Abstract
Exosomes are membrane vesicles released by reticulocytes during their maturation into erythrocytes. They have a clearing function because of their enrichment with some proteins known to decrease or disappear from the cell surface during maturation, eg, acetylcholinesterase (AChE) and transferrin receptor (TfR), respectively. To better understand the molecular events leading to protein sorting in exosomes, we analyzed the expression of glycosylphosphatidylinositol (GPI)-anchored proteins on the exosome surface through a technique involving bead coupling and flow cytometry immunodetection. The presence of AChE, decay-accelerating factor (DAF), membrane inhibitor of reactive lysis (MIRL), and lymphocyte function-associated antigen 3 (LFA-3) on the surface of exosomes obtained from normal and paroxysmal nocturnal hemoglobinuria (PNH) reticulocytes, suggests that (1) the GPI anchor is efficiently sorted during exosome formation, (2) exosome release could account for the observed discrepancy in GPI-protein expression between reticulocytes and erythrocytes from PNH patients, and (3) exosomes could have another physiologic function related to controlling membrane attack complex formation.
DURING RETICULOCYTE maturation, some membrane activities are lost through their release in the extracellular medium, as they are associated with small lipid vesicles called exosomes. The major membrane protein lost by this physiologic process is the transferrin receptor (TfR), which completely disappears from the red blood cell (RBC) surface during maturation.1 Other membrane proteins such as acetylcholinesterase (AChE) and transporters of nucleosides, glucose, and amino acids have been shown to be sorted in exosomes.1The signals involved in this sorting process are still unknown. Nevertheless, we recently showed that antibody-induced molecule clustering increases TfR and AChE release within exosomes,2suggesting that the aggregation of some membrane constituents may be involved in this physiologic process. Different forms of AChE have been described for various cells. RBC AChE was shown to be membrane-bound through a glycosylphosphatidylinositol (GPI) anchor.3,4AChE detected in exosomes therefore probably does not span the membrane, excluding a direct interaction with a cytosolic protein (eg, hsc 70) suggested to be involved in TfR sorting.5 On the other hand, GPI-anchored proteins are known to be sorted into specific lipid domains in different cell compartments such as the trans-Golgi network or caveolae.6,7 Glycosphingolipids such as the ganglioside GM1 were shown to be enriched in these lipid domains.8
Other GPI-anchored proteins are present on the surface of RBCs. These include the decay-accelerating factor (DAF, CD55) and membrane inhibitor of reactive lysis (MIRL, CD59), two proteins that inhibit formation of the membrane attack complex on autologous cell surfaces.9 10
In hematologic disorder, paroxysmal nocturnal hemoglobinuria (PNH), impaired transfer of N-acetylglucosamine to phosphatidylinositol leads to a synthetic defect in GPI and thus to a deficiency in the surface expression of all GPI-anchored proteins.11 Analysis of many patients with PNH has shown that somatic mutation in the X-linked gene PIG-A is responsible for the GPI-anchor deficiency in PNH.12 Blood cells of PNH patients are affected differently,13,14 but are generally more susceptible to complement-mediated attack. Hemolysis of RBCs is the major clinical indication of the disease, although the lack of DAF and/or MIRL on the cell surface can vary markedly between patients. Moreover, there is often a difference in the extent of erythrocytes and reticulocytes affected in the RBC population.15
In the present study, we showed that the GPI-anchored proteins AChE, DAF, MIRL, and LFA-3 were released in exosomes by reticulocyte during in vitro maturation into erythrocytes. The extent of GPI-anchored proteins released within exosomes was not highly modified when reticulocytes of PNH patients were matured in vitro, indicating that the GPI-anchor was efficiently sorted during vesicle formation. Exosome release could thus account for the observed discrepancy in GPI-protein expression between reticulocytes and erythrocytes from PNH patients.15 Moreover, the presence of DAF and MIRL within the exosomal membrane could provide vesicles released in the blood circulation with another physiologic function related to the regulation of membrane attack complex formation.
MATERIALS AND METHODS
Donors.
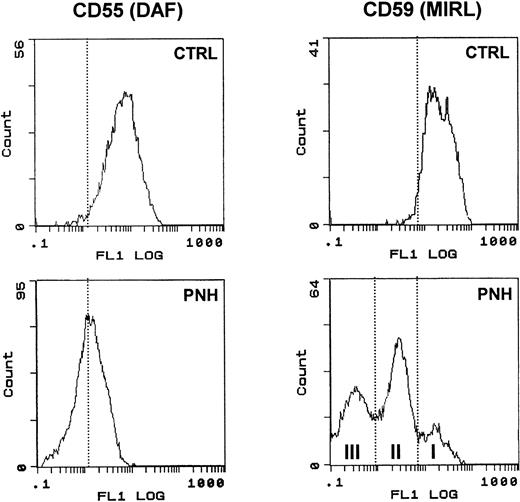
The two patients with PNH had marked deficiencies of CD55 and CD59 antigens. In particular, the presence of partially CD59 deficient (type II) and completely deficient (type III) RBCs was detected for case 1 (see Fig 5). The reticulocyte count was determined by flow cytometric analysis with thiazole orange labeling of RNA. Controls involved patients with a high percentage of reticulocytes (always >6%) due to other pathologic states, such as hemolytic anemia.
Surface expression of DAF and MIRL on the surface of RBC. RBCs from a control subject (CTRL) and a patient (PNH) were analyzed for CD55 and CD59 expression as described in Materials and Methods. Note the typical heterogeneous pattern of CD59 staining obtained with PNH cells compared with the uniformly positive staining of control RBC.
Surface expression of DAF and MIRL on the surface of RBC. RBCs from a control subject (CTRL) and a patient (PNH) were analyzed for CD55 and CD59 expression as described in Materials and Methods. Note the typical heterogeneous pattern of CD59 staining obtained with PNH cells compared with the uniformly positive staining of control RBC.
Reagents.
Magnetic beads with covalently bound affinity purified sheep antimouse IgG (Dynabeads M-450*SAM) or antihuman TfR (Dynabeads M-450*CD71) were from Dynal France S.A. (Compiègne, France). Monoclonal antibodies (MoAb) against human and rat transferrin receptors were obtained from Monosan (Uden, The Netherlands) and Chemicon (Temecula, CA), respectively. Culture supernatants of OKT9 cells (American Type Culture Collection, Rockville, MD) were used to detect human TfR by Western blot. MoAbs specific to CD55, CD58, and CD59 antigens were from Cymbus Biotechnology Ltd (Hants, UK), Chemicon and Monosan, respectively. Fluorescein isothiocyanate (FITC)-conjugated goat antimouse IgG (GAM-FITC) and goat antirabbit IgG (GAR-FITC), FITC-conjugated cholera toxin B subunit (FITC-CTB), and wheat germ agglutinin (FITC-WGA) were purchased from Sigma (St Louis, MO). Antibodies to rat acetylcholinesterase16 were a generous gift of Jean Massoulié (E.N.S., Paris, France). Phosphatidylinositol-specific phospholipase C (PI-PLC) from Bacillus cereus was obtained from Boehringer Mannheim (Meylan, France).
Exosome preparation.
Peripheral blood was collected from phenylhydrazine-treated rats (Sprague-Dawley) by cardiac puncture as previously described.17 After removing the buffy coat, RBCs (reticulocyte count >70%) were washed three times with phosphate-buffered saline (PBS) and subcultured (3% suspension) for 24 hours at 37°C in maturation medium (RPMI supplemented with 5 mmol/L glutamine, 5 mmol/L adenosine, 10 mmol/L inosine). After cell pelleting, the culture supernatant was centrifuged (20,000g for 20 minutes) to remove cellular debris. Exosomes were separated from the supernatant by centrifugation (100,000g for 90 minutes). The pellet (vesicular fraction) was washed once by centrifugation and resuspended in 250 mmol/L sucrose, 10 mmol/L Tris-HCl (pH 7.4).
Venous peripheral blood was drawn from the PNH patient and anemic controls into EDTA-containing tubes. After centrifugation and two washes with PBS, the packed cells were resuspended (3%) and subcultured in maturation medium. Exosomes were collected as described above.
Immunobinding of exosomes to magnetic beads.
A total of 100 μL of Dynabeads M-450 coupled with SAM (4 × 108 beads/mL) were washed twice with 4 mL of PBS and resuspended in the same volume. The magnetic beads were then incubated with 10 μL of MoAbs (antihuman or antirat) TfR overnight at 4°C with gentle rotation. After three washes in PBS, the beads were incubated with the different exosome preparations. A total of 100 μL of exosomes, containing 2 to 5 mg protein/mL, were added for at least 2 hours at 4°C. Exosome-bead complexes, in some cases blocked with rabbit antimouse IgG, were then washed twice using a magnet, with 4 mL of PBS containing 1% fetal calf serum (PBS-FCS).
Complexes with various exosome densities on the bead surface were obtained by incubation of increasing exosome amounts (1, ×5, ×25) with the same number of beads. After coupling, the complexes were analyzed for the presence of CD59 by flow cytometry and for AChE activity after detergent solubilization. The higher exosome density per bead corresponded to the conditions used throughout this work and was thus set arbitrarily at 1. The shift of fluorescence peak was obtained for each exosome/bead ratio by subtracting the peak position value of GAM-FITC alone from the fluorescence resulting from CD59 labeling.
Electron microscopy.
Exosome-bead conjugates were fixed in glutaraldehyde 2% for 1 hour at room temperature. Exosomes immunologically bound to magnetic beads were fixed in suspension with 2% glutaraldehyde in PBS for 1 hour at room temperature and then centrifuged for 10 minutes at 13,000g and postfixed in osmium. The pellet was stained in bloc with magnesium uranyl acetate, dehydrated in graded ethanol, and embedded in epon.
Sections were stained with uranyl acetate and lead citrate and observed on a Hitachi 7000-100 electron microscope (Hitachi Scientific Instruments, Dusseldorf, Germany).
Flow cytometric analysis.
A total of 10 μL of exosome-bead complexes in 100 μL PBS-FCS were incubated with the different primary antibodies for 30 minutes at room temperature, washed three times using a magnet with PBS-FCS, and incubated with corresponding FITC-labeled secondary antibodies for 30 minutes at room temperature. In the indicated experiments, fluorescent lectins (FITC-WGA and FITC-CTB) were incubated with the exosome-bead complexes for 1 hour at 37°C. After washing, the samples were analyzed using an Epics XL flow cytometer (Coulter, Hialeah, FL) equipped with an ion Argon LASER (488 nm). Beads or exosome-bead complexes were assessed from the dot plot representation of forward scatter (size) versus side scatter (granularity), which were set at logarithmic gain. Only single beads or complexes were gated for fluorescence analysis (logarithmic scale). Twenty thousand events were analyzed for each sample.
PI-PLC sensitivity of AChE.
Exosomes (approximately 2 mg protein/mL) were extracted with 1% Triton X-100, 10 mmol/L Tris/HCl pH 7.4, 1 mmol/L EGTA, 5 mmol/L EDTA, and proteinase inhibitors (Boehringer Mannheim): aprotinin (2 μg/mL), leupeptin (0.5 μg/mL), and pepstatin (0.7 μg/mL) for 15 minutes at 4°C with intermittent stirring. After a 15-minute centrifugation at 10,000g, AChE was recovered in the supernatant. This exosome extract was used as control or treated for 1 hour at 37°C with PI-PLC (5 U/mL). Nondenaturing polyacrylamide gel electrophoresis was performed as described previously.18 Briefly, gels and running buffers contained 50 mmol/L Tris/glycine (pH 8.9) and 0.5% Triton X-100. Samples (15 μL) were run for 3 hours at 10 V/cm. Gels were rinsed in distilled water and stained for AChE activity according to Karnovsky and Roots.19
Sedimentation analyses were performed in 11 mL 5% to 20% linear sucrose gradients containing 10 mmol/L Tris/HCl (pH 7.4), 150 mmol/L NaCl, plus or minus 1% Triton X-100. Samples of 190-μL exosome extracts were loaded and centrifuged for 19 hours at 36,000 rpm and 4°C in a SW41 rotor (200,000g). Fractions were collected from the bottom of each gradient and assayed for AChE at pH 7. One unit is defined as the amount catalyzing the hydrolysis of 1 μmol acetylthiocholine substrate/minute at 20°C.18
RESULTS
Exosomal AChE is anchored through a GPI.
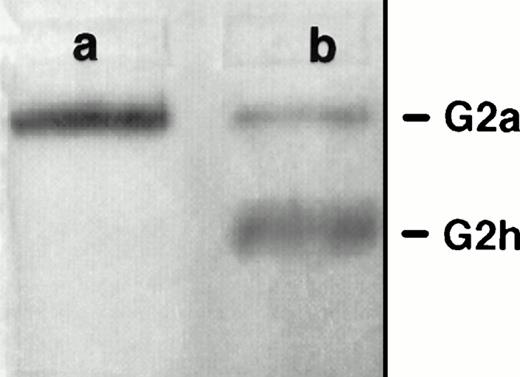
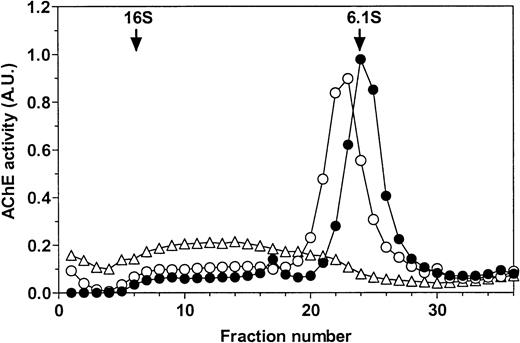
PI-PLC treatment of rat exosomes induced a more mobile form of AChE in nondenaturing polyacrylamide gel electrophoresis (Fig 1A). This is typical of partial conversion of the amphiphilic dimer (G2a) into the hydrophilic dimer (G2h) form of RBC AChE.18 This was confirmed by sucrose gradient centrifugation analysis (Fig 1B). Using an untreated detergent extract of rat exosomes, a single AChE activity peak was obtained, which sedimented at 6 Svedberg (S) in the presence of 1% Triton X-100. When the detergent was omitted in the gradient, AChE activity was recovered as a broad peak around 12 S, indicative of the formation of micellar aggregates. However, AChE activity sedimented in the same gradient as a well-defined peak shifted to 6.8 S after incubation of exosomal detergent extract with PI-PLC. The amount of AChE activity recovered in this 6.8S peak, typical of the hydrophilic dimer of AChE, indicated that conversion of amphiphilic AChE by PI-PLC was complete. Taken together, these results showed that AChE was secreted in the extracellular medium, associated with the exosome membrane through a GPI anchor.
Sensitivity of rat exosomal AChE to PI-PLC. (A) Nondenaturing gel electrophoresis. Lane a, detergent extract of rat exosomes; lane b, detergent extract of exosomes treated by PI-PLC as outlined in Materials and Methods. (B) Sedimentation analysis. Samples of exosomal detergent extracts, treated (○) or not (•, ▵) by PI-PLC, were layered on 5% to 20% sucrose gradients containing (•) or not (○, ▵) 1% Triton X-100, and centrifuged for 19 hours at 36,000g. Collected fractions were assayed for AChE activity as described in Materials and Methods. The vertical arrows indicate the positions of E coli β-galactosidase (16S) and alkaline phosphatase (6.1S).
Sensitivity of rat exosomal AChE to PI-PLC. (A) Nondenaturing gel electrophoresis. Lane a, detergent extract of rat exosomes; lane b, detergent extract of exosomes treated by PI-PLC as outlined in Materials and Methods. (B) Sedimentation analysis. Samples of exosomal detergent extracts, treated (○) or not (•, ▵) by PI-PLC, were layered on 5% to 20% sucrose gradients containing (•) or not (○, ▵) 1% Triton X-100, and centrifuged for 19 hours at 36,000g. Collected fractions were assayed for AChE activity as described in Materials and Methods. The vertical arrows indicate the positions of E coli β-galactosidase (16S) and alkaline phosphatase (6.1S).
Exosome-bead complex construction.
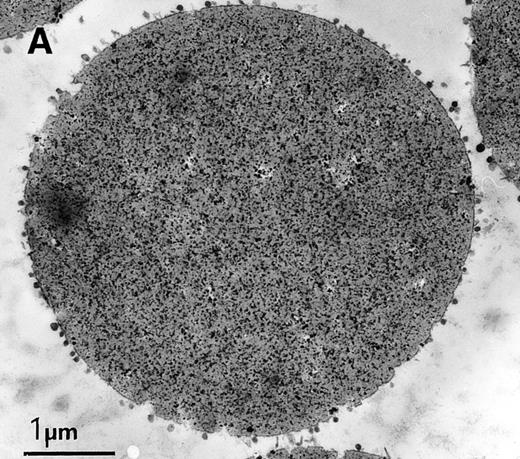
Coupling exosomes to beads was dictated by requirement of antibody binding/washing steps during the immunophenotypic labeling. TfR was ideally suited to conjugate the exosomes to beads, because of its high amount on the vesicle surface and specificity as an exosome marker. We set up the conditions, using highly-available exosomes derived from rat reticulocytes and magnetic beads coupled with SAM, and a mouse monoclonal antirat TfR as a secondary antibody. Coupling the two types of particles was very efficient and homogeneous, as assessed by electron microscopy (Fig 2A). It also confirmed the size homogeneity of exosomes (diameter around 60 nm) (Fig2B). Another effect of coupling, apart from simplifying washing of the complex, was that the exosomal signal could be distinguished from the background detection (nonexosomal vesicles, small particles, etc) during flow cytometry analysis because of the larger size of the beads (4.5 μm) as compared with exosomes (Fig3A). As the beads were precisely calibrated, populations that were double or triple the size of the beads could be detected, probably because of exosomal cross-linking (Fig 3A). In subsequent experiments, only single beads or complexes were gated for fluorescence analysis. FITC-labeled wheat germ agglutinin (WGA), a lectin binding N-acetyl-glucosamine-containing glycoproteins and glycoconjugates, was used as a control to nonspecifically stain the exosome-bead complexes. The addition of detergent diminished the WGA-FITC labeling of exosomes-bead complexes to the fluorescent level obtained by WGA-FITC labeling of anti-TfR antibody-conjugated beads, showing the exosomal origin of the staining (Fig 3B). Finally, an antirat acetylcholinesterase16 was used to test the detection potential of the specific antigen on our construction. The complexes were AChE-positive, as shown by a shift in the fluorescence peak (Fig3C).
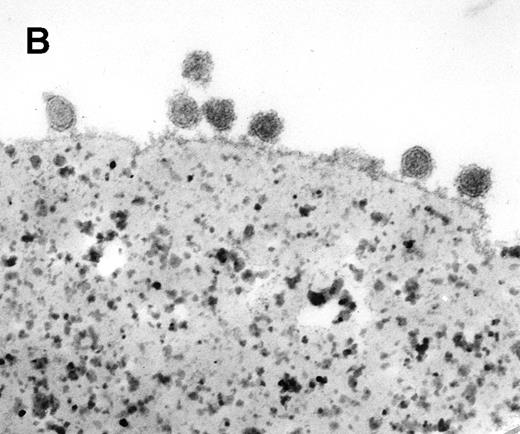
Characterization of rat exosome-bead conjugates by electron microscopy. Electron micrographs of magnetic beads immunologically coated with exosomes. Beads were coated with vesicles of 60 nm average diameter. Most vesicles were filled with electron-dense material (A) (bar, 1 μm). The beads were uniformly coated with vesicles, with most of them directly attached to the beads. Some vesicles appeared to be extracted and partially flattened (B).
Characterization of rat exosome-bead conjugates by electron microscopy. Electron micrographs of magnetic beads immunologically coated with exosomes. Beads were coated with vesicles of 60 nm average diameter. Most vesicles were filled with electron-dense material (A) (bar, 1 μm). The beads were uniformly coated with vesicles, with most of them directly attached to the beads. Some vesicles appeared to be extracted and partially flattened (B).
Characteristics of rat exosome-bead conjugates. (A) The size/structure of exosome-bead conjugates was analyzed using a Coulter Epics XL. The window set as in the left panel allowed visualization of populations of conjugates with single (s), double (d), and triple (t) sized beads (right panel). The window was then set to measure only fluorescence associated with single-bead complexes. (B) Representative flow cytometric analysis of WGA-FITC labeled exos-beads. Conjugates were incubated with lectin, washed, and FACS analysis was performed before (solid line) and after (dotted line) the addition of detergent. (C) Surface expression of AChE on conjugates (solid line). Background staining was obtained by labeling the exosome-bead conjugates only with GAR-FITC (dotted line).
Characteristics of rat exosome-bead conjugates. (A) The size/structure of exosome-bead conjugates was analyzed using a Coulter Epics XL. The window set as in the left panel allowed visualization of populations of conjugates with single (s), double (d), and triple (t) sized beads (right panel). The window was then set to measure only fluorescence associated with single-bead complexes. (B) Representative flow cytometric analysis of WGA-FITC labeled exos-beads. Conjugates were incubated with lectin, washed, and FACS analysis was performed before (solid line) and after (dotted line) the addition of detergent. (C) Surface expression of AChE on conjugates (solid line). Background staining was obtained by labeling the exosome-bead conjugates only with GAR-FITC (dotted line).
DAF, MIRL, and LFA-3 are present on the exosome surface.
The presence of three other GPI-anchored proteins, DAF (CD55), MIRL (CD59), and LFA-3 (CD58) within exosomes was investigated. For this immunophenotypic analysis, human reticulocytes were incubated in vitro in maturation conditions, and exosomes released in the medium were collected and coupled to either SAM-magnetic beads, using mouse MoAb antihuman TfR or directly to Dynabeads M-450*CD71. As shown in Fig 4A, exosomes were labeled with anti-CD55, anti-CD59, and anti-CD58 antibodies. Note that, in the three cases, the fluorescence peaks were very sharp and markedly shifted as compared with control (GAM-FITC alone), indicating a very homogenous phenotype. Staining also showed a negative population only with LFA-3. By using the cross-reactivity of antirat AChE with the human protein,16 we analyzed the staining of these human exosomes for AChE (Fig 4B). As was the case with rat exosomes, the fluorescence peak obtained was higher than for the other GPI-anchored proteins. Note also that the fluorescence intensity of GAM-FITC staining was higher than with GAR-FITC alone, probably due to the labeling of anti-TfR mouse antibody on the magnetic bead surface.
Flow cytometric analysis of DAF, MIRL, LFA-3, and AChE expression on human exosome-bead conjugates. Staining for DAF/MIRL/LFA-3 (A) and AChE (B) was performed as detailed in Materials and Methods. Labeling only with the respective FITC-conjugated anti-IgG was used as background staining. Typical labeling patterns obtained from the same preparation of exosome-bead conjugates are presented.
Flow cytometric analysis of DAF, MIRL, LFA-3, and AChE expression on human exosome-bead conjugates. Staining for DAF/MIRL/LFA-3 (A) and AChE (B) was performed as detailed in Materials and Methods. Labeling only with the respective FITC-conjugated anti-IgG was used as background staining. Typical labeling patterns obtained from the same preparation of exosome-bead conjugates are presented.
Sorting of DAF and MIRL is very efficient in exosomes.
We used blood from PNH patients to further analyze the sorting of these GPI-anchored molecules in exosomes. Figure5 shows the results of the flow cytometry analysis of DAF and MIRL on the surface of RBCs from a PNH (case 1) and a control patient. As compared with normal RBC, which had uniform, positive staining with anti-CD59, there was high heterogeneity of MIRL expression in RBC of this PNH patient. Approximately 40% of the circulating RBCs showed negative expression (type III), around 50% showed intermediate expression (type II), and 10% showed normal expression (type I). Interestingly, the deficiency of DAF expression was less marked than for the other GPI-anchored protein. RBCs from this PNH patient and from an anemic patient (reticulocyte count, 12.5% and 6%, respectively) were cultured in vitro, and the released vesicles were analyzed for the presence of TfR by Western blot. As shown in Fig 6, the amount of TfR associated with exosomes was very similar. As previously described,2,20 the dimeric form of TfR could be detected, even after electrophoresis in reducing conditions. Similarly, a lower molecular weight band was observed, probably resulting from TfR proteolysis.21 22Moreover, the protein and phospholipid (PL) contents of the released vesicles were similar (4.5 mg protein/mL and 1.2 mmol/L PL v5.4 mg protein/mL and 1 mmol/L PL, for the PNH patient vcontrol, respectively). In contrast, as expected, AChE activity was reduced (fivefold) in exosomes collected from PNH cells (0.028 U/mg protein v 0.147 U/mg protein). When analyzed by flow cytometry, this low-AChE phenotype was observed with PNH exosomes as compared with exosomes from control patients (Fig 7). Surprisingly, expression of other GPI-anchored proteins on the surface of exosomes from PNH or control patients was very similar (DAF) or identical (MIRL). To further analyze these results, we performed a dose-response experiment using increasing exosome/bead ratios during the coupling reaction. The amount of exosomes bound was quantified biochemically using AChE activity and found to correspond perfectly to the ratios used for the coupling reaction (Fig 8). When the complexes were analyzed by flow cytometry labeling with anti-CD59, increasing amounts of CD59 (shift of fluorescence peak) were detected with higher exosome/bead ratios, although the relationship between these ratios and the fluorescence peak shift was not linear.
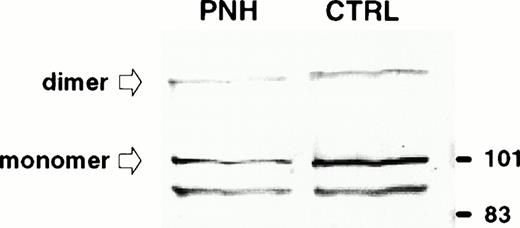
Immunodetection of TfR in exosomes released from control and PNH reticulocytes. Samples (50 μg protein) were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) in a 10% acrylamide gel and proteins were transferred to nitrocellulose. TfR was detected by Western blot using a MoAb (OKT9) raised against human TfR and an alkaline phosphatase-conjugated rabbit antimouse antibody. The molecular mass (kD) standards are indicated on the right.
Immunodetection of TfR in exosomes released from control and PNH reticulocytes. Samples (50 μg protein) were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) in a 10% acrylamide gel and proteins were transferred to nitrocellulose. TfR was detected by Western blot using a MoAb (OKT9) raised against human TfR and an alkaline phosphatase-conjugated rabbit antimouse antibody. The molecular mass (kD) standards are indicated on the right.
Comparison of GPI-anchored protein (AChE, DAF, MIRL) expression onto control and PNH exosomes. Exosomes released from reticulocytes of a control subject (CTRL) and a patient (PNH) were analyzed, as described in Materials and Methods, for the presence of the different GPI-anchored proteins. Background staining obtained with only the FITC-labeled antibodies are presented.
Comparison of GPI-anchored protein (AChE, DAF, MIRL) expression onto control and PNH exosomes. Exosomes released from reticulocytes of a control subject (CTRL) and a patient (PNH) were analyzed, as described in Materials and Methods, for the presence of the different GPI-anchored proteins. Background staining obtained with only the FITC-labeled antibodies are presented.
Relationship between fluorescence peak shift and antigen densities on bead surface. Complexes with various exosome amounts on the bead surface were obtained as described in Materials and Methods and analyzed for CD59 expression by flow cytometry (○) and AChE activity (□).
Relationship between fluorescence peak shift and antigen densities on bead surface. Complexes with various exosome amounts on the bead surface were obtained as described in Materials and Methods and analyzed for CD59 expression by flow cytometry (○) and AChE activity (□).
GM1 is present in rat reticulocyte exosomes.
GM1 is not present in human RBCs, but is found in rat erythrocytes.23 We thus analyzed the binding of FITC-conjugated cholera toxin B subunit to exosomes. Vesicles from human or rat reticulocytes were labeled with increasing amounts of FITC-CTB (Fig 9). The shift in the fluorescence peak was plotted as a function of the amount of added FITC-CTB. As expected, human exosomes used as negative control did not bind significant amounts of FITC-CTB. Conversely, a clear titration curve was obtained with rat exosomes. GM1 was thus released in rat reticulocyte exosomes, together with the TfR and GPI-anchored proteins.
Binding of FITC-CTB on rat reticulocyte exosomes. Exosomes from rat (•) or human (○) reticulocytes were conjugated to magnetic beads. Increasing amounts of the FITC-CTB were incubated with 100 μL of exosome-bead conjugates and analyzed by flow cytometry as described in Materials and Methods. The fluorescence peak position is plotted as a function of added FITC-CTB.
Binding of FITC-CTB on rat reticulocyte exosomes. Exosomes from rat (•) or human (○) reticulocytes were conjugated to magnetic beads. Increasing amounts of the FITC-CTB were incubated with 100 μL of exosome-bead conjugates and analyzed by flow cytometry as described in Materials and Methods. The fluorescence peak position is plotted as a function of added FITC-CTB.
DISCUSSION
Exosomes are mainly known as vesicles involved in the clearance of obsolete proteins from the erythrocyte surface, such as the TfR. Other transmembrane proteins are also sorted in these vesicles, but the molecule sorting mechanism is still unknown. We have shown that the sorting step could involve parameters such as the aggregation state of molecules on the membrane surface, and also the nature of lipid components of the bilayer. Indeed, we showed that molecule (TfR, AChE) aggregation reroutes molecules towards exosomal release, instead of recycling to the plasma membrane.2 Moreover, N-(lissamine rhodamine B sulfonyl) phosphatidyl ethanolamine (N-Rh-PE), after insertion into the plasma membrane of reticulocytes, was shown to be selectively sorted from other lipid and accumulated in exosomes. We proposed that such sorting is triggered by the presence of the fluorescent analog in the membrane as small molecular clusters. As natural lipids such as glycosphingolipids are also able to interact by hydrogen bonding, the fate of N-Rh-PE in reticulocytes may represent amplification of the physiologic route of specific membrane lipids.24 Moreover, glycosphingolipids are known to be molecular partners of lipid domains found in the trans-Golgi network or caveolae,6,7 where proteins are specifically sorted. It has been shown that GPI-anchored proteins are enriched in these domains.7 AChE is sorted in exosomes and as shown here, sensitive to PI-PLC treatment, demonstrating its GPI-anchoring to the exosomal membrane. We propose that the GPI-anchor could be a sorting signal for AChE, and thus that other GPI-anchored proteins must also be sorted in reticulocyte exosomes.
The presence of three other GPI-anchored proteins (DAF, MIRL, and LFA-3) in exosomes is shown here. This indicates involvement of the GPI-anchor during the intracellular sorting step of exosomal vesicles. This was further shown in experiments using exosomes collected from PNH patients. In this disease, GPI-anchored proteins are almost or completely missing from the cell surface because of a defect in the biosynthesis of the GPI-anchor.11 The well established heterogeneity in GPI-anchored protein expression in cells of PNH patients was also noted here, as RBC of these patients showed very deficient MIRL (CD59) expression, while being closer to normal in DAF (CD55). We confirmed the clinical phenotypic analysis through quantitation of AChE released in exosomes from reticulocytes by two independent methods, namely AChE activity and flow cytometry immunodetection. Note that, depending on the quantitation method used, the extent of the AChE decrease in PNH versus control exosomes differed. This was confirmed by a dose-response curve using various amounts of exosomes coupled per bead, showing that the relationship between the antigen density and the fluorescence peak shift obtained with the fluorescence-activated cell sorting (FACS)-based methodology was not linear. However, as in the case of AChE (Fig 7, upper panel), a fivefold decrease in CD59 was detectable by immunolabeling.
Complexes were then stained for DAF and MIRL. The results were very similar or identical, respectively, when comparing normal exosomes with PNH exosomes. Although the methodology is not completely quantitative, it nevertheless clearly shows that the densities of GPI-anchored proteins were much higher on the exosome membrane than on the cell surface. Although only 10% of PNH RBCs showed normal expression (type I), while 40% were negative (type III), especially for CD59, the final expression on the exosomal surface was identical between PNH and control complexes (Fig 7, lower panel). We conclude from these results that sorting of GPI-anchored proteins in exosomes is a very efficient mechanism.
This also means that, during reticulocyte maturation, the release of exosomes contributes to the loss of a certain amount of GPI-anchored proteins. This exosomal loss may account for the previously noted paradox15 between percentages of type II reticulocytes and type II mature erythrocytes. Indeed, type II erythrocytes (ie, partially CD59-deficient) were often found in PNH patients with virtually no type II reticulocytes. In our data, type II erythrocytes could thus have been derived, at least partly, from the maturation of type I reticulocytes. This is also in agreement with a very recent study suggesting that disappearance of PNH II erythroblasts during maturation to erythrocytes is related to other mechanisms in addition to hypersensitivity to the complement.25
These results highlight the efficiency of GPI-anchored protein sorting during exosome formation. In other words, the little that is expressed as GPI-anchored proteins on the reticulocyte surface is very efficiently sorted during reticulocyte maturation. This is in agreement with the presence, in rat exosomes, of GM1, a ganglioside that is found in high quantities in caveolae.8 In fact, the efficiency of sorting on membrane surface might reflect a cause of the exosome formation rather than a consequence. Indeed, enrichment of GPI-anchored proteins could be expected to participate in the budding phenomenon. Considerable evidence favors this hypothesis: (1) coat proteins have not been described in exosomes, suggesting that some membrane components may be involved, (2) RBCs from PNH patients cannot properly vesiculate when submitted to Ca2+ ionophore A23187,26 (3) the membrane (and budding) topology is identical in these cases (exosome formation and calcium-induced plasma membrane shedding) but, contrary to caveolae formation, which requires the involvement of the protein caveolin not found here,27(4) similar vesicles released by other cells, such as prostasomes derived from prostate epithelial cells, are enriched with GPI-anchored proteins (CD59)28, (5) exosomes derived from B lymphocytes were shown to be devoid of TfR, but contain LFA-3 (CD58).29
GPI-anchored proteins may contribute to exosome formation suggesting a new vesicle function. Exosomes from reticulocytes could participate in complement regulation, acting directly with complement proteins through DAF and MIRL, or by representing a circulating reservoir of GPI-anchored proteins that could transfer from the exosomal membrane to plasma membrane of cells. GPI-anchored proteins can be transferred directly from the plasma membrane of RBCs to endothelial cells.30,31 It is thus possible that transfer of DAF and MIRL to these target membranes is facilitated by the high curvature of the exosome membrane as compared with the plasma membrane. Here it should be noted that integrin α4β1 is downregulated during normal erythroid differentiation,32 but expressed on circulating reticulocytes in sickle cell anemia.33 It is thus possible that exosomes express α4β1, which may be involved in GPI-anchored protein transfer through interaction with vascular cell adhesion molecule-1 (VCAM-1) present on the surface of endothelial cells. Indeed, it was shown with prostasomes that a close apposition between the vesicle and plasma membrane is a prerequisite for GPI-protein transfer.34 Experiments are currently under way to assess the possibility of a molecular transfer of GPI-anchored proteins, especially DAF and MIRL, from exosomes to target cell membranes.
Finally, note that some viruses have been found to bud from major histocompatability complex (MHC) class II compartments (MIICs) in B lymphocytes, and to be released as exosome-like virions (G. Raposo, personal communication, July 1996). MIICs are similar to reticulocyte multivesicular endosomes in terms of several characteristics.35 This may be a way by which viruses incorporate CD55 and CD59 onto their envelope and escape inactivation by human complement.36
Supported by the Centre National de la Recherche Scientifique and by grants from the Université Montpellier II and the Hôpital St Eloi.
Address reprint requests to Michel Vidal, PhD, UMR 5539, Univ. Montpellier II, cc107, Montpellier 34095, France.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal