Abstract
In genetic hemochromatosis (GH), iron overload affects mainly parenchymal cells, whereas little iron is found in reticuloendothelial (RE) cells. We previously found that RE cells from GH patients had an inappropriately high activity of iron regulatory protein (IRP), the key regulator of intracellular iron homeostasis. Elevated IRP should reflect a reduction of the iron pool, possibly because of a failure to retain iron. A defect in iron handling by RE cells that results in a lack of feedback regulation of intestinal absorption might be the basic abnormality in GH. To further investigate the capacity of iron retention in RE cells of GH patients, we used inflammation as a model system as it is characterized by a block of iron release from macrophages. We analyzed the iron status of RE cells by assaying IRP activity and ferritin content after 4, 8, and 24 hours of incubation with lipopolysaccharide (LPS) and interferon-γ (IFN-γ). RNA-bandshift assays showed that in monocytes and macrophages from 16 control subjects, IRP activity was transiently elevated 4 hours after treatment with LPS and IFN-γ but remarkably downregulated thereafter. Treatment with NO donors produced the same effects whereas an inducible Nitric Oxide Synthase (iNOS) inhibitor prevented them, which suggests that the NO pathway was involved. Decreased IRP activity was also found in monocytes from eight patients with inflammation. Interestingly, no late decrease of IRP activity was detected in cytokine-treated RE cells from 12 GH patients. Ferritin content was increased 24 hours after treatment in monocytes from normal subjects but not in monocytes from GH patients. The lack of downregulation of IRP activity under inflammatory conditions seems to confirm that the control of iron release from RE cells is defective in GH.
IN RECENT years considerable advances have been made in the knowledge of the molecular events regulating iron delivery to cells by transferrin. Most cells acquire iron through receptor-mediated internalization of iron-laden transferrin and then either use iron for metabolic needs or store excess metal in ferritin.1 Intracellular iron homeostasis is therefore maintained through regulation of ferritin and transferrin receptor synthesis in a coordinated and opposite manner.2 This is achieved by two cytoplasmic proteins, iron regulatory proteins 1 and 2 (IRP-1 and IRP-2), which bind in an iron-dependent way to iron responsive elements (IRE) in untranslated regions of ferritin and transferrin receptor mRNA. IRP-1 has two mutually exclusive functions that are switched by changes in a 4Fe-4S cluster. Under conditions of iron deficiency in the cellular labile iron pool (LIP), the cluster is disassembled, and IRP-1 binds to IRE and decreases the synthesis of ferritin but enhances that of transferrin receptor, thus providing the cell with readily available iron. Conversely, when iron is abundant, the cluster is reconstituted, IRP-1 dissociates from IRE and thus acquires aconitase function, and iron sequestration prevails over iron uptake.3-5 IRP-2 controls the expression of ferritin and transferrin receptor mRNAs with a specificity and efficacy similar to those of IRP-1, but it lacks an iron sulfur cluster, is regulated by iron through proteolysis, and is expressed differently in various tissues.5,6 In addition, IRP-2 is modulated differentially under some pathophysiological situations.7-10 According to the above notions, it can easily be seen that IRP is the main and most reliable indicator of cellular iron status even though factors other than iron levels themselves, such as bioradicals, can directly or indirectly influence IRP activity.8-12
In contrast to the improvements in molecular analysis of the regulatory pathways of cellular iron acquisition and storage, little progress has been made in elucidating the mechanisms underlying iron egress from the cell.13 Knowledge of how iron release is controlled is particularly important for the understanding of iron metabolism in reticuloendothelial (RE) cells, which process more than 80% of the iron entering the plasma each day and thus represent a fundamental compartment in systemic iron metabolism.14 Furthermore, the abnormalities in RE iron metabolism that appear in certain diseases seem related to alterations in iron extrusion from the cell. Indeed, the hypoferremia that accompanies chronic inflammation and anemia of chronic disease (ACD) is mainly caused by enhanced iron retention in RE cells,15 even though the molecular events responsible for the block in the release of iron from macrophages are not completely understood.16 Alterations of RE iron metabolism are also present in genetic hemochromatosis (GH), a common inherited disorder of iron metabolism characterized by unregulated iron absorption.17 In fact, both in GH patients and in β2 microglobulin knockout mice representing a rodent model of hemochromatosis,18 the metal accumulates preferentially in parenchymal cells, with little iron stored in RE cells until late in the disease.19,20 The pathogenetic biochemical defect of GH is unknown, and even the identification of a strong candidate gene (HFE) encoding an HLA-like protein21 with a peculiar pattern of expression in the gastrointestinal tract22 has given no clues to the underlying metabolic derangement. A defect in iron handling by RE cells that causes both excess deposition in parenchymal cells and lack of feedback regulation of intestinal absorption might be the basic abnormality in GH. Indeed, our demonstration of an inappropriately high IRP activity in RE cells from GH patients suggested a low iron level in the LIP, possibly caused by enhanced iron release.23 The latter observation constituted molecular evidence to support previous results showing inappropriately high rates of iron release in patients with GH24 and therefore supported the idea that defective iron handling by the RE compartment is critical for the development of this disease.
In the present study, we used inflammation, which is characterized by a block of iron release from macrophages,13 15 as a model system to further investigate the capacity of iron retention in RE cells of GH patients. We analyzed the iron status of RE cells from both control subjects and GH patients by assaying IRP activity and ferritin content in monocytes and monocyte-derived macrophages after treatment with inflammatory agents.
MATERIALS AND METHODS
Reagents.
RPMI 1640, minimum essential medium (MEM), and fetal calf serum (FCS) were purchased from ICN Biomedicals (Opera, Milano, Italy); Ficoll-Paque and Percoll from Pharmacia Biotech (Cologno Monzese, Milano, Italy); Dynabeads M-450 and anti-human CD14 antibody from Unipath (Garbagnate, Milano, Italy); Enzymun-test for serum ferritin immunoassay from Boehringer Mannheim (Milano, Italy); Magic-Fer radioimmunoassay kit for ferritin from Ciba Corning (Cassina de' Pecchi, Milano, Italy); human recombinant IFN-γ (Imukin) from Boehringer Ingelheim (Firenze, Italy); Desferrioxamine, NG-Monomethyl-L-arginine monoacetate (NMMA), S-nitroso-N-acetyl-D,L-penicillamine (SNAP), N-acetyl-D,L-penicillamine (NAP) and lipopolysaccharide fromEscherichia coli serotype 0111:B4 (LPS) from Sigma Chemical Co (Milano, Italy); and antiserum to mouse macrophage inducible Nitric Oxide Synthase (iNOS) from Alexis Corp (Inalco SpA, Milano, Italy). The kit for tumor necrosis factor-α (TNF-α) determination was supplied by Genzyme srl. (Cinisello B, Italy) and Hybond membranes, ECL Plus, and α-32P UTP by Amersham Co (Milano, Italy).
Subjects.
Thirty-nine subjects were studied, and their iron indexes are reported in Table 1. Informed consent was obtained from them all, and the study protocol was approved by the Ethics Committee of the University of Milano.
Iron Indexes of Study Groups
| . | No. . | Hb (g/dL) . | Serum Iron (μg/dL) . | Transferrin Saturation (%) . | Serum Ferritin (μg/L) . |
|---|---|---|---|---|---|
| Control | 16 | 14 ± 1 | 90 ± 26 | 19 ± 6 | 19 ± 6 |
| GH | 12 | 14 ± 1 | 166 ± 47-151 | 67 ± 25-150 | 1478 ± 972-150 |
| SH | 3 | 12 ± 2 | 154 ± 5-151 | 56 ± 2-150 | 1046 ± 795-150 |
| Inflammation | 8 | 12 ± 1 | 37 ± 6-150 | 12 ± 6 | 287 ± 279-150 |
| . | No. . | Hb (g/dL) . | Serum Iron (μg/dL) . | Transferrin Saturation (%) . | Serum Ferritin (μg/L) . |
|---|---|---|---|---|---|
| Control | 16 | 14 ± 1 | 90 ± 26 | 19 ± 6 | 19 ± 6 |
| GH | 12 | 14 ± 1 | 166 ± 47-151 | 67 ± 25-150 | 1478 ± 972-150 |
| SH | 3 | 12 ± 2 | 154 ± 5-151 | 56 ± 2-150 | 1046 ± 795-150 |
| Inflammation | 8 | 12 ± 1 | 37 ± 6-150 | 12 ± 6 | 287 ± 279-150 |
Values are mean ± SD.
P < .001 versus controls.
P < .05 versus controls.
The control group consisted of 16 healthy blood donors (11 men and 5 women, age range 26 to 64 years) without a clinical history of disorders of iron metabolism and with normal serum iron indexes.
The GH group consisted of 12 patients (10 men and 2 women, age range 32 to 63 years), 8 untreated and 4 on a phlebotomy program. The diagnosis of GH was established according to standard criteria as previously reported.25 Nine subjects were homozygous for the major C282Y mutation in the HFE gene, and 3 were negative. Removal of excess body iron was defined as normalization of serum iron indexes.23
The secondary hemochromatosis (SH) group consisted of 3 patients (all men, age range 28 to 53 years), 2 with thalassemia intermedia and 1 with alcoholic liver disease. None of them were undergoing phlebotomy therapy at the time of the study.
The inflammation group consisted of 8 patients (6 men and 2 women, age range 23 to 57 years) with inflammation secondary to inflammatory bowel disease in 5 and pneumonia in 3. Serum inflammatory indexes were as follows: white blood cell count (11,287 ± 2,606/μL), erythrocyte sedimentation rate (64 ± 22), C-reactive protein concentration (8 ± 5 mg/dL), mucoprotein concentration (181 ± 31 mg/dL), and fibrinogen level (561 ± 130 mg/dL).
All the subjects except 3 GH patients were unrelated.
Biochemical evaluation.
Serum iron, total iron binding capacity, and transferrin saturation index were determined by standard techniques as previously reported.25 Serum ferritin was measured by an enzyme immunoassay. Hepatic iron stores were evaluated and graded microscopically (0 to 4) by two independent observers and chemically by atomic absorption spectrophotometry as described previously.25
Serum inflammatory indexes were measured by standard techniques.
Isolation, culture, and treatment of monocytes.
Monocytes were purified as already described.23 Buffy coats were prepared from venous heparinized blood, and mononuclear cells were separated on Ficoll-Paque solution. Monocytes were then separated from lymphocytes by density gradient centrifugation on a solution composed of RPMI 1640 medium (54%) and 285 mosm Percoll (46%). Yield, purity, viability, and recovery of monocytes were as previously reported.23 The cells were either pelletted and stored at −80°C in aliquots or cultured (see below). Monocytes from patients with inflammation were separated from 50 mL of blood using magnetic beads coated with anti-human CD14 antibody.26Purity and viability of monocytes were similar as above.23For culturing, monocytes were resuspended in RPMI 1640 medium containing 2 mmol/L glutamine, antibiotics, and 20% heat-inactivated human serum and kept in 5% CO2 at 37°C.23Medium and all reagents were endotoxin-free.
To induce differentiation to macrophages, monocytes (approximately 1.5 × 106 cells) were maintained in culture for 6 days. Both monocytes and macrophages were stimulated for different time periods (4, 8, and 24 hours) with 1 μg/mL LPS plus 100 U/mL IFN-γ in the presence and absence of 250 μmol/L NMMA and 50 μmol/L DFO. Cells were also exposed to 0.5 mmol/L SNAP or NAP for various time periods as reported above. At the end of the treatments cells were obtained, pelletted, and stored at −80°C. J774 mouse macrophage cells were grown in MEM supplemented with 10% heat-inactivated FCS, 2 mmol/L glutamine, 100 U/mL penicillin, and 0.1 ng/mL streptomycin at 37°C in 5% CO2 and treated with cytokines as described above.
Tumor necrosis factor-α (TNF-α) concentration was measured in cell supernatants using a specific enzyme-linked immunoassay according to the manufacturer's instructions.
In vitro RNA transcription.
The pSPT-fer plasmid containing the IRE of the human ferritin H chain27 was linearized with BamHI and transcribed in vitro with T7 RNA polymerase in the presence of 100 μCi of (α-32P) UTP (800 Ci/mmol).
RNA-protein bandshift assay.
Cells were lysed in the buffer described by Leibold and Munro,28 the lysate was centrifuged at 16,000g for 5 minutes, and the supernatant was used for RNA-protein bandshift assays. Equal amounts of protein (2 μg as determined using the Bio-Rad [Segrate, Milano, Italy] protein assay kit) were incubated with a molar excess of IRE probe in the absence and presence of 2% 2-mercaptoethanol and treated sequentially with RNase T1 and heparin as already described.7 After separation on 6% nondenaturing polyacrylamide gels, RNA-protein complexes were visualized by autoradiography. For quantitation of IRP activity, radioactivity of bands excised from dried gels was determined by liquid scintillation counting.29
Determination of ferritin content.
Ferritin intracellular concentration was determined in aliquots of the cytoplasmic extracts used for bandshift assays using a radioimmunoassay kit (Magic-Fer, Ciba Corning) based on an anti-human liver ferritin antibody.
Western blot analysis.
Aliquots of the cytosolic extracts used for the determination of IRP activity containing equal amounts of proteins were electrophoresed in 10% acrylamide-sodium dodecyl sulfate (SDS) gels, electroblotted to Hybond membranes, and incubated with anti-serum to mouse iNOS. iNOS was detected by chemiluminescence using an immunodetection kit (ECL Plus, Amersham Co) according to instructions.
Statistical analysis.
Values are expressed as means ± SD. The significance of differences was evaluated with the t test using the Stat View 4.0 program (Abacus Concept Inc, Berkeley, CA).
RESULTS
Time course of IRP activity in monocytes and monocyte-derived macrophages from control subjects stimulated with LPS/IFN-γ.
To investigate the response of iron metabolism to inflammatory stimuli in human cells of the macrophage lineage, we assessed IRP activity in extracts of cytokine-stimulated RE cells. A representative RNA-bandshift assay is shown in Fig 1A, and Table 2 summarizes the results of all experiments; despite some variability from patient to patient, the trend was similar in all individuals. In monocytes from control subjects stimulated with LPS/IFN-γ, IRP activity rose transiently (1.5-fold) at 4 hours, returned to control levels at 8 hours, and was clearly downregulated at 24 hours. Incubation for the same periods of time in the absence of cytokines did not significantly alter IRP activity (Fig 1B). Macrophages derived from monocytes after 6 days of culture had higher IRP binding activity23 but showed the same pattern of response to cytokine stimulation (Fig 1C). Treatment of extracts with 2% 2-mercaptoethanol fully activated IRE binding activity, indicating that the effect of cytokines was mediated by a post-translational switch (Fig 1, lower panels). As previously reported,23 the small remaining differences in total IRP activity are possibly caused by the presence in the IRE-IRP complex of IRP-2, which cannot be separated from IRP-1 in extracts of human cells and which is not sensitive to reducing agents.5,6 The production of TNF-α, an indicator of inflammatory response,30 was consistently enhanced after cytokine stimulation.
Time course of IRP activity in monocytes and monocyte-derived macrophages from control subjects treated with LPs/IFN-γ. Monocytes from healthy blood donors were cultured in RPMI 1640 medium containing 20% autologous serum for 4, 8, and 24 hours in the presence (A) and absence (B) of cytokines (100 U/mL IFN-γ + 1 μg/mL LPS). Cells were also maintained in culture for 6 days to allow differentiation to macrophages and were then treated with cytokines for the same time periods (C). Cytoplasmic extracts (2 μg protein) were analyzed for IRE-binding activity by RNA-bandshift assay with excess32P-labeled IRE probe in the absence (upper panels) and presence (lower panels) of 2% 2-mercaptoethanol. TNF-α production was assayed in the culture medium by enzyme-linked immunoassay. After a transient increase, IRP activity returned to that of untreated cells and was eventually downregulated at 24 hours after stimulation with LPS/IFN-γ in both monocytes and monocyte-derived macrophages.
Time course of IRP activity in monocytes and monocyte-derived macrophages from control subjects treated with LPs/IFN-γ. Monocytes from healthy blood donors were cultured in RPMI 1640 medium containing 20% autologous serum for 4, 8, and 24 hours in the presence (A) and absence (B) of cytokines (100 U/mL IFN-γ + 1 μg/mL LPS). Cells were also maintained in culture for 6 days to allow differentiation to macrophages and were then treated with cytokines for the same time periods (C). Cytoplasmic extracts (2 μg protein) were analyzed for IRE-binding activity by RNA-bandshift assay with excess32P-labeled IRE probe in the absence (upper panels) and presence (lower panels) of 2% 2-mercaptoethanol. TNF-α production was assayed in the culture medium by enzyme-linked immunoassay. After a transient increase, IRP activity returned to that of untreated cells and was eventually downregulated at 24 hours after stimulation with LPS/IFN-γ in both monocytes and monocyte-derived macrophages.
IRP Activity and Ferritin Content in Monocytes From Controls and GH Patients Treated With Cytokines
| . | Medium . | LPS/IFN-γ . | LPS/IFN-γ/NMMA . | SNAP . | LPS/IFN-γ/DFO . |
|---|---|---|---|---|---|
| Control subjects (n = 16) | |||||
| IRP (%) | 100 | 47 ± 12* | 94 ± 16 | 43 ± 9* | 146 ± 21* |
| Ft content (ng/mg prot.) | 620 ± 257 | 798 ± 232† | 667 ± 321 | 803 ± 221† | 482 ± 52† |
| GH patients (n = 12) | |||||
| IRP (%) | 100 | 93 ± 16 | 97 ± 14 | 91 ± 11 | 153 ± 19* |
| Ft content (ng/mg prot.) | 1023 ± 627 | 1124 ± 735 | 1107 ± 642 | 1078 ± 756 | 651 ± 32* |
| . | Medium . | LPS/IFN-γ . | LPS/IFN-γ/NMMA . | SNAP . | LPS/IFN-γ/DFO . |
|---|---|---|---|---|---|
| Control subjects (n = 16) | |||||
| IRP (%) | 100 | 47 ± 12* | 94 ± 16 | 43 ± 9* | 146 ± 21* |
| Ft content (ng/mg prot.) | 620 ± 257 | 798 ± 232† | 667 ± 321 | 803 ± 221† | 482 ± 52† |
| GH patients (n = 12) | |||||
| IRP (%) | 100 | 93 ± 16 | 97 ± 14 | 91 ± 11 | 153 ± 19* |
| Ft content (ng/mg prot.) | 1023 ± 627 | 1124 ± 735 | 1107 ± 642 | 1078 ± 756 | 651 ± 32* |
Monocytes from control subjects and GH patients were left untreated (medium) or treated for 24 hours with 100 U/mL IFN-γ plus 1 μg/mL LPS, in the presence and absence of 0.1 mmol/L NMMA and 0.05 mmol/L Desferrioxamine (DFO). Cells were also incubated with 0.5 mmol/L SNAP for 24 hours. IRP activity was quantitated by liquid scintillation counting. Ferritin content was measured by a radioimmunoassay based on antihuman liver ferritin antibody. Values are given as mean ± SD.
P < .001 versus controls.
P < .05 versus controls.
Involvement of the iNOS pathway in the modulation of IRP activity in immunostimulated monocytes from control subjects.
A functional interaction between iron and NO has been shown16 with modulation of IRP activity by NO.8,10,11 Moreover, LPS and IFN-γ synergize to induce NO synthesis.31 Therefore, as NO production by human RE cells is virtually undetectable by chemical determination of NO metabolites in the medium, whereas induction of iNOS expression has been documented,32 we evaluated iNOS induction by Western blot analysis to assess the role of the iNOS pathway in the changes of IRP activity reported above. Immunoblot assay of human monocyte proteins with anti-iNOS antibody (Fig 2) revealed induction of a band of approximately 130 kD molecular mass in cytokine treated cells. This band comigrated with immunoreactive material in extracts of activated mouse J774 macrophages, although in much smaller amount. To further investigate the role of the iNOS pathway, we treated RE cells with LPS/IFN-γ in the presence of NMMA, an iNOS inhibitor. In this condition, the changes of IRE-binding activity triggered by cytokine treatment were largely prevented in both monocytes (Fig3A and Table 2) and monocyte-derived macrophages (data not shown) from control subjects. IRP activity was not appreciably modified by incubation in the presence of NMMA alone (Fig 3B). Continuing our assessment of the involvement of NO in the modulation of IRP activity, we treated monocytes with the NO donor SNAP. The addition of SNAP, but not of its inactive non-nitrosylated counterpart NAP (data not shown), profoundly affected IRP activity with a time course similar to that obtained during treatment with LPS/IFN-γ (Fig 3C and Table 2). Comparable results were obtained with monocyte-derived macrophages (data not shown). Treating RE cells with cytokines for 24 hours in the presence of the iron chelator DFO prevented the decrease of IRP activity, suggesting that cytokines and NO may act indirectly by increasing iron availability (Table2).
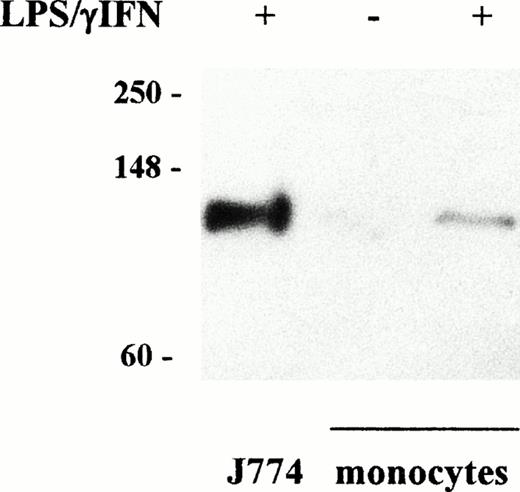
Induction of iNOS accumulation in LPS/IFN-γ–treated monocytes. Cytosolic extracts were prepared from untreated monocytes and from monocytes and murine J774 macrophages treated with cytokines for 24 hours as described in Fig 1. Proteins (100 μg) of treated and untreated monocytes and 25 μg of J774 cells were analyzed on SDS 10% polyacrylamide gels and blotted to filters that were incubated with primary (anti-iNOS, 1:500 dilution) and secondary antibody as described in Materials and Methods. Bands were visualized by chemiluminescence. Migration of molecular mass markers (myosin, phosphorylase B, and glutamic dehydrogenase, 250, 148, and 60 kD, respectively) loaded on the same gel is shown on the left. Similar results were obtained in all the experiments on cytokine stimulation of both monocytes and monocyte-derived macrophages. Accumulation of iNOS was detected in human monocytes after LPS/IFN-γ stimulation, although to a lower extent than in mouse J774 macrophages.
Induction of iNOS accumulation in LPS/IFN-γ–treated monocytes. Cytosolic extracts were prepared from untreated monocytes and from monocytes and murine J774 macrophages treated with cytokines for 24 hours as described in Fig 1. Proteins (100 μg) of treated and untreated monocytes and 25 μg of J774 cells were analyzed on SDS 10% polyacrylamide gels and blotted to filters that were incubated with primary (anti-iNOS, 1:500 dilution) and secondary antibody as described in Materials and Methods. Bands were visualized by chemiluminescence. Migration of molecular mass markers (myosin, phosphorylase B, and glutamic dehydrogenase, 250, 148, and 60 kD, respectively) loaded on the same gel is shown on the left. Similar results were obtained in all the experiments on cytokine stimulation of both monocytes and monocyte-derived macrophages. Accumulation of iNOS was detected in human monocytes after LPS/IFN-γ stimulation, although to a lower extent than in mouse J774 macrophages.
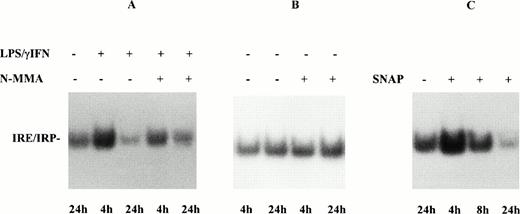
Effect of NO on IRP activity in monocytes from control subjects. (A) Monocytes of control subjects were treated with 100 U/mL IFN-γ plus 1 μg/mL LPS for 4 and 24 hours, in the presence and absence of 0.1 mmol/L NMMA. IRP activity and TNF-α were determined as described in Fig 1. (B) Monocytes of control subjects were incubated for 4 and 24 hours in the presence and absence of 0.1 mmol/L NMMA. (C) Monocytes of control subjects were treated with 0.5 mmol/L SNAP for 4, 8, and 24 hours. Lysates were assayed for IRP activity as described in Fig 1. The results of treatment with the iNOS inhibitor NMMA and with the NO donor SNAP indicated a role for NO in the modulation of monocyte IRP activity by cytokines.
Effect of NO on IRP activity in monocytes from control subjects. (A) Monocytes of control subjects were treated with 100 U/mL IFN-γ plus 1 μg/mL LPS for 4 and 24 hours, in the presence and absence of 0.1 mmol/L NMMA. IRP activity and TNF-α were determined as described in Fig 1. (B) Monocytes of control subjects were incubated for 4 and 24 hours in the presence and absence of 0.1 mmol/L NMMA. (C) Monocytes of control subjects were treated with 0.5 mmol/L SNAP for 4, 8, and 24 hours. Lysates were assayed for IRP activity as described in Fig 1. The results of treatment with the iNOS inhibitor NMMA and with the NO donor SNAP indicated a role for NO in the modulation of monocyte IRP activity by cytokines.
Effect of cytokine stimulation on ferritin content of monocytes from control subjects.
To investigate whether ferritin content inversely reflects variations of IRP activity, as predicted by the model of IRP-regulated cellular iron metabolism, we measured intracellular ferritin concentration in monocytes at various times after treatment with LPS/IFN-γ. At 4 hours no appreciable effects on ferritin content were evident (data not shown). On the contrary, the amount of ferritin in RE cells, in spite of some variability among subjects, was significantly enhanced by cytokine treatment at 24 hours, concomitantly with the downregulation of IRP activity (Table 2). This indicated that the effect of in vitro treatment of monocytes and monocyte-derived macrophages with LPS/IFN-γ mirrors the retention of iron in RE cells, which has been reported to occur under inflammatory conditions in vivo.15
IRP activity in monocytes from patients with inflammation.
To confirm that observations made in vitro after treating cells with LPS/IFN-γ correctly mimicked an in vivo inflammatory status, we analyzed IRP activity in monocytes purified from patients with various diseases causing acute and chronic inflammation (Table 1). Figure 4 shows a representative bandshift assay demonstrating that in subjects with inflammation, with serum iron indexes compatible with enhanced iron retention in RE cells, IRP activity was consistently lower than in control subjects and only slightly higher than in patients with SH, a condition associated with RE iron overload.
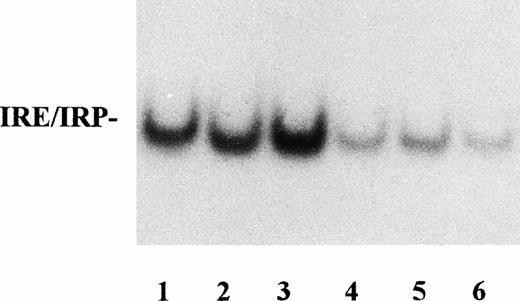
IRP activity in monocytes from control subjects and patients with SH or inflammation. Lysates of monocytes from control subjects (lanes 1 through 3), patients with inflammation disorders (lanes 4 and 5), and patients with SH (lane 6) were assayed for IRP activity as described in Fig 1. IRP activity was lower in patients with inflammation than in control subjects and was comparable with that of patients with SH.
IRP activity in monocytes from control subjects and patients with SH or inflammation. Lysates of monocytes from control subjects (lanes 1 through 3), patients with inflammation disorders (lanes 4 and 5), and patients with SH (lane 6) were assayed for IRP activity as described in Fig 1. IRP activity was lower in patients with inflammation than in control subjects and was comparable with that of patients with SH.
IRP activity and ferritin content in immunostimulated monocytes from GH patients.
Having established the effect of cytokine stimulation on IRP activity in RE cells from control subjects, we investigated the response in GH patients. As shown by a typical bandshift reported in Fig 5A, IRP activity in monocytes from GH patients stimulated with LPS/IFN-γ rose transiently at 4 hours but was not downregulated at 24 hours when it returned only to the level of unstimulated samples (Fig 5A). As previously shown for monocytes from control subjects (Fig 1B), incubation of GH monocytes without cytokines for up to 24 hours did not affect IRP activity (Fig 5B). Quantitation of the results obtained in all the patients showed that, in contrast with the findings in controls, IRE binding activity was not significantly altered by incubation with cytokines for 24 hours (Table2). Similar results were observed when cells were treated with SNAP (Table 2). On the contrary, IRP downregulation at 24 hours was detected in cells from SH patients with a tissue iron burden equivalent to that of GH patients (Fig 5C). TNF-α production stimulated by LPS/IFN-γ was similar in all the groups of subjects studied, thus indicating that other indexes of inflammatory response were not impaired in GH subjects. A similar pattern was also obtained when monocyte-derived macrophages from GH patients were examined (data not shown). No significant differences were detected either between untreated and phlebotomy-treated patients or between patients positive and negative for the C282Y mutation in the HFE gene (data not shown). In agreement with the lack of changes of IRP activity, ferritin concentration in monocytes from GH patients did not vary appreciably after treatment with cytokines or SNAP (Table 2).
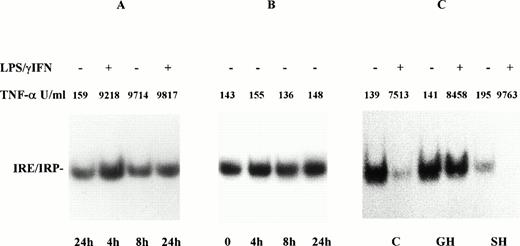
Effect of LPS/IFN-γ on IRP activity in monocytes from GH patients. Monocytes of GH patients were incubated for 4, 8, and 24 hours in the presence (A) and in absence (B) of cytokines (100 U/mL IFN-γ + 1 μg/mL LPS). Monocytes of control subjects (C), patients with GH, and secondary hemochromatosis (SH) were treated with LPS/IFN-γ for 24 hours (C). IRP activity and TNF-α were determined as described in Fig 1. IRP activity in monocytes from patients with GH rose transiently 4 hours after stimulation with LPS/IFN-γ but was not downregulated at 24 hours. On the contrary, in cells from SH patients IRP downregulation was observed at 24 hours.
Effect of LPS/IFN-γ on IRP activity in monocytes from GH patients. Monocytes of GH patients were incubated for 4, 8, and 24 hours in the presence (A) and in absence (B) of cytokines (100 U/mL IFN-γ + 1 μg/mL LPS). Monocytes of control subjects (C), patients with GH, and secondary hemochromatosis (SH) were treated with LPS/IFN-γ for 24 hours (C). IRP activity and TNF-α were determined as described in Fig 1. IRP activity in monocytes from patients with GH rose transiently 4 hours after stimulation with LPS/IFN-γ but was not downregulated at 24 hours. On the contrary, in cells from SH patients IRP downregulation was observed at 24 hours.
DISCUSSION
The role of cells of the macrophage lineage in the systemic changes of iron metabolism that accompany inflammatory states is pivotal. Indeed, low serum iron levels in chronic inflammation are in part caused by decreased iron absorption in the gut but are mainly caused by enhanced iron retention in RE cells.15 However, the molecular events responsible for the block in the release of iron from macrophages are not completely understood. Moreover, the effects of inflammatory agents such as cytokines and NO on iron metabolism have primarily been studied in murine cell lines, and data on human RE cells are lacking.16 Analysis of the regulation of macrophage iron traffic could also give insights into the mechanisms underlying the defective control of iron retention by RE cells in GH,23,24,33 34 which could represent the basic defect of this disorder. In the present study we assessed the effects of inflammatory stimuli on the regulatory mechanisms of iron metabolism in human monocytes and macrophages by assaying the activity of IRP, the key regulator of cellular iron homeostasis. We found that the main effect of inflammation was downregulation of IRP accompanied by increased iron retention, as shown by greater ferritin content. We also obtained evidence for a lack of response in GH patients.
A link between NO and iron metabolism exists16 and, in particular, stimulation of IRP binding activity by NO has been observed in various cellular systems,10,11,35,36 including RE cells,37-39 but how NO-mediated activation of IRP in murine macrophagic cell lines and mouse primary macrophages correlates with alterations of systemic iron traffic occurring in human chronic inflammatory disease is not clear. In fact, one would expect enhanced IRP binding activity to result in reduced ferritin expression and, in turn, in decreased iron storage capacity, a picture that is not consistent with the recognized enhancement of iron retention in RE cells under inflammatory conditions.13,15,16 This study showed that in human RE cells from a large number of control subjects, stimulation with inflammatory cytokines caused only a transient early increase in IRP activity, whereas a marked downregulation was observed at later times of treatment. Experiments with an iNOS inhibitor and a NO donor seem to suggest that modulation of IRP activity is NO dependent. The decrease of IRP activity reported here, which was accompanied by increased ferritin content, fits better with the enhanced iron retention that is the physiologic effect of cytokine action on iron metabolism in RE cells.13,15,16 The conclusion that IRP downregulation by in vitro treatment with cytokines is truly representative of an in vivo inflammatory condition is strongly supported by the finding that IRP activity is severely depressed in monocytes from patients with inflammation. Chronic inflammation may lead to ACD in which iron availability to erythroid precursors for hemoglobin synthesis is restricted despite normal total body iron content.15 Evidence that NO has a role in the overall alterations of iron metabolism present in ACD has recently been suggested by studies on K562 cells.36,40 41 However, it is still not fully understood how derangements triggered by NO might lead to reduced iron utilization by the bone marrow. The finding of reduced IRP activity in monocytes from patients with inflammation provides a novel insight into the molecular mechanisms underlying enhanced iron sequestration in the RE system and hence the reduced iron availability for hematopoiesis in ACD.
As to the mechanistic aspects of the action of cytokines on IRP, cytokine-mediated transcriptional induction42 could trigger ferritin synthesis which, in turn, could cause sequestration of iron from the labile pool into ferritin and thus increase IRP activity at 4 hours. However, as we did not detect increased ferritin accumulation at 4 hours of treatment, this interpretation seems unlikely. Alternatively, the early activation of IRP may depend on direct interaction of NO with the 4Fe-4S cluster.43 On the other hand, the slow, iron dependent-like kinetics of inactivation at later times suggests that NO may act indirectly by increasing iron availability in the LIP. IRP downregulation is likely to be the expression of an expansion of the iron pool; indeed, we showed that the fall of IRP activity is prevented by treatment with an iron chelator and that ferritin accumulation is enhanced by cytokine treatment.
A complex and sequential interplay between NO and iron involving IRP-mediated modulation of transferrin receptor expression has been invoked to account for enhanced intracellular iron levels in the presence of increased IRP activity.16 A number of studies44 have been performed on the regulation of transferrin receptor in activated macrophages, but the results were frequently discordant. However, in our opinion transferrin receptor-mediated iron uptake may be of limited importance in RE cells. In fact, erythrophagocytosis is the most important way for these cells to acquire iron, as also shown by the relatively low number of receptors present on the surface of macrophages.44 Thus, in an alternative model, enhanced iron accumulation, and hence IRP downregulation, could instead be caused by the block of metal release previously described in stimulated murine macrophages13 and in patients with inflammation.24 The finding that both NMMA and SNAP can, in opposite ways, interact with this process seems to indicate that the poorly characterized mechanisms of iron release from the cell are under the control of the NO pathway.
The present results also show that RE cells of GH patients do not downregulate IRP activity after cytokine treatment. The lack of response does not seem to be caused by an impaired general response to inflammation, as shown by the observation that TNF-α levels in supernatants of mononuclear-phagocytes from GH subjects increased after stimulation with LPS/IFN-γ (Fig 5A). Moreover, in contrast to the previously reported low concentration of TNF-α in LPS-treated monocytes from GH patients,45 we did not find significant differences in TNF-α production between GH subjects and healthy controls after LPS/IFN-γ stimulation. If, as discussed above, inflammatory stimuli cause the fall of IRP activity by increasing iron levels in the LIP, the lack of downregulation in GH patients could be caused by a reduced or no expansion of the pool. Only these iron-dependent mechanisms of control of IRP seem altered in GH; in fact, the NO-mediated enhancement of IRP activity at 4 hours, which may depend on direct interaction of NO with the cluster, is maintained also in GH patients. A further indication of the impaired capacity of RE cells of GH patients to retain iron in response to inflammation is provided by the lack of enhanced ferritin accumulation in response to cytokines. High levels of intracellular iron, which could act as an NO scavenger, should not be responsible for the lack of response in RE cells of GH subjects, as a remarkable downregulation of IRP activity was observed in monocytes from SH patients with an iron burden similar to that of GH patients. Moreover, IRP remained insensitive to cytokine or SNAP treatment also in RE cells from iron-depleted GH patients.
Analysis of the activation state of the IRP, the most reliable indicator of intracellular iron status, showed that the LIP is inappropriately reduced in RE cells from GH patients.23This could be because of abnormally high rates of iron release, as shown by radioiron kinetics experiments.24 The present data indicate that the capacity of RE cells of GH patients to retain iron is impaired not only under normal conditions but even in response to a stimulus that induces a block of iron release, such as inflammation. In this regard, the localization of HFE, the product of the recently identified GH gene, on the basolateral membrane in most cell types22 suggests that this protein, possibly in cooperation with other members of the Nramp family of iron-transporting proteins ,46 47 may play a role in controlling the poorly characterized mechanisms underlying iron release from the cell. In GH, lack of cell-surface presentation of HFE21,48 could thus contribute to the reduced iron storage capacity in cells of the RE system. This will eventually result in lack of feedback regulation of intestinal iron absorption and in high levels of circulating nontransferrin-bound iron. The latter is avidly taken up by parenchymal cells and iron overload and tissue damage ensue.
ACKNOWLEDGMENT
We thank F. Ravagnani for providing buffy coats of control subjects, D. Taramelli for determination of TNF-α, L. Kuhn for the gift of the pSPT-Fer plasmid, and A. Pietrangelo for reading the manuscript and helpful discussion.
Supported by grants from Ministero Università e Ricerca Scientifica e Tecnologica (MURST), Consiglio Nazionale delle Ricerche (CNR), and from IRCCS Ospedale Maggiore, Milano, Italy.
Address reprint requests to Dr Gaetano Cairo, Centro di Studio sulla Patologia Cellulare CNR, Via Mangiagalli 31, 20133 Milano, Italy.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal