Abstract
Mycophenolate mofetil (MMF) was evaluated either alone or combined with cyclosporine (CSP) for preventing graft-versus-host disease (GVHD) in dogs given 9.2 Gy total body irradiation and DLA-nonidentical unrelated marrow grafts. Marrow autograft studies showed gut toxicity as limiting MMF side effects. Four groups were studied for GVHD prevention: six dogs in group 1 received MMF 10 mg/kg twice daily subcutaneously (SC) on days 0 to 27. They died between 8 to 28 days from infection or GVHD; survival was better than that of 72 controls given no immunosuppression (P = .04), but not different from 19 dogs given CSP. Four dogs in group 2 received MMF as described, along with CSP at 10 to 15 mg/kg twice daily on days 0 to 27. They died at 6 to 98 days from CSP-associated toxicity, weight loss, or infection. Nine dogs in group 3 received MMF SC twice daily 6 mg/kg/d for 3 days, followed by 10 mg/kg twice daily until day 27, along with CSP as described; four died between 7 to 106 days with intussusception, infection, or GVHD, and five became long-term survivors. Six dogs in group 4 received shortened MMF (21 days) and reduced doses of CSP given through day 100. Three died with GVHD or infection between days 38 to 119, and three became long-term survivors. Results support the notion of synergism between MMF and CSP, as evidenced by stable graft-host tolerance in greater than 50% of dogs.
THE PURPOSE of immunosuppressive drugs administered after allogeneic marrow grafts is to prevent graft-versus-host disease (GVHD) and to facilitate the induction of graft-host tolerance that will persist even after immunosuppression has been discontinued. We have evaluated immunosuppressive drugs for GVHD prevention in a model of marrow allografting from dog leukocyte antigen (DLA)-nonidentical unrelated donor dogs. Without immunosuppression, hyperacute and fatal GVHD occurred uniformly. Significant delay of GVHD and prolongation of survival were seen when methotrexate (MTX), cyclosporine (CSP), FK506, azathioprine, deoxyspergualin, 6-mercaptopurine, or succinylacetone were used as single agents, while other drugs were ineffective.1-3 GVHD prevention was most impressive when MTX was combined with either CSP2 or FK506,3 but other drug combinations were not effective.1 The advantages of MTX/CSP and MTX/FK506 were confirmed clinically first in Phase I/II studies and then in randomized trials.4-6 Nevertheless, while the drug combinations worked well in transplants from HLA-identical sibling donors, results were less impressive with marrow grafts from alternative donors, either phenotypically HLA-identical unrelated individuals or HLA-haploidentical relatives. Therefore, the search for alternative immunosuppressive drugs, which are more effective in GVH prevention, has continued.
Mycophenolic acid (MPA) is an antimetabolite with a broad range of activities that include antitumor, antifungal, antibacterial, antiviral activities, and immunosuppression.7-9 MPA selectively inhibits inosine 5′-monophosphate dehydrogenase (IMPDH), the key enzyme that controls the de novo synthesis of guanosine monophosphate (GMP).9,10 Lymphocyte activation and proliferation depends on the supply of guanine nucleotides (GTP) for protein and nucleic acid synthesis through the de novo pathway while other cells have a salvage biosynthesis pathway.10,11MPA blocks T- and B-cell proliferation in response to antigens and mitogens, thereby suppressing cellular and humoral immune responses.12,13 In addition, MPA inhibits lymphocyte binding and downregulates expression of adhesion molecules, and that way reduces lymphocyte migration and interaction with macrophages.12,13 Mycophenolate mofetil (MMF), a semisynthetic derivative of MPA, has been studied in solid organ transplants and has been shown to prolong allografts of skin, lung, small intestine, heart, liver, and kidney in animals and in humans.14
The present study evaluated the usefulness of MMF for GVHD prevention in dogs given marrow grafts from DLA-nonidentical unrelated donors. MMF alone delayed the onset of acute GVHD and resulted in a modest prolongation of survival. When MMF was combined with CSP, approximately half of the dogs studied became long-term survivors.
MATERIALS AND METHODS
Dogs and DLA typing.
Litters of beagles, harriers, Walker hounds, and crossbred dogs were used in this study. Dogs were either bred at the Fred Hutchinson Cancer Research Center or purchased from Department of Agriculture licensed vendors located in the states of Washington and Michigan. Dogs were immunized against leptospirosis, distemper, hepatitis, and parvovirus, dewormed, and observed for disease for at least 2 months before being entered on study. Dogs weighed from 5.8 to 18.6 (median, 10) kg and were 7 to 36 (median, 10) months old. The experimental protocols and the facilities used were approved by the Fred Hutchinson Cancer Research Center's (FHCRC) Internal Animal Care and Use Committee per guidelines stipulated in the Experimental Animal Welfare Act of 1985 administrated through the National Institutes of Health.
MMF.
MMF was provided by Roche Bioscience (formerly Syntex Discovery Research, Palo Alto, CA) as lyophilized powder (intravenous [IV] formula without TWEEN 80). The drug was prepared as a fresh suspension in 5% Dextoses at a concentration of 25 mg/mL and stored at room temperature until use.
Total body irradiation (TBI), marrow transplantation, and supportive care.
For both autologous and allogenic grafts, marrow cells were aspirated under general anesthesia through needles inserted into humeri and femora and stored in heparinized tissue culture medium at 4°C for no more than 6 hours.17 Recipients were administered TBI at a single dose of 920 cGy delivered at 7 cGy/minute from two opposing cobalt-60 sources.17 Within 4 hours of TBI, harvested marrow cells were infused IV into recipients at doses of 1.3 to 4.1 (mean, 3.2) × 108 nucleated cells/kg. The day of marrow grafting was designated as day 0. All allogeneic recipients were given, in addition, IV infusions of peripheral blood buffy coat cells obtained by leukapheresis from the marrow donor on days 1 and 2, at doses of 6.3 to 19.6 (mean, 12.7) × 108 nucleated cells/kg to assure consistent hematopoietic engraftment.18
Supportive care included oral and systemic antibiotics, subcutaneous fluids with electrolytes, and platelet transfusions.17Complete blood counts were obtained daily before and for the first 3 weeks after transplant and twice weekly thereafter. Serum chemistry levels were obtained once weekly to monitor kidney and liver functions. Dogs were checked and weighed daily, and the percentage of weight loss calculated as the weight at day 0 (W0) minus the least weight after transplant (Wl) divided by the weight at day 0 (W0): [W0 − Wl] / W0 × 100%.
Hematopoietic engraftment was assessed by sustained increases in granulocyte and platelet counts after the postirradiation nadir, by documentation of donor originated cells with microsatellite marker studies in specimens from peripheral blood and marrow,19 by histologic features of the marrow from biopsy or autopsy specimens, and by clinical and histopathologic findings of GVHD in allogeneic recipients.
Clinical signs of GVHD included severe diarrhea due to gut involvement (G), conjunctival or skin erythema (S), and elevations of liver enzymes and bilirubin (L). Acute GVHD was defined as disease manifested before day 100, and chronic GVHD was defined as disease present after day 100. Dogs either died of or were euthanized because of complications of the study, or they were euthanized after completion of the study. The study was considered complete once dogs lived more than 200 days after transplant with or without clinical evidence of GVHD.
The immune function in long-term survivors was assessed by established techniques. These included in vitro testing of lymphocyte responsiveness to alloantigens in a standard mixed lymphocyte culture and to concanavalin A,20 natural killer (NK) cell function,21 cytotoxic T-lymphocyte function, and flow cytometry for lymphocyte phenotyping.22 Also, antibody responses to sheep red blood cells (SRBC) in vivo were determined. For that purpose, dogs were given an IV injection of 1 mL of a 10% suspension of SRBC in 0.15 mol/L NaCl. Antibody titers were determined as described.20
Statistical methods.
Survival times and times to granulocyte or platelet recoveries were compared between treatment groups using log-rank statistics. Granulocyte and platelet recoveries were defined as the first days after the postirradiation decline when granulocyte counts rose above 500/μL and platelet counts above 20,000/μL for two consecutive measurements. Pearson's χ2 test was used to compare proportions experiencing graft failure in different groups.
Historical and concurrent controls.
Results in current recipients were compared with those in previous and concurrent DLA-nonidentical recipients receiving either no immunosuppression (n = 72), a long course of CSP for 100 days (n = 19), or a short course of MTX along with 45 days of CSP (n = 6) (unpublished), and 100 days of CSP (n = 10).2
RESULTS
Dose finding and toxicity studies in dogs with marrow autografts.
Five dogs given 9.2 Gy TBI and autologous marrow grafts were scheduled to receive MMF from day 0 to day 27 after transplant delivered either as a continuing IV infusion or SC. All three dogs given 40 mg MMF/kg/d developed severe diarrhea along with dehydration and loss of 20% to 29% of body weight. They had poor hematopoietic engraftment and were euthanized on days 11, 12, and 13 because of poor general condition. Marrow cellularity was <5%, <5%, and 20% of normal, respectively. The fourth dog was given MMF 20 mg/kg/d as a continuous IV infusion, and the fifth dog was given MMF 10 mg/kg SC three times a day on days 0 to 8 and twice a day on days 9 to 27. Both dogs developed transient diarrhea and 14% to 19% of body weight loss, and both had prompt and complete hematopoietic recovery. No significant toxicities in central nervous system, kidney, and liver were observed in the five autologous transplant recipients.
DLA-nonidentical, unrelated recipients.
Given the findings in dogs with autografts, a dose of MMF of 10 mg/kg twice daily (20 mg/kg/d) SC was thought to be safe for the GVHD prevention studies. Because of the pronounced weight loss seen with MMF, we administered the drug for no more than 4 weeks. Owing to the considerable cost of the drug, we elected to discontinue CSP on the same day we stopped MMF. The CSP doses used were arrived at empirically, and they were shown in previous studies to enhance hematopoietic engraftment in dogs given suboptimal conditioning programs.23 24
Results are summarized in Table 1. Six dogs in group 1 were scheduled to receive MMF alone at a dose of 10 mg/kg SC twice daily on days 0 to 27 after transplant. Two of the six dogs had persistent severe diarrhea from day 0. One of two was euthanized on day 8 because of poor clinical condition, while the other died on day 12 of pneumonia and intestinal bleeding. Both dogs had a severely hypoplastic marrow at autopsy and no evidence of GVHD. The remaining four dogs had successful allografts as indicated by rapidly rising granulocyte and platelet counts and by microsatellite marker studies. They developed clinical and histologic evidence of severe GVHD and died between days 18 and 28. All six dogs had reduced food intake and lost between 17% and 36% of their body weight. Serum chemistries showed normal levels of creatinine, urea nitrogen, bilirubin, and slightly increased levels of aspartate aminotransferase and/or alanine aminotransferase (data not shown).
Dogs Administered 9.2 Gy TBI, Hematopoietic Grafts From DLA-Nonidentical, Unrelated Donors, Followed by MMF With or Without CSP Treatment
| Dog No. . | Donor Cells Infused (×108/kg) . | Marrow Engraftment . | Marrow Cellularity (%) . | GVHD . | Survival (d) . | Weight Loss (%) . | Cause of Death . | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Clinical . | Histological . | |||||||||
| Marrow . | Buffy Coat . | Acute . | Chronic . | |||||||
| Group 1 | ||||||||||
| D876 | 3.8 | 19.6 | Yes | 75-150 | S, G | NA | S, L, G | 20 | 28 | GVHD, ET1 |
| D935 | 1.5 | 12.8 | Yes | 100-150 | S, L, G | NA | S, L, G | 23 | 33 | GVHD, ET1 |
| D954 | 1.3 | 13.2 | No | 0 | — | NA | ;— | 8 | 25 | ET1 |
| D957 | 4.0 | 16.1 | Yes | 100-150 | S, G | NA | S, L, G | 28 | 33 | GVHD, ET1 |
| D958 | 3.5 | 9.1 | Yes | 35-150 | S, G | NA | S, L, G | 18 | 36 | Pneumonia |
| D972 | 2.9 | 13.1 | No | 0 | — | NA | ;— | 12 | 17 | Infection, GI bleeding |
| Group 2 | ||||||||||
| D868 | 4.0 | 13.2 | Yes | 10-150 | — | NA | ;— | 6 | 15 | MMF and CSP toxicities, ET1 |
| D910 | 3.6 | 9.8 | Yes | 60-150 | G | NA | S±, L±, G | 28 | 40 | Weight loss, ET1 |
| D911 | 2.8 | 11.7 | Yes | 100-150 | — | NA | S±, L±, G | 98 | 22 | Myocarditis |
| D936 | 2.1 | 16.5 | Yes | 5-150 | — | NA | ;— | 8 | 15 | MMF and CSP toxicities, ET1 |
| Group 3 | ||||||||||
| D823 | 4.1 | 15.2 | Yes | 100-150 | — | S | S, L, G± | 106 | 34 | Skin GVHD, ET1 |
| D960 | 3.3 | 12.6 | Yes | 100-150 | — | NA | S, L, G | 72 | 25 | Pneumonia and meningitis |
| D962 | 2.6 | 15 | Yes | 5-150 | — | NA | ;— | 7 | 17 | Intussusception, septicemia, ET1 |
| D967 | 3.7 | 10.3 | Yes | 100-150 | S, G | NA | S, L, G | 48 | 38 | Pneumonia, ET1 |
| D988 | 3.4 | 11.9 | Yes | 100-150 | — | — | (chronic) G | >540 | 30 | ET2 |
| D992 | 3.6 | 9.8 | Yes | 100-150 | — | — | S, L, (chronic) G± | >420 | 19 | ET2 |
| E071 | 3.8 | 11.2 | Yes | 100-150 | — | — | L, G± | >420 | 38 | ET2 |
| E072 | 4.0 | 13.6 | Yes | 100-150 | — | — | S±, L, (chronic) G± | >510 | 29 | ET2 |
| E223 | 3.7 | 15.8 | Yes | 100-150 | — | S± | S, L, (chronic) G | >420 | 26 | ET2 |
| Group 4 | ||||||||||
| E119 | 2.2 | 9.2 | Yes | 100-150 | — | — | S±, L, (chronic) G | >316 | 23 | ET2 |
| E168 | 2.7 | 8.8 | Yes | 100-150 | — | S | S, L | 119 | 18 | Skin GVHD, ET1 |
| E171 | 3.6 | 9.4 | Yes | 100-150 | — | S | S, L, G± | >224 | 8 | ET2 |
| E207 | 4.0 | 6.3 | Yes | 100-150 | — | S± | S, L, (chronic) G | >216 | 21 | ET2 |
| E210 | 4.1 | 17.1 | Yes | 100-150 | L | NA | S, L, (chronic) G | 95 | 41 | Infection, GVHD, renal failure |
| E211 | 3.5 | 15.5 | Yes | 100-150 | — | NA | S±, G | 38 | 25 | Meningitis, ET1 |
| Dog No. . | Donor Cells Infused (×108/kg) . | Marrow Engraftment . | Marrow Cellularity (%) . | GVHD . | Survival (d) . | Weight Loss (%) . | Cause of Death . | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Clinical . | Histological . | |||||||||
| Marrow . | Buffy Coat . | Acute . | Chronic . | |||||||
| Group 1 | ||||||||||
| D876 | 3.8 | 19.6 | Yes | 75-150 | S, G | NA | S, L, G | 20 | 28 | GVHD, ET1 |
| D935 | 1.5 | 12.8 | Yes | 100-150 | S, L, G | NA | S, L, G | 23 | 33 | GVHD, ET1 |
| D954 | 1.3 | 13.2 | No | 0 | — | NA | ;— | 8 | 25 | ET1 |
| D957 | 4.0 | 16.1 | Yes | 100-150 | S, G | NA | S, L, G | 28 | 33 | GVHD, ET1 |
| D958 | 3.5 | 9.1 | Yes | 35-150 | S, G | NA | S, L, G | 18 | 36 | Pneumonia |
| D972 | 2.9 | 13.1 | No | 0 | — | NA | ;— | 12 | 17 | Infection, GI bleeding |
| Group 2 | ||||||||||
| D868 | 4.0 | 13.2 | Yes | 10-150 | — | NA | ;— | 6 | 15 | MMF and CSP toxicities, ET1 |
| D910 | 3.6 | 9.8 | Yes | 60-150 | G | NA | S±, L±, G | 28 | 40 | Weight loss, ET1 |
| D911 | 2.8 | 11.7 | Yes | 100-150 | — | NA | S±, L±, G | 98 | 22 | Myocarditis |
| D936 | 2.1 | 16.5 | Yes | 5-150 | — | NA | ;— | 8 | 15 | MMF and CSP toxicities, ET1 |
| Group 3 | ||||||||||
| D823 | 4.1 | 15.2 | Yes | 100-150 | — | S | S, L, G± | 106 | 34 | Skin GVHD, ET1 |
| D960 | 3.3 | 12.6 | Yes | 100-150 | — | NA | S, L, G | 72 | 25 | Pneumonia and meningitis |
| D962 | 2.6 | 15 | Yes | 5-150 | — | NA | ;— | 7 | 17 | Intussusception, septicemia, ET1 |
| D967 | 3.7 | 10.3 | Yes | 100-150 | S, G | NA | S, L, G | 48 | 38 | Pneumonia, ET1 |
| D988 | 3.4 | 11.9 | Yes | 100-150 | — | — | (chronic) G | >540 | 30 | ET2 |
| D992 | 3.6 | 9.8 | Yes | 100-150 | — | — | S, L, (chronic) G± | >420 | 19 | ET2 |
| E071 | 3.8 | 11.2 | Yes | 100-150 | — | — | L, G± | >420 | 38 | ET2 |
| E072 | 4.0 | 13.6 | Yes | 100-150 | — | — | S±, L, (chronic) G± | >510 | 29 | ET2 |
| E223 | 3.7 | 15.8 | Yes | 100-150 | — | S± | S, L, (chronic) G | >420 | 26 | ET2 |
| Group 4 | ||||||||||
| E119 | 2.2 | 9.2 | Yes | 100-150 | — | — | S±, L, (chronic) G | >316 | 23 | ET2 |
| E168 | 2.7 | 8.8 | Yes | 100-150 | — | S | S, L | 119 | 18 | Skin GVHD, ET1 |
| E171 | 3.6 | 9.4 | Yes | 100-150 | — | S | S, L, G± | >224 | 8 | ET2 |
| E207 | 4.0 | 6.3 | Yes | 100-150 | — | S± | S, L, (chronic) G | >216 | 21 | ET2 |
| E210 | 4.1 | 17.1 | Yes | 100-150 | L | NA | S, L, (chronic) G | 95 | 41 | Infection, GVHD, renal failure |
| E211 | 3.5 | 15.5 | Yes | 100-150 | — | NA | S±, G | 38 | 25 | Meningitis, ET1 |
Group 1: MMF 10 mg/kg SC twice daily (20 mg/kg/d), from days 0-27.
Group 2: MMF 10 mg/kg SC twice daily (20 mg/kg/d), from days 0-27, CSP 10 mg/kg IV twice daily (20 mg/kg/d) from days 0-9, 15 mg/kg by mouth, twice daily (30 mg/kg/d) from days 10-27.
Group 3: MMF 6 mg/kg SC twice daily (12 mg/kg/d), from days 0-2, 10 mg/kg SC twice daily (20 mg/kg/d) from days 3-27; CSP 10 mg/kg IV twice daily (20 mg/kg/d) from days 0-9, 15 mg/kg by mouth twice daily (30 mg/kg/d) from days 10-27.
Group 4: MMF 6 mg/kg SC twice daily (12 mg/kg/d), from days 0-2, 10 mg/kg SC twice daily (20 mg/kg/d) from days 3-20; CSP 5 mg/kg IV twice daily (10 mg/kg/d) from days 0-5, 15 mg/kg by mouth twice daily (30 mg/kg/d) from days 6-20, 7.5 mg/kg twice daily (15 mg/kg/d) from days 21-45, 5 mg/kg by mouth twice daily (10 mg/kg/d) from days 46-75, 3 mg/kg by mouth twice daily (6 mg/kg/d) from days 76-100.
Abbreviations: ET1, euthanized due to poor condition; ET2, euthanized at completion of the study; G, gut; L, liver; S, skin.
All donor engraftment, as determined by microsatellite markers.19
Four dogs in group 2 were given MMF at 10 mg/kg twice daily SC from days 0 to 27 and CSP IV at 10 mg/kg twice daily from days 0 to 9, followed by CSP by mouth at 15 mg/kg twice daily from days 10 to 27. Two dogs were euthanized because of MMF and CSP-associated toxicities at days 6 and 8, which included intussusception and massive diarrhea. The two remaining dogs had prompt engraftment. One of the two had clinical signs of gut GVHD and was euthanized at day 28 due to severe weight loss (over 40%), and the other died of myocarditis of unknown etiology at day 98 without clinical signs of GVHD.
Because of the observed drug toxicities in dogs of group 2, the dose of MMF in dogs of group 3 was reduced from 10 to 6 mg/kg twice daily SC for the first 3 days followed by 10 mg/kg twice daily SC until day 27. The CSP dose was that described for dogs in group 2. All 9 dogs had prompt engraftment. Four of the 9 died: 1 died of pneumonia and meningitis on day 72, 1 was euthanized on day 7 because of septicemia secondary to intussusception (a CSP-associated toxicity in dogs), 1 because of acute GVHD with 38% weight loss on day 48, and 1 because of severe chronic skin GVHD on day 106. The remaining 5 dogs became long-term survivors without clinical manifestation of GVHD, even though all had histopathologic evidence of GVHD on autopsy at the completion of study (n = 5). All 9 dogs in this group had weight loss ranging from 17% to 38% while on MMF. In dog E071, MMF was discontinued prematurely on day 23 because of weight loss. Dogs recovered their body weight after immunosuppression was stopped.
To further reduce MMF- and CSP-related toxicities, the six dogs in group 4 were given the same regimen of MMF as was used in dogs of group 3 except that MMF was stopped after 21 instead of 27 days, and the IV CSP dose was reduced to 5 mg/kg twice daily for the first 6 days, followed by CSP by mouth at 15 mg/kg twice daily until day 20. Given that GVHD was observed between days 27 and 100 in dogs of groups 2 and 3 in which CSP was stopped on day 27, we elected to continue CSP until day 100 using a gradual taper schedule as outlined in the footnote of Table 1. All six dogs had prompt engraftment. Three died. One died on day 95 of GVHD, systemic infection, and renal failure. CSP was discontinued early (day 66) in this dog because of extensive papillomata on all extremities, which were related to prolonged administration of CSP. The second dog developed severe skin GVHD and was euthanized on day 119. In this dog, CSP was also stopped prematurely (day 79) because of papillomata. The third dog was euthanized on day 38 because of continuous seizures caused by a purulent meningitis. The three remaining dogs all had a complete course of posttransplant immunosuppression and they became long-term survivors. Skin GVHD developed in one of the long-term survivors on day 140 and resolved after 2 weeks of treatment with CSP at 10 mg/kg/d by mouth.
Peripheral blood leukocytes.
Table 2 shows the flow cytometric analyses of leukocytes after hematopoietic reconstitution in four allografted dogs from group 3 tested between 80 to 120 days after transplant. The proportions of B and T cells were in the normal range, and the CD4/CD8 ratios were inverted in all four dogs.
Flow Cytometric Analysis of Peripheral Blood Leukocytes From Normal Dogs and Dogs With Marrow Allografts
| MoAb . | % of Total Leukocytes . | ||||
|---|---|---|---|---|---|
| Normal Dogs (n = 4) . | Allograft Recipients (Dog No.) . | ||||
| D823 . | D988 . | E071 . | E072 . | ||
| CD21+ | 5.2-14.4 | 7.9 | 3.1 | 6.3 | 5.4 |
| CD4+ | 34.3-40.9 | 23.8 | 36.3 | 33 | 22.5 |
| CD8+ | 16.5-32.4 | 38.7 | 36.7 | 41.9 | 28 |
| CD4+:CD8+ | 1.06-2.48 | 0.61 | 0.99 | 0.79 | 0.8 |
| MoAb . | % of Total Leukocytes . | ||||
|---|---|---|---|---|---|
| Normal Dogs (n = 4) . | Allograft Recipients (Dog No.) . | ||||
| D823 . | D988 . | E071 . | E072 . | ||
| CD21+ | 5.2-14.4 | 7.9 | 3.1 | 6.3 | 5.4 |
| CD4+ | 34.3-40.9 | 23.8 | 36.3 | 33 | 22.5 |
| CD8+ | 16.5-32.4 | 38.7 | 36.7 | 41.9 | 28 |
| CD4+:CD8+ | 1.06-2.48 | 0.61 | 0.99 | 0.79 | 0.8 |
Flow cytometric analysis of leukocytes was done between 80 to 120 days after transplant. Allograft recipients were treated with MMF and CSP from days 0-27. The normal range was obtained from four normal dogs.
Abbreviation: MoAb, monoclonal antibody.
Antibodies to SRBC.
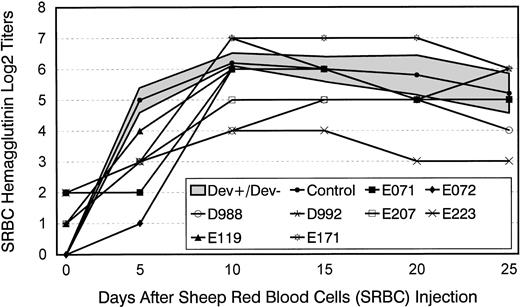
Figure 1 illustrates the eight long-term survivors' primary humoral antibody titers in response to SRBC. Results were compared with those from five concurrently injected normal dogs. All but one of the marrow graft recipients showed lower than normal hemagglutinin titers on day 5 after injection. Subsequently, the majority of dogs reached antibody titers in the normal range, while at least three of the animals continued to show subnormal antibodies during the 25-day period of serum collection.
Hemagglutinin titers after a single injection of SRBC in five normal control dogs and eight long-term survivors after marrow transplantation. Shown for controls are mean titers ± 1 standard deviation (SD) (shaded area). Transplant recipients were administered SRBC between days 80 and 120 after transplant.
Hemagglutinin titers after a single injection of SRBC in five normal control dogs and eight long-term survivors after marrow transplantation. Shown for controls are mean titers ± 1 standard deviation (SD) (shaded area). Transplant recipients were administered SRBC between days 80 and 120 after transplant.
NK and T-cell function.
Data on NK function in four of the long-term surviving dogs and four normal dogs are summarized in Table 3. NK function was within the normal range in all but one dog (E072) in which low NK function was seen at effector:target cell ratios of 80:1 and 40:1, respectively. Cytotoxic T-cell function as determined by cell-mediated lympholysis assays was within the normal range in the same four dogs as were the lymphocyte responses to alloantigens in mixed leukocyte culture and to concanavalin A (data not shown).
NK Function in Normal Dogs and in Four Dogs With DLA-Nonidentical, Unrelated Hematopoietic Grafts
| % of Specific Lysis of Target Cells . | ||||||
|---|---|---|---|---|---|---|
| Effector:Target Cell Ratio . | Normal Dogs (n = 4) . | Allograft Recipients (Dog No.) . | ||||
| Range . | Median . | D823 . | D988 . | E071 . | E072 . | |
| 80:1 | 21.0-29.2 | 24.2 | 29.1 | 32.7 | 23.6 | 8.3 |
| 40:1 | 18.5-32.4 | 20.4 | 24.1 | 27.8 | 25 | 13.2 |
| 20:1 | 13.2-30.8 | 15.5 | 14.7 | 17 | 11.9 | 15.8 |
| 10:1 | 6.7-22.8 | 8.3 | 9.6 | 10.7 | 11.7 | 14.5 |
| 5:1 | 5.0-13.6 | 7.4 | 9.8 | 7.4 | 3.3 | 7.6 |
| % of Specific Lysis of Target Cells . | ||||||
|---|---|---|---|---|---|---|
| Effector:Target Cell Ratio . | Normal Dogs (n = 4) . | Allograft Recipients (Dog No.) . | ||||
| Range . | Median . | D823 . | D988 . | E071 . | E072 . | |
| 80:1 | 21.0-29.2 | 24.2 | 29.1 | 32.7 | 23.6 | 8.3 |
| 40:1 | 18.5-32.4 | 20.4 | 24.1 | 27.8 | 25 | 13.2 |
| 20:1 | 13.2-30.8 | 15.5 | 14.7 | 17 | 11.9 | 15.8 |
| 10:1 | 6.7-22.8 | 8.3 | 9.6 | 10.7 | 11.7 | 14.5 |
| 5:1 | 5.0-13.6 | 7.4 | 9.8 | 7.4 | 3.3 | 7.6 |
51Cr labeled CTAC20 were used as the target cells to quantitate NK activity in four normal dogs and four allograft recipients between 80 to 120 days after transplant. Allograft recipients were treated with MMF and CSP from day 0 to 27. The incubation time for the assay was 15 hours. The data represent the mean of triplicate wells in the assay.
Granulocyte and platelet recoveries in MMF/CSP-treated dogs.
Figure 2 compares granulocyte and platelet recoveries in dogs given MMF/CSP (n = 19) to those in 16 dogs treated with another antimetabolite, MTX, combined with CSP. No significant differences between the two groups of dogs were found, within regard to the tempo of platelet (P = .27) or granulocyte recoveries (P = .21).
Granulocyte and platelet recovery in dogs administered hematopoietic grafts from DLA-nonidentical, unrelated donors followed by either MMF/CSP (n = 19; solid lines) or MTX/CSP (n = 16; interrupted lines).
Granulocyte and platelet recovery in dogs administered hematopoietic grafts from DLA-nonidentical, unrelated donors followed by either MMF/CSP (n = 19; solid lines) or MTX/CSP (n = 16; interrupted lines).
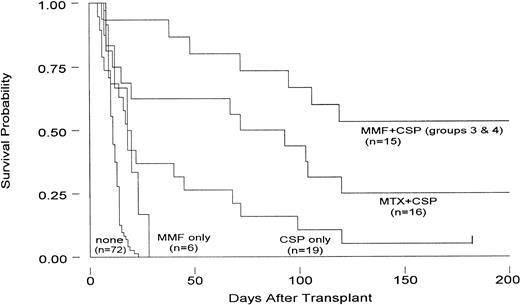
Comparison of current results to those in concurrent and historical controls given either no immunosuppression, CSP alone, or MTX/CSP combined.
Table 4 and Fig 3 summarize the results. Dogs in group 1 given MMF alone survived significantly longer than dogs not given immunosuppression (P = .04).1 25 Dogs in group 2 were not used for statistical comparisons given that the study was prematurely stopped after four dogs were entered owing to the drug toxicities encountered.
Comparison of Survivals in Dogs Administered 9.2 Gy TBI and Hematopoietic Grafts From DLA-Nonidentical Unrelated Donors With or Without Posttransplant Immunosuppression
| Immunosuppression3-150 . | No. of Dogs Studied . | Graft Failure . | Survival Days . | Surviving >200 days . | References . | |
|---|---|---|---|---|---|---|
| Median . | Range . | |||||
| None | 72 | 6 | 11 | 7-23 | 0 | 1, 25 and concurrent |
| CSP3-151 | 19 | 8 | 18 | 4->182 | 1/193-152 | 2 and concurrent |
| MTX + CSP3-153 | 16 | 1 | 88 | 5->372 | 4/16 | 2 and concurrent |
| MMF (Group 1)3-155 | 6 | 2 | 18 | 8-28 | 0 | Current study |
| MMF + CSP (Group 2)¶ | 4 | 0 | 8 | 6-98 | 0 | Current study |
| MMF + CSP (Group 3)# | 9 | 0 | >420 | 7->540 | 5/9 | Current study |
| MMF + CSP (Group 4)3-160 | 6 | 0 | >119 | 38->316 | 3/6 | Current study |
| Immunosuppression3-150 . | No. of Dogs Studied . | Graft Failure . | Survival Days . | Surviving >200 days . | References . | |
|---|---|---|---|---|---|---|
| Median . | Range . | |||||
| None | 72 | 6 | 11 | 7-23 | 0 | 1, 25 and concurrent |
| CSP3-151 | 19 | 8 | 18 | 4->182 | 1/193-152 | 2 and concurrent |
| MTX + CSP3-153 | 16 | 1 | 88 | 5->372 | 4/16 | 2 and concurrent |
| MMF (Group 1)3-155 | 6 | 2 | 18 | 8-28 | 0 | Current study |
| MMF + CSP (Group 2)¶ | 4 | 0 | 8 | 6-98 | 0 | Current study |
| MMF + CSP (Group 3)# | 9 | 0 | >420 | 7->540 | 5/9 | Current study |
| MMF + CSP (Group 4)3-160 | 6 | 0 | >119 | 38->316 | 3/6 | Current study |
Long-term survival (comparison between the regimens by 2-sided log-rank test): Group 1 versus no immunosuppression: P = .04, Groups 3 and 4 versus no immunosuppression: P < .0001, Groups 3 and 4 versus CSP: P = .0002, Groups 3 and 4 versus MTX + CSP:P = .078.
Graft failure (proportions compared using Pearson's χ2test): Groups 2 + 3 + 4 versus CSP only: P < .001.
Results are from historical and concurrent controls and studies.
CSP: Cyclosporine, given at doses of 10-12.5 mg/kg twice daily IM on days 0-7 and by mouth on days 8-25 (n = 13) or 10 mg/kg twice daily IV on days 0-9 and 15 mg/kg twice daily on days 10-27 (n = 6).
One dog was clinically free of GVHD and euthanized at day 182. We assume that dog would have become a long-term survivor.
MTX 0.4 mg/kg/d IV at days 1, 3, 6 and 11 in all dogs. In 10 dogs CSP was given at 7.5 mg/kg twice daily IM on days 0-7 and by mouth on days 8-26; 5 mg/kg twice daily by mouth on days 27-50, 2.5 mg/kg twice daily by mouth on days 51-75 and then every other day 2.5 mg/kg twice daily until day 100. In 6 dogs, CSP was given at 10 mg/kg twice daily IV on days 0-6, 15 mg/kg twice daily by mouth on days 7-45.
MMF 10 mg/kg SC twice daily (20 mg/kg/d) on days 0-27.
¶MMF 10 mg/kg SC twice daily (20 mg/kg/d) on days 0-27, CSP 10 mg/kg IV twice daily (20 mg/kg/d) on days 0-9, 15 mg/kg by mouth twice daily (30 mg/kg/d) on days 10-27.
#MMF 6 mg/kg SC twice daily (12 mg/kg/d) on days 0-2, 10 mg/kg SC twice daily (20 mg/kg/d) on days 3-27; CSP 10 mg/kg IV twice daily (20 mg/kg/d) on days 0-9, 15 mg/kg by mouth twice daily (30 mg/kg/d) on days 10-27.
MMF 6 mg/kg SC twice daily (12 mg/kg/d) on days 0-2, 10 mg/kg SC twice daily (20 mg/kg/d) on days 3-20; CSP 5 mg/kg IV twice daily (10 mg/kg/d) on days 0-5, 15 mg/kg by mouth twice daily (30 mg/kg/d) on days 6-20, 7.5 mg/kg twice daily (15 mg/kg/d) on days 21-45, 5 mg/kg by mouth twice daily (10 mg/kg/d) on days 46-75, 3 mg/kg by mouth twice daily (6 mg/kg/d) on days 76-100.
Survival of dogs administered 9.2 Gy TBI followed by hematopoietic grafts from DLA-nonidentical donors. Dogs were administered either no immunosuppression, MMF alone, CSP alone, MTX+CSP, or MMF+CSP. For details on the immunosuppressive regimens, see Tables 1 and 4.
Dogs in groups 3 and 4 given the combination of MMF/CSP survived significantly better than dogs not given immunosuppression (P< .001). Also, there was a suggestion that their survival was better than that of dogs given MTX/CSP (P = .078). Of note, the addition of MMF to CSP reduced the risk of graft failure in dogs given CSP alone, a finding that was comparable to that previously made with CSP after addition of MTX.2
DISCUSSION
The model used to test the efficacy of immunosuppressive agents in controlling GVHD involves dogs given hematopoietic grafts from DLA-nonidentical unrelated donors. By combining marrow and peripheral blood buffy cells for the transplant, engraftment, and subsequent development of hyperacute and fatal GVHD are the rule,18thus making this a stringent model for studies of GVHD prevention.1
Many single immunosuppressive agents have been tested in the model, and seven drugs—MTX, CSP, FK506, azathioprine, deoxyspergualin, succinylacetone, and corticotropin releasing factor—have led to significant prolongations of recipient survival.1-3,26However, long-term survival after discontinuation of immunosuppression was not seen with any of the drugs except for MTX (<10% of dogs). As seen with most other single agents, dogs treated with MMF alone lived significantly longer than controls, but there were no long-term survivors. Previously, best survivals were seen when MTX was combined with either CSP or FK506.25 We interpreted this to represent synergisms between these agents in regard to controlling GVHD, an assumption that was also supported by subsequent clinical trials.4 5 We failed to observe synergism when combining another antimetabolite, azathioprine, with CSP.27
The current results with a combination of MMF and CSP are encouraging. Eight of 15 dogs studied in groups 3 and 4 that received MMF for 21 to 27 days on a reduced schedule and CSP either for 27 days or for 100 days became long-term survivors, a result that may have been better than that seen with MTX/CSP. Even though all immunosuppression was discontinued, most dogs survived either with no clinical evidence of chronic GVHD or only mild skin disease. However, when dogs were euthanized at the completion of the studies, autopsy findings showed subtle histologic signs of GVHD in various organs. It is unknown whether chronic GVHD would have become clinically manifested had the animals been permitted to live. While no data on synergism between MMF and CSP have been reported for the marrow transplant setting, there is at least one publication showing synergism between the two drugs in a rat hind limb transplant model.28
Two of six dogs given MMF alone experienced graft failure, a figure which was not statistically different from the 53% seen previously in dogs given CSP alone. Apparently, both MMF and CSP when administered alone block the graft enhancing effect of the donor T cells, while not abrogating the host-versus-graft reaction which, in this model, is thought to be effected by large granular lymphocytes with NK function.29 30 Of interest, when the two drugs were combined (groups 2 to 4), no graft failure was seen among 19 dogs studied.
MMF given for 21 to 27 days in the doses applied to dogs in groups 3 and 4 along with CSP did not result in a delay of hematopoietic recovery after transplant over that seen in dogs given a short course of MTX (days 1, 3, 6, 11) along with CSP. Thus, no limiting hematopoietic toxicity of MMF in this setting was seen. The major limiting toxicity of MMF in this model has been gastrointestinal, although it is not clear how much of this toxicity was contributed by CSP. All animals developed some degree of diarrhea after transplant, and three allografted dogs died with intussusception or severe gut hemorrhage. All dogs experienced significant weight loss which, however, corrected itself after discontinuation of the agents. Another problem related to the profound postgrafting immunosuppression has been papilloma virus infection, usually manifested as extensive warts on the dog's paws. This finding has resulted in a papilloma virus vaccination program at FHCRC.
In previous studies, we administered immunosuppressive therapy for approximately 3 months after transplant. This was based on observations in MTX-treated dogs, which showed superior immunosuppression when the drug was administered for an extended period of time. In the current study, we reduced the immunosuppressive therapy to approximately 4 to 5 weeks after transplant in an effort of cost containment, except for dogs in group 4. Comparing the results in dogs of group 3 given MMF and CSP for 28 days with those in group 4 given MMF and CSP for 22 and 101 days, respectively, there was no statistically significant difference in survival. It appears that effective GVHD prevention in this model can be accomplished with MMF and CSP administered for only 4 weeks. Nevertheless, a number of dogs exhibited signs of mild chronic skin GVHD, as well as histologic evidence of chronic GVHD in skin, gut, and liver on autopsy. Presumably, these findings are related to the severe degree of histoincompatibility between current unrelated pairs. Despite the persisting subclinical chronic GVHD, immune functions in long-term survivors were either in the near normal or normal range. As in previous studies in dogs and humans, the T4/T8 ratio was inverted.
In conclusion, MMF was effective in delaying the onset of or completely preventing acute GVHD and prolonging survival in dogs given DLA-nonidentical unrelated hematopoietic grafts. When combined with CSP, slightly more than half of the dogs so treated became long-term survivors, even though both MMF and CSP were discontinued as early as day 27. The limiting toxicities of this dose regimen in dogs were gastrointestinal. The significant synergism between MMF and CSP observed in the current study and confirmed in an independent study on hematopoietic engraftment23 warrants exploration of this drug combination in clinical marrow transplantation.
ACKNOWLEDGMENT
We gratefully acknowledge Dr George Sale for providing pathology reports, Jim Works and Dr John Wagner for their expertise in DLA typing. We would also like to thank Lori Ausburn, Doug Jones, Eric Bell, Alix Smith, Bonnie Larson, Harriet Childs, Dr Barbara Johnston, and the technicians of the hematology/pathology and bacteriology laboratories for their excellent assistance. We also would like to thank Dr Tom Matthews at Roche Bioscience for providing MMF.
Supported in part by Grants No. CA15704, CA31787, HL36444, and DK42716 from the National Institutes of Health, Department of Health and Human Services (DHHS), Bethesda, MD. The laboratory was also supported through a prize from the Josef Steiner Krebsstiftung, Bern, Switzerland, awarded to R.S.
Presented in part at the 37th Annual Meeting of the American Society of Hematology, Seattle, WA, December 1-5, 1995.
Address reprint requests to Cong Yu, MD, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, D1-100, PO Box 19024, Seattle, WA 98109-1024.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal