151 or PLP 178-191. Splenocytes from naive mice had no detectable IFN-γ production.
BMT in chronic phase. Day 142 in vivo DTH reaction. Horizontal line is the mean ear swelling in 3 naive mice.
BMT in chronic phase. Day 142 in vivo DTH reaction. Horizontal line is the mean ear swelling in 3 naive mice.
BMT in Chronic Phase: Histology
| Group . | Score . | Glial Scarring . |
|---|---|---|
| Control | 2+ (8/10), 3+ (2/10) | Severe |
| Bone marrow only | 1+ (4/10), 2+ (6/10) | Moderate |
| TBI/BMT | 1+ (8/10), 2+ (2/10) | Mild-moderate |
| Cy/TBI/BMT | 1+ (1/10), 2+ (9/10) | Mild-moderate |
| Group . | Score . | Glial Scarring . |
|---|---|---|
| Control | 2+ (8/10), 3+ (2/10) | Severe |
| Bone marrow only | 1+ (4/10), 2+ (6/10) | Moderate |
| TBI/BMT | 1+ (8/10), 2+ (2/10) | Mild-moderate |
| Cy/TBI/BMT | 1+ (1/10), 2+ (9/10) | Mild-moderate |
Day 152 histology score shown for 10 spinal cord sections from 1 animal in each group.
BMT in chronic phase. Day 152 RT-PCR of spinal cord for IFN-γ. HPRT, housekeeping gene, hypoxanthine phosphoribosyl transferase. 1, naive; 2, control; 3, BMC; 4, TBI/BMT; 5, Cy/TBI/BMT.
BMT in chronic phase. Day 152 RT-PCR of spinal cord for IFN-γ. HPRT, housekeeping gene, hypoxanthine phosphoribosyl transferase. 1, naive; 2, control; 3, BMC; 4, TBI/BMT; 5, Cy/TBI/BMT.
DISCUSSION
Hematopoietic stem cell transplantation as a treatment for MS has recently been initiated by several centers.19,20 Although no animal model is a perfect correlate for MS, in general, new therapies for MS are tested and reported in R-EAE before initiating clinical trials. Other investigators have reported on the clinical effects of immune ablation and hematopoietic stem cell transplantation in both rat21-24 and mouse25 models of EAE. Van Gelder et al22-24 reported improvement in the clinical course or relapse rate after hematopoietic stem cell transplantation in rats with EAE. Importantly, the outcome depended on the type of graft. The extent of remission and decrease in the recurrence rate were better with an allogeneic stem cell source from a resistant strain than with a syngeneic or pseudoautologous graft.22-24 Karussis et al25 have reported amelioration of clinical EAE after syngeneic transplantation in SJL/J mice. Their study also investigated proliferative responses to myelin after transplantation. However, a thorough evaluation of immune responsiveness following stem cell transplantation for an autoimmune disease has not been reported. We therefore chose syngeneic unmanipulated stem cells rather than an allogeneic stem cell source to evaluate autoimmunity without interference from graft-versus-host disease following infusion of the graft.
We were concerned that TBI may cause an exacerbation of neurologic symptoms in inflammatory demyelinating neurologic diseases, because macrophages may be activated by TBI to produce cytokines such as tumor necrosis factor that could exacerbate neuropathology.26 27We therefore compared different TBI fraction sizes alone or combined with either cyclophosphamide or methylprednisolone. No acute neurologic exacerbations occurred with any of these conditioning regimens. In addition, there were no differences in clinical outcome between the different regimens.
In animals undergoing transplantation during the acute phase of R-EAE, the mean clinical scores were significantly improved. However, when SBMT was performed during the chronic phase, there was no significant improvement in overall disease severity. We believe that SBMT during chronic disease is a more appropriate analogy for patients with MS, since most of these individuals would have had symptoms for some period with progressive deterioration in baseline values before being considered for aggressive experimental therapies. To the best of our knowledge, no prior investigator has studied the responses of the immune system in SJL/J mice with R-EAE following transplantation in the chronic phase of disease. The failure of transplantation to improve disease severity when performed in chronic phase appears to be due to irreversible glial scarring.
Our results suggest that SBMT early in the disease is capable of improving or ameliorating disease severity but does not cure it, and does not abolish the immune responsiveness to myelin. These results are in contrast with a previous report by Karussis et al.25 In their study, R-EAE was induced in SJL/J mice by adoptive transfer of lymphoblasts primed with guinea pig myelin basic protein, a weak encephalitogenic stimulus in the SJL/J mouse. They performed SBMT during acute disease and reported decreased immune proliferative responses to intact guinea pig myelin basic protein.25 In our study, proliferative responses were measured to small (12 to 13 amino acids) highly immunodominant disease-initiating and relapse-associated peptides. Our results suggest that the immune system is capable of responding to components of myelin after SBMT.
The difference in proliferative responses between their study25 and ours is not readily explained by differences in the myeloablative regimens. Their study used a 900- to 1,100-cGy single-dose conditioning regimen, which is approximately equivalent to the immunosuppressive and myeloablative effect of our TBI, MP/TBI, and Cy/TBI conditioning regimens.
In the early posttransplant period, the immune system has a suppressor immunophenotype with subtle deficits despite normal cell numbers.28 To avoid potential interference from defects in immune reconstitution, we performed proliferation assays more than 120 days after transplantation in acute-phase R-EAE and 70 days after transplantation in chronic-phase R-EAE. Karussis et al25performed proliferation assays 60 days after transplantation for acute-phase R-EAE. It is therefore unlikely that differences in the results between our study and theirs are due to differences in immune reconstitution.
The severity of disease at the time of transplantation may have contributed to the differences in results between the Karussis study and our own. In their study, some of the experiments had a mean clinical score at the time of treatment of 1.5; this compares with 2.4 and 3.3 in our study. Their clinical scoring system was also markedly different from ours. In general, their grading system scored the same neurologic deficit 1 point higher in severity compared with our scoring system. For example, they defined a score of 2 as “tail paralysis,” which in our grading system would be a score of 1. For any given clinical score, these differences would result in an overall more mild disease course in their study. Therefore, our animals developed more severe disease.
The differences in results between our study and the Karussis study may also be due to differences in the homing of lymphocytes between the lymph nodes, spleen, and target organ.29-31 Karussis et al measured proliferative responses in lymph node cells after adoptive transfer of disease. We were uncertain if disease-initiating lymphocytes would home to unprimed lymph nodes following adoptive transfer. Therefore, we used splenocytes to measure proliferative responses. To further explore the homing of cells to the CNS, we performed RT-PCR for IFN-γ RNA on spinal cord homogenate. R-EAE is a Th1-mediated disease in which IFN-γ is released by disease-causing lymphocytes. IFN-γ RNA levels are not detectable in the CNS of naive mice. In mice with chronic R-EAE, despite transplantation, IFN-γ remains detectable within the CNS. This suggests continued homing of disease-mediating lymphocytes to the CNS.
Transplantation resulted in no significant improvement in proliferative responses or IFN-γ production by ELISA to PLP epitopes. However, there was significant improvement in DTH responses, as well as slight improvement in histology scores and IFN-γ RNA levels within the CNS. The dichotomy between proliferative responses and DTH has been noted in prior therapies for R-EAE.32 For example, IFN-β has been reported to cause significant inhibition of DTH and improvement in histology but no change in the proliferative response to disease-initiating PLP 139-151.32 Furthermore, it has been previously reported that proliferative responses in R-EAE do not necessarily correlate with clinical changes.32 In our study, clinical improvement in the acute phase of disease occurred despite persistent proliferative responses. The continued proliferation of lymphocytes to myelin epitopes despite clinical improvement may indicate a regulatory or suppressor subset of cells capable of proliferation but not cytotoxicity. In R-EAE, proliferation assays are poor indicators of clinical disease. However, since splenocytes from naive mice do not proliferate to PLP, the persistent proliferative responses after transplantation support a failure of the conditioning regimen to completely ablate the pretransplant immune system. It is possible that a relatively radiation-resistant subpopulation of disease-causing lymphocytes survived the conditioning regimen. If this is the case, it would be difficult to further maximize the immune ablative regimen without substantially increasing other organ toxicity. Alternatively, reinfusion of mature lymphocytes with the marrow graft may contribute to the recurrence of disease. If this is the case, infusion of a lymphocyte-depleted graft may be helpful in reestablishing an immune system unresponsive to myelin. Finally, even if the graft had all mature lymphocytes removed, regeneration of the neonatal immune repertoire from marrow hematopoietic progenitor cells may regenerate autoreactive T cells.
Summary.
The clinical outcome after SBMT for immune-mediated demyelinating CNS diseases may depend on the stage and severity of disease at the time of transplant. In specific, if SBMT is performed in the acute phase of disease, marked neurologic improvement can be expected. Alternatively, if SBMT is performed in the chronic phase, little or no neurologic improvement may occur. The difference in clinical outcome between transplantations performed in the acute and chronic phase of R-EAE may be due to axonal injury and failure of tissue repair. Regardless of disease duration and in contrast to previous reports, SBMT did not diminish proliferative responsiveness to myelin epitopes. This may be explained by either a failure of the conditioning regimen to completely eradicate disease-causing lymphocytes or a regeneration of myelin-reactive lymphocytes from the infused graft. Furthermore, if irreversible organ damage has not occurred, in vivo DTH and histology are more likely to correlate with clinical disease than in vitro proliferation assays. This indicates that in vitro proliferation assays may not be valid as a marker for disease activity in patients with MS.
These experiments also suggest that intense immunosuppression in the chronic phase of an immune-mediated neurologic disease will not improve neurologic deficits. Theoretically, the outcome may be improved by posttransplant growth factors that promote remyelination and/or axonal regeneration. They also suggest that a naive state of immune unresponsiveness to myelin will not occur after intense myeloablation and syngeneic transplantation. However, this outcome may be changed by manipulation of the graft. For example, the graft may be engineered preinfusion by lymphocyte depletion or posttransplantation by immune modulation with cytokines such as IL-4 that promote a Th2phenotype.
Address reprint requests to Richard K. Burt, MD, Northwestern University Medical School, Department of Medicine, Wesley Pavilion, Room 1456, 250 East Superior, Chicago, IL 60611.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal