Abstract
Eosinophils, prominent cells in asthmatic inflammation, have been shown to synthesize, store, and release an array of up to 18 cytokines and growth factors, including interleukin-6 (IL-6). In this report, we show that IL-6 immunofluorescence localizes to the matrix of the crystalloid granule in peripheral blood eosinophils from atopic asthmatics using confocal laser scanning microscopy (CLSM). Granule localization of IL-6 was confirmed using dot-blot analysis and enzyme-linked immunosorbent assay (ELISA) on subcellular fractions of highly purified eosinophils produced from density centrifugation across a 0% to 45% Nycodenz gradient. IL-6 was found to coelute with eosinophil crystalloid granule marker proteins, including eosinophil peroxidase (EPO), major basic protein (MBP), arylsulfatase B, and β-hexosaminidase. Immunoreactivity to IL-6 colocalized with granule-associated IL-2 and IL-5 in subfractionated eosinophils. We also made the novel and compelling observation that interferon γ (IFNγ), a Th1-type cytokine, stimulated an early elevation in eosinophil IL-6 immunoreactivity. A 2.5-fold enhancement of IL-6 immunoreactivity in eosinophil granules was observed within 10 minutes of IFNγ treatment (500 U/mL), as determined by subcellular fractionation and CLSM. These findings suggest that IFNγ has short-term effects on human eosinophil function and imply that a physiologic role exists for Th1-type cytokine modulation of Th2-type responses in these cells.
EOSINOPHILS, prominent cells in allergic inflammation and asthma, have been shown to synthesize, store, and release up to 18 inflammatory and regulatory cytokines and growth factors,1 including interleukin-2 (IL-2), IL-4, IL-5, and granulocyte/macrophage colony-stimulating factor (GM-CSF).2-5 We have previously reported that human asthmatic peripheral blood eosinophils express mRNA for IL-6.6 In addition, IL-6–positive eosinophils have been detected in blood from normal donors, suggesting that IL-6 may be constitutively synthesized and stored in unstimulated eosinophils.7 The site of storage of IL-6 in eosinophils could not be determined by immunocytochemical staining, although it appeared to be associated with the crystalloid secretory granule, because IL-6 immunoreactivity showed a granular pattern of staining.6
IL-6 is a pleiotropic lymphokine shown to be released from a wide range of tissue cell types, particularly fibroblasts, T cells, and peripheral blood mononuclear cells.8,9 Its expression is usually induced in cells by viral infection, lipopolysaccharide, or other cytokines, depending on the cell type concerned. The biologic effects of IL-6 range from stimulation of B-cell hybridoma and mouse plasmacytoma growth (leading to enhanced monoclonal antibody production), B-cell terminal differentiation, T-cell proliferation, and induction of cytotoxicity, to proliferation of hematopoietic progenitor cells.8,9 Many actions of IL-6 occur in synergy with other cytokines, principally IL-1, IL-3, and GM-CSF. IL-6 has been shown to be a cofactor that potentiates IgE production from switched B cells by enhancing the effects of IL-4,10 suggesting a role for IL-6 in atopy and Th2-type responses. Recent evidence suggests that IL-6 may be involved in the pathophysiology of bronchial asthma.11,12 Elevated levels of circulating IL-6 were observed in asthmatic subjects (both symptomatic and asymptomatic) compared with normal controls. IL-6 levels were further increased during natural exacerbation of asthma compared with asymptomatic periods.11 Furthermore, bronchoalveolar lavage levels of IL-6 were found to increase after occupational allergen challenge.12
We have previously shown that mRNA encoding IL-6 and the released product (by in situ hybridization and enzyme-linked immunosorbent assay [ELISA], respectively) were upregulated after treatment of highly purified human asthmatic eosinophils with interferon γ (IFNγ).6 The effect of IFNγ on stimulating the release of other eosinophil-derived cytokines and chemokines has also been observed.13-15 IFNγ is a cytokine described as the prominent product of Th1-type lymphocytes in both mouse and human,16,17 and is usually associated with a wide range of bacterial and viral infections. Serum levels of IFNγ have been shown to be elevated in acute severe asthma.18 Previous studies have demonstrated that IFNγ can influence human eosinophil cytotoxicity and receptor expression following long-term (>12 hours) stimulation.19 20 The observation that IFNγ, a Th1-type cytokine, may be able to stimulate the release of a Th2-type cytokine (IL-6) from human eosinophils suggests that a shift is needed in our current appreciation of the relationship between these two types of immune responses, at least at the level of the eosinophil.
The aim of the present study is to determine the intracellular site of storage for eosinophil-derived IL-6, and to analyze the possible effects of IFNγ on IL-6 mobilization within the eosinophil. We hypothesized that (1) IL-6 is associated with the matrix of the secretory granules in human eosinophils, and (2) IL-6 storage and release in human eosinophils is regulated by IFNγ in a time-dependent manner. We tested these hypotheses using a combination of in vitro assays, confocal laser scanning microscopy (CLSM), and subcellular fractionation on peripheral blood eosinophils obtained from atopic asthmatic subjects. We examined the colocalization of IL-6 with known granule proteins in the presence or absence of IFNγ stimulation. Our results suggest an early effect of IFNγ on eosinophil-derived IL-6 storage and mobilization within the cell.
MATERIALS AND METHODS
Materials.
Di-isopropyl fluorophosphate (DFP), phenylmethylsulfonyl fluoride (PMSF), leupeptin, aprotinin,Nα-p-tosyl-l-arginine methyl ester (TAME), 4-methylumbelliferyl sulfate, 4-methylumbelliferylN-acetyl-β-d-glucosaminide, β-nicotinamide adenine dinucleotide (reduced form), Coomassie Blue G, and sodium pyruvate were purchased from Sigma (Poole, Dorset, UK). Adenosine triphosphate (ATP) was obtained from Boehringer (Mannheim, Germany). Nycodenz was purchased from Nycomed Pharma (Oslo, Norway) and from GIBCO-BRL Life Technologies (Grand Island, NY).
Preparation of eosinophils.
A sample of peripheral blood (100 mL) was obtained from mild atopic asthmatic subjects displaying eosinophilia more than 10% and who were not receiving oral corticosteroids. Red blood cells were removed by dextran sedimentation, with the remaining cells subjected to density centrifugation on Ficoll to obtain a granulocyte pellet. Eosinophils were then purified by immunomagnetic selection using the MACS system (Becton Dickinson, Cowley, UK). This method uses the expression of CD16 antigen on neutrophils, because this antigen is absent from resting eosinophils, as previously described.2,3,5,6,20 21 Briefly, anti-CD16 monoclonal antibody (MoAb) bound to micromagnetic beads (Miltenyi Biotec, Bergisch-Gladbach, Germany) was incubated with the granulocyte pellet for 40 minutes at 4°C. Contamination by mononuclear cells was avoided by coincubating anti-CD14– and anti-CD3–coated micromagnetic beads (Lab Impex, Teddington, Middlesex, UK; and Miltenyi Biotec). The mixture was then passed through a ferrous matrix column held in the field of a permanent magnet. Highly purified CD16− eosinophils (>99%) were obtained by negative selection, depleted of the immunomagnetically positive neutrophils (CD16+).
Subcellular fractionation.
Eosinophils were subjected to subcellular fractionation as previously described.2 5 Briefly, at least 5 × 107purified eosinophils were treated with 2 mmol/L DFP, a serine protease inhibitor, for 5 minutes at room temperature before sedimenting at 240g for 5 minutes. The pellet was resuspended in ice-cold 0.25 mol/L HEPES-buffered sucrose (containing 10 mmol/L HEPES, 1 mmol/L EGTA, and 5 μg/mL each of leupeptin, aprotinin, and TAME, pH 7.4), and the cells were centrifuged again at 4°C. Cells were resuspended in homogenization buffer (HEPES-buffered sucrose supplemented with 2 mmol/L MgCl2 and 1 mmol/L ATP) to optimal subfractionating density, between 10 and 15 × 106/mL, and subjected to 10 to 15 passes through a ballbearing homogenizer (EMBL, Heidelberg, Germany) possessing 11 μm clearance. The homogenate was centrifuged at 400g for 10 minutes, and the resulting postnuclear supernatant was layered onto an 8-mL linear Nycodenz gradient (0% to 45% Nycodenz dissolved in HEPES-buffered sucrose with protease inhibitor cocktail) in a Beckman 14 × 89-mm Ultra-Clear centrifuge tube (Beckman, High Wycombe, UK). The postnuclear supernatant was subjected to equilibrium density centrifugation at 100,000g for 1 hour at 4°C. Twenty-four 0.4-mL fractions were collected from each preparation and stored at 4°C no longer than overnight or −80°C until used. The density of each fraction was calculated from its respective refractive index.
Marker enzyme assays.
A total of four marker enzyme assays were used for locating specific subcellular organelles to fractions collected from density gradient centrifugation. Arylsulfatase B and β-hexosaminidase activities were measured in each fraction as markers for secretory granules and lysosomes, using the method described by Levi-Schaffer et al.5 Briefly, 50 μL diluted fraction (1:10 in HEPES-buffered sucrose) was added to a black 96-well microplate and mixed with 50 μL arylsulfatase B substrate solution (10 mmol/L 4-methylumbelliferyl sulfate in 0.2 mol/L acetate buffer, 6 mmol/L lead acetate, and 0.1% Triton X-100, pH 5.6) or 50 μL β-hexosaminidase substrate solution (1 mmol/L 4-methylumbelliferylN-acetyl-β-d-glucosaminide in 0.2 mol/L citrate buffer and 0.1% Triton X-100, pH 4.5) before incubating at 37°C for 1 hour. The reaction was terminated by addition of 150 μL ice-cold 0.2 mol/L Tris, and the fluorescence was measured in a Titertek Fluoroskan (Huntsville, AL) microplate reader (excitation 340 nm and emission 450 nm). For marking the presence of secretory granules, we measured eosinophil peroxidase (EPO) activity adapted from White et al22 for microtiter plates, and in later experiments, tetramethylbenzidine substrate solution ([TMB] Sigma, Oakville, Ontario, Canada) was used as a safer substrate for EPO in place of the more commonly used o-phenylenediamine HCl. Cytosolic activity was detected by assay of lactate dehydrogenase (LDH) as previously described.5 Plasma membrane activity was assessed by the dot-blot method with antibody to CD9 as previously described.5 Protein measurements were made using the Bradford dye-binding protein assay, with bovine serum albumin as the standard.
Cytokine ELISA.
IL-6 and IL-2 were assayed in fractions using Quantikine ELISA kits (British Biotechnology, Oxford, England; and R & D Systems, Minneapolis, MN). These assays have a detection sensitivity of 0.08 and 6 pg/mL, respectively. Assays were performed in duplicates (whenever possible) of undiluted fractionated material. IL-5 was quantified using an anti–human IL-5 MoAb as previously described.23 The detection sensitivity of the assay was 6.25 pg/mL.
Dot-blot analysis.
This technique was used to confirm the presence of IL-6 shown by ELISA and to detect the granule-associated proteins, major basic protein (MBP) and eosinophil cationic protein (ECP), and the eosinophil plasma membrane marker CD9. For detection of IL-6, an anti–human IL-6 MoAb was used in parallel with a rabbit anti-mouse antibody used as a negative control (British Biotechnology). MBP was detected by an in-house mouse MoAb BMK-13 (supernatant IgG1) that has been carefully validated, whereas ECP was detected using EG2 MoAb (supernatant IgG1; Pharmacia, Uppsala, Sweden). Anti-CD9 MoAb (purified IgG1) was purchased from Becton Dickinson. Both anti-CD3 MoAb (supernatant IgG1) and anti-CD5 MoAb (purified IgG1) were used as irrelevant negative controls for these MoAbs (Becton Dickinson). A total of 2 μL of the supernatants were placed on nitrocellulose strips, allowed to dry, and blocked in 5% milk powder (Sigma). The blocked membrane strips were incubated with appropriate antibodies, and after extensive washings in PBS-Tween 20, they were incubated with biotinylated anti-mouse or anti-goat antibody followed by streptavidin-alkaline phosphatase, washed, and developed. In the IL-6 dot-blot assay, recombinant IL-6 was used as a positive control. Fractional activities of the markers were assessed by staining density, assigned arbitrary units, and converted to the percentage of total activity for each specific protein.
Double labeling and CLSM.
Cytospins of eosinophils (100 μL 0.5 × 106 cells/mL in RPMI supplemented with 20% FCS) were made by vortexing slides in a Cytospin 2 (Shandon, UK) centrifuge (800 rpm for 2 minutes) followed by fixation in 2% paraformaldehyde in PBS for 10 minutes. Slides were subjected to a wash step (five washes in Tris-buffered saline [TBS]), followed by incubation in blocking solution (2% human IgG [Sigma Reagent Grade] in H2O) in a humidified container for 1 hour. After a second wash step, TBS containing 1% rat anti–human IL-6 MoAb (50 μg/mL) labeled with FITC (Pharmingen, San Diego, CA) was added, and the slides were incubated for 1 hour. Slides were subjected to a wash step again before adding 1% BMK-13 in TBS and incubating for 1 hour. Bound BMK-13 antibody was detected by addition of 1% (50 μg/mL) Texas Red–labeled goat anti-mouse antibody (Pharmingen) to washed slides and incubation for 1 hour. For comparison, 1% FITC-labeled rat IgG2a was used as an isotype control (Pharmingen). After a final wash step, 10 μL antibleaching agent (0.4%n-propyl gallate [Sigma] in 3:1 glycerol:TBS) was dropped onto each slide before cover slip attachment. Slides were examined using a 100× objective under a Leica confocal laser scanning microscope (Heidelberg, Germany). This instrument contained a krypton-argon laser to allow simultaneous scanning of two excitation wavelengths (488 and 568 nm) so that two images could be acquired from a single pass to minimize bleaching of the slides. Differences between the photomultiplier tube sensitivities of the two fluorochrome emission spectra were compensated during collection of the data to obtain images of equivalent brightness. Images were stored on computer and transferred to Adobe Photoshop (Adobe Systems Inc, Mountain View, CA) for cropping and sizing.
Data presentation.
The bioactivity of eosinophil granule, membrane, and cytosol constituents after fractionation, including IL-6 quantitation by ELISA (picograms per milliliter) and dot blot, is expressed as the frequency distribution as previously described,5 except for the time course of IFNγ stimulation, where IL-6 is quantitatively displayed as picograms per fraction.
RESULTS
Immunocytochemistry using APAAP.
Highly purified eosinophils from asthmatic subjects were examined using the APAAP staining technique for the presence of IL-6 immunoreactivity. Anti–IL-6 binding revealed cytoplasmic and/or granular staining of IL-6 in a subpopulation of cells (25% to 50%; Fig1a), suggesting that IL-6 may be stored inside the secretory granules, in the same manner as previously observed for other eosinophil-derived cytokines.2,3 5Staining of eosinophils with an isotype control antibody was negative (Fig 1b).
Photomicrograph of a human eosinophil detected in a buffy coat cytospin. (a) The eosinophil was stained specifically with mouse monoclonal anti–human IL-6 using the APAAP technique, showing a granular pattern of immunoreactivity as compared with the negative isotype control (b) (original magnification ×100).
Photomicrograph of a human eosinophil detected in a buffy coat cytospin. (a) The eosinophil was stained specifically with mouse monoclonal anti–human IL-6 using the APAAP technique, showing a granular pattern of immunoreactivity as compared with the negative isotype control (b) (original magnification ×100).
Subcellular fractionation.
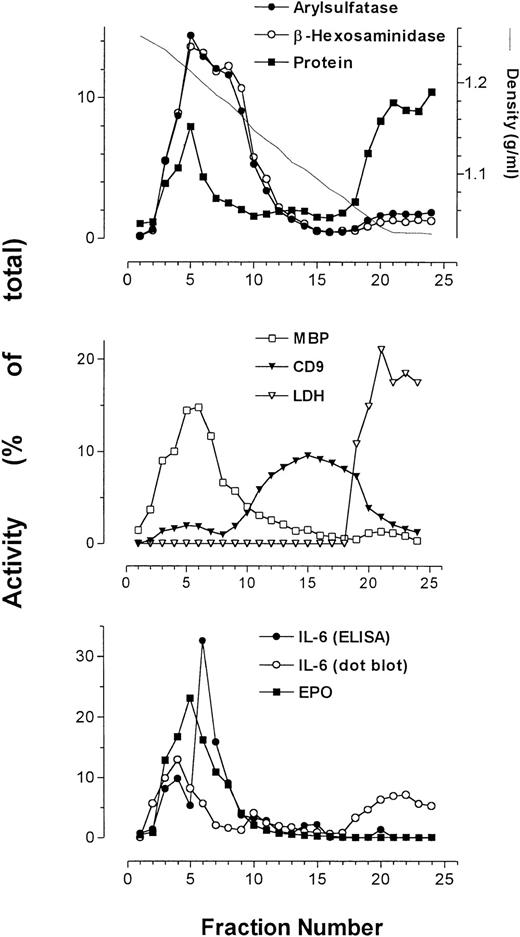
Using subcellular fractionation of eosinophils, we successfully separated some of the organelles in these cells, allowing analysis of organelle-specific protein expression. The secretory granules, plasma membrane, and cytosol were clearly resolved after density gradient centrifugation (Fig 2). Secretory granule activity was detected by assays for EPO, ECP (Fig 3), and MBP, and was usually confined to a single peak. We have previously shown that the pellet produced from pooled fractions corresponding to peak granule protein activity contains enriched secretory granules.5
Average of profiles (from 4 patients) of arylsulfatase B, β-hexosaminidase, protein, MBP, CD9, LDH, and EPO together with IL-6 immunoreactivity as determined by ELISA and dot-blot analysis. Measurements of activities were averaged and plotted as a function of collected fractions.
Average of profiles (from 4 patients) of arylsulfatase B, β-hexosaminidase, protein, MBP, CD9, LDH, and EPO together with IL-6 immunoreactivity as determined by ELISA and dot-blot analysis. Measurements of activities were averaged and plotted as a function of collected fractions.
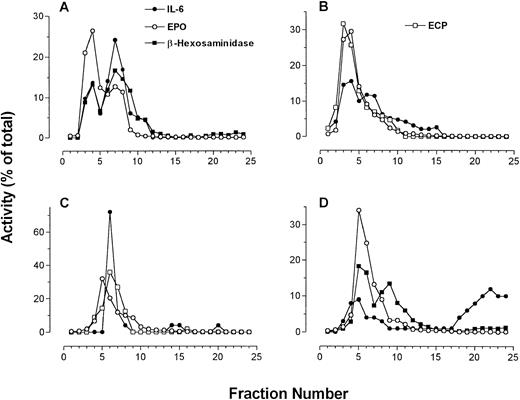
Four individual fractionations of human eosinophils in which IL-6 immunoreactivity is compared with β-hexosaminidase and EPO activities along with ECP immunoreactivity (using EG2 MoAb in dot blot). (A to C) IL-6 measured by ELISA; (D) IL-6 measured by dot-blot analysis.
Four individual fractionations of human eosinophils in which IL-6 immunoreactivity is compared with β-hexosaminidase and EPO activities along with ECP immunoreactivity (using EG2 MoAb in dot blot). (A to C) IL-6 measured by ELISA; (D) IL-6 measured by dot-blot analysis.
The plasma membrane and cytosol detected by peak anti-CD9 binding and LDH activity, respectively, sedimented at densities much lower than those for secretory granules. Immunoreactivity for the plasma membrane antigen CD9 resolved into two distinct peaks. The first peak, present in higher-density fractions, sedimented in fractions containing maximal secretory granule activity. The second peak of CD9 immunoreactivity, which was much larger than the first, appeared at the expected range of densities (1.04 to 1.17 g/mL) for plasma membranes.24 The protein assay showed enrichment of cellular protein in two peaks across the gradient, corresponding to fractions containing secretory granule activity and cytosolic activity. The cytosol, as identified by the presence of LDH activity, was associated with fractions of a density range of 1.03 to 1.07 g/mL, coeluting with the second peak of total protein.
β-Hexosaminidase and arylsulfatase B activity, which coincided with that of EPO, consistently overlapped and usually produced bisected peaks, suggesting that at least two subpopulations of secretory granules exist in eosinophils. Also displayed in Fig 2 is the profile of IL-6 as measured by ELISA in a representative sample, which peaked at or near the same density (∼1.2 g/mL) as MBP and EPO.
The sedimentation profile of IL-6 immunoreactivity by ELISA and dot blot, along with that of ECP, EPO, and β-hexosaminidase, for four separate fractionations are presented in Fig3. In one of four preparations, IL-6 immunoreactivity could be resolved as a single well-defined peak. Otherwise, two peaks of IL-6 immunoreactivity could be resolved on the gradient in two preparations, whereas in the final preparation only a shoulder on a single peak was apparent (Fig 3). These split peak profiles overlapped with those of β-hexosaminidase and arylsulfatase B activities. In all cases, IL-6 immunoreactivity colocalized with fractions possessing peak granule protein activity. Taken together, these results suggest that IL-6 is associated with eosinophil secretory granules, and may be present in more than one subpopulation of granules. Using whole-cell samples, unstimulated eosinophils were found to store an average of 25 ± 6 pg IL-6/106 cells (n = 4). We have previously established that unstimulated eosinophils are able to release an average of 190 ± 18 pg/mL IL-6 in supernatants, which increased to 403 ± 214 pg/mL after 24 or 48 hours of IFNγ stimulation (106 cells per sample).6
Dot-blot analysis was used to confirm the results of our ELISA for IL-6 (Fig 3D). All profiles of dot-blot analysis for IL-6 showed two peaks of immunoreactivity, the first appearing in fractions containing secretory granule activity and the second present in cytosolic fractions as indicated by LDH activity. The most likely explanation for the appearance of IL-6 immunoreactivity in the cytosolic fractions is granule breakage during homogenization. In three of four cases, dot-blot results correlated with those in ELISA determinations. The fourth preparation of eosinophils yielded erratic values of IL-6 immunoreactivity by ELISA, although the dot-blot analysis for this sample was similar to what was observed in the other three preparations (Fig 3D).
Coelution of IL-2, IL-5, and IL-6 with granule markers.
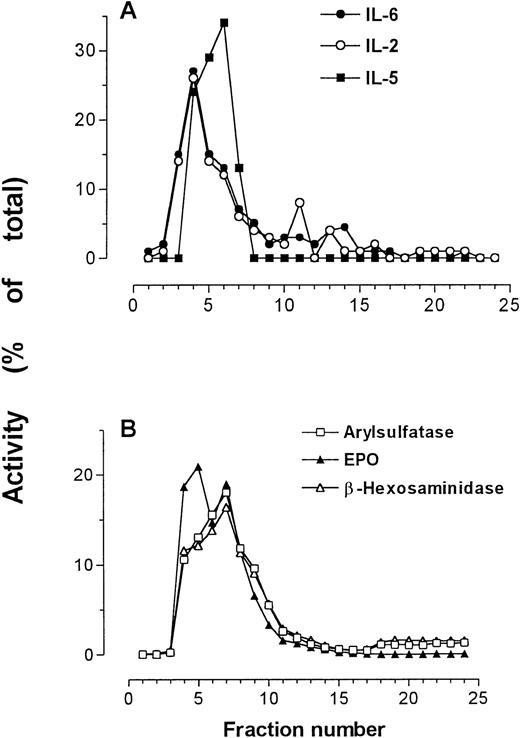
In one preparation, IL-2, IL-5, and IL-6 immunoreactivities were all determined by relevant specific ELISAs, and were detected in the same fractions as those containing peak secretory granule activity (Fig4). This finding supports previous results showing IL-5 immunoreactivity in the secretory granules of eosinophils.25
IL-2, IL-5, and IL-6 immunoreactivities in subcellular fractions of eosinophils shown in comparison with arylsulfatase B, β-hexosaminidase, and EPO activities. (A) IL-2, IL-5, and IL-6 measured by ELISA; (B) marker enzyme assays for secretory granules.
IL-2, IL-5, and IL-6 immunoreactivities in subcellular fractions of eosinophils shown in comparison with arylsulfatase B, β-hexosaminidase, and EPO activities. (A) IL-2, IL-5, and IL-6 measured by ELISA; (B) marker enzyme assays for secretory granules.
CLSM.
In support of our findings using subcellular fractionation, unstimulated eosinophils showed colocalization of IL-6 and MBP immunoreactivity to the crystalloid granules using CLSM in double-labeled cells (Fig 5A to C). Yellow regions in combined fluorescent imaging correspond to overlapping green and red images, and suggest that the two stained proteins reside within the same intracellular compartment. Isotype controls displayed minimal fluorescence background after subtraction of autofluorescence (Fig 5D and E). Interestingly, anti–IL-6 fluorescence produced characteristic doughnut-like shapes corresponding to the granule matrix (Fig 5A, F, G, and K). Upon increased magnification of combined images, these granular shapes were found to possess red centers (crystallized MBP) surrounded by green fluorescence, corresponding to the core and matrix of the crystalloid granules, respectively (Fig 5F). These observations suggest that eosinophil IL-6 is stored within the matrix of the specific secretory granule.
Confocal microscopy of double-labeled eosinophils. (A to C) Representative unstimulated eosinophils with the FITC channel corresponding to IL-6 (A), the Texas Red channel corresponding to MBP (B), and the combined fluorescence (C). (D to E) Isotype controls for FITC fluorescence and Texas Red fluorescence, respectively. (F) Close-up of granules from an unstimulated cell showing doughnut-shaped IL-6 immunoreactivity surrounding red centers of MBP immunoreactivity. (G to J) Time course of IFNγ effects on IL-6 immunoreactivity in eosinophils after (G) 10 minutes, (H) 6 hours, (I) 12 hours, and (J) 18 hours of stimulation by 500 U/mL IFNγ. (K to N) Same time course as G to J, showing combined images for IL-6 and MBP (original magnification ×100).
Confocal microscopy of double-labeled eosinophils. (A to C) Representative unstimulated eosinophils with the FITC channel corresponding to IL-6 (A), the Texas Red channel corresponding to MBP (B), and the combined fluorescence (C). (D to E) Isotype controls for FITC fluorescence and Texas Red fluorescence, respectively. (F) Close-up of granules from an unstimulated cell showing doughnut-shaped IL-6 immunoreactivity surrounding red centers of MBP immunoreactivity. (G to J) Time course of IFNγ effects on IL-6 immunoreactivity in eosinophils after (G) 10 minutes, (H) 6 hours, (I) 12 hours, and (J) 18 hours of stimulation by 500 U/mL IFNγ. (K to N) Same time course as G to J, showing combined images for IL-6 and MBP (original magnification ×100).
Effects of IFNγ stimulation.
The effect of IFNγ stimulation was examined in subfractionations of unstimulated and stimulated eosinophils from the same donor at 10 minutes and 16 hours of IFNγ incubation. After 10 minutes of stimulation by 500 U/mL IFNγ, immunoreactivity to IL-6 was elevated over that of unstimulated cells (Fig 6),although it remained within the secretory granule fractions. The granule-associated IL-6 was reduced to prestimulation levels after 16 hours of incubation with IFNγ.
Eosinophils that were subfractionated to determine effects of IFNγ incubation (500 U/mL) on IL-6 concentration or localization. Quantification of IL-6 in each fraction was made by ELISA. Marker enzyme assays used in these experiments were EPO and β-hexosaminidase. The experiments were conducted at different times using purified blood eosinophils from the same donor. (A) Unstimulated eosinophils, followed by eosinophils stimulated for (B) 10 minutes and (C) 16 hours by IFNγ. An average of 50 × 106eosinophils were used for each preparation.
Eosinophils that were subfractionated to determine effects of IFNγ incubation (500 U/mL) on IL-6 concentration or localization. Quantification of IL-6 in each fraction was made by ELISA. Marker enzyme assays used in these experiments were EPO and β-hexosaminidase. The experiments were conducted at different times using purified blood eosinophils from the same donor. (A) Unstimulated eosinophils, followed by eosinophils stimulated for (B) 10 minutes and (C) 16 hours by IFNγ. An average of 50 × 106eosinophils were used for each preparation.
By confocal microscopy, 10 minutes of stimulation by IFNγ produced an apparent intensification of anti–IL-6 fluorescence in the secretory granules (Fig 5G). Anti–IL-6 fluorescence became diminished at 6 hours of stimulation (Fig 5H) and dispersed throughout the cytoplasm after 18 hours of incubation (Fig 5J). By 24 hours of IFNγ stimulation, the morphology of the cells deteriorated and staining became sporadic (data not shown). During incubation with IFNγ, IL-6 immunoreactivity in CLSM results became progressively dissociated from that of MBP in combined images, suggesting that IL-6 had mobilized into separate intracellular compartments (Fig 5K to N). However, we were unable to confirm this finding in results from subfractionation, which may have been a result of the very low levels of IL-6 in the individual fractions.
DISCUSSION
In this study, we have shown for the first time that IL-6 is localized to the matrix of secretory granules in eosinophils from atopic asthmatics. We have based our findings on the comprehensive analysis of subcellular fractions of eosinophils, correlation of cellular components with IL-6 immunoreactivity, and fluorescent–labeled cells using CLSM. The use of subcellular fractionation and CLSM combined provides a powerful tool for determining the processes of storage and intracellular trafficking of important proteins in inflammatory cells. In subcellular fractionation, maximal IL-6 immunoreactivity was found to coelute specifically with granule protein markers. We have previously shown that fractions containing eosinophil granule proteins are concentrated in crystalloid secretory granules.2,3 5IL-6 immunofluorescence was found to localize to the matrix of the crystalloid granule in CLSM analysis of cytospin preparations of human eosinophils. Eosinophils are among the few inflammatory cells capable of storing cytokines and chemokines in their secretory granules. These stored products may be rapidly mobilized from intracellular sites of storage and released locally during inflammation. Whether these proteins are bioactive and exert their influence on the inflammatory milieu in situ by paracrine, autocrine, or juxtacrine signaling remains to be elucidated.
It is unlikely that IL-6 immunoreactivity detected in granule-containing fractions results from internalized IL-6–bound receptors, because secretory granules sediment at a much greater buoyant density than anticipated for endosomal structures. These structures would contain any endocytosed ligand-bound receptors, and would be expected to sediment at a density equivalent to plasma membrane.24 Moreover, earlier results have shown that eosinophils transcribe and translate the gene encoding IL-6,6 implying that IL-6 is synthesized within the eosinophil.
The significance of the association of IL-6 immunoreactivity with secretory granules lies in the possibility that IL-6 may be released by regulated exocytosis from the eosinophil. Eosinophils have been shown to undergo degranulation, measured by the release of β-hexosaminidase and by patch-clamp analysis, in response to intracellularly applied agonists.26,27 So far, the mechanism of eosinophil-derived cytokine release has not been investigated, although it is possible to evoke secretion of IL-6 by IFNγ,6 suggesting that IL-6 is released by receptor-mediated secretion.
We observed an early effect of IFNγ on the intensification of IL-6 immunoreactivity using two separate experimental approaches. This finding compelled us to reevaluate our current appreciation of the short-term effects of this cytokine. By subcellular fractionation of eosinophils and CLSM, a 2.5-fold enhancement of IL-6 immunoreactivity was observed in eosinophil granules within 10 minutes of IFNγ treatment. Such short-term effects of IFNγ on IL-6 mobilization within eosinophils from atopic asthmatics may occur in patients with acute severe asthma. These patients exhibit high levels of serum IFNγ.18 Whether this is also relevant to viral exacerbation of asthma remains to be established.28
The most plausible explanation for the observed short-term effects of IFNγ on IL-6 immunoreactivity is that IFNγ may activate a conformational change in the structure of IL-6 within the granules. Alternatively, IFNγ may stimulate the “unmasking” of putative prepro–IL-6, which is not recognized by the MoAbs used in this investigation. The specificity of the antibody for the prepro form of IL-6 as opposed to the secreted form of this protein is not known. It is unlikely that de novo IL-6 synthesis and vesicular trafficking into the secretory granules would occur within such a short time to account for the enhanced IL-6 immunoreactivity in response to IFNγ.
The CLSM results showing dissociation of IL-6 from MBP-positive granule cores during prolonged stimulation by IFNγ suggest that some form of translocation of IL-6 may occur between the secretory granules and the plasma membrane, an observation that we were unable to confirm with subcellular fractionation. However, the sensitivity of detection of IL-6 immunoreactivity using CLSM is at least one order of magnitude greater than that of ELISA measurement of IL-6 activity in subcellular fractions. Based on CLSM results, extragranular anti–IL-6 staining may be related to (1) newly synthesized IL-6 in the Golgi and its transport to the secretory granules and/or (2) a smaller class of secretory vesicles engaged in the process of piecemeal degranulation. The latter process has been previously suggested for physiological release of eosinophil granule proteins.29 The biosynthetic pathway of IL-6, as well as other cytokines, in eosinophils remains unknown and is currently being investigated by our laboratory.
The putative role of eosinophil-derived IL-6 remains the subject of speculation in asthma and allergic inflammation. IL-6 has been shown to be associated with symptomatic and asymptomatic asthma and with natural or induced exacerbations.11,12 The precise mechanisms involving IL-6 in asthma are not clear, but it is likely that eosinophils are an important source of this cytokine in asthmatic responses. IL-6 is an important helper cytokine for primary antigen–dependent T-cell activation and proliferation.30Infiltration of CD4+ T cells has been shown to be an important component of the inflammatory response in antigen-induced late-phase allergic reactions in human skin,31lung,32 and nose.33 IL-6 participates in IL-4–dependent IgE synthesis from B cells10 and acts in synergism with IL-3 and GM-CSF to enhance the proliferation and growth of hematopoietic progenitors in humans.34,35 Furthermore, IL-6 is involved in promoting secretion of IgA in mucosal tissue during inflammatory reactions,30 and secretory IgA has been shown to be a potent trigger of eosinophil degranulation.36 In fibroblasts, IL-6 has been shown to inhibit cell growth,37so the “repair” function of these cells may be influenced by IL-6 during allergic inflammation. IL-6 is released in association with protective responses against infectious and parasitic agents,9 and this may be related to the elevated eosinophil numbers seen in helminth-induced infection.38 In summary, eosinophils may serve as an important cellular source of IL-6 in allergic and inflammatory reactions.
Eosinophils also store a number of other cytokines in their secretory granules, such as IL-2, IL-4, IL-5, and GM-CSF.2,3,5,25,39,40 Our results showing expression and intracellular localization of IL-5 to the specific secretory granules are in agreement with other reports describing detection of IL-2 and IL-5 mRNA and their related products in eosinophils from patients with atopic asthma.2 39-41
Storage of cytokines may lend eosinophils the potential to release these preformed regulatory proteins rapidly and allow them to act locally during inflammation. In addition, eosinophil-derived cytokines may perpetuate inflammatory responses and prolong the survival of these cells with damaging sequelae. Eosinophil synthesis and storage of cytokines, chemokines, and growth factors in health and disease requires further investigation to determine how these cells contribute to the regulation of allergic inflammation.
ACKNOWLEDGMENT
We would like to thank Dr David Huston and Richard Dickason (Baylor College of Medicine, Houston, TX) for assistance in measuring IL-5 in one of our subfractionations; Professor Bastien Gomperts (University College, London, UK) for invaluable advice, support, and supervision of P.L. during the early phase of this research; and Matthew Wakelin and Janet North (National Heart and Lung Institute, London, UK) for technical assistance.
Supported by The Wellcome Trust, UK; the Medical Research Council, UK; Medical Research Council, Canada; and Alberta Heritage Foundation for Medical Research.
Address reprint requests to Redwan Moqbel, PhD, Pulmonary Research Group, 574 Heritage Medical Research Center, University of Alberta, Edmonton, Alberta, Canada T6G 2S2.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal