Abstract
Primary effusion lymphoma (PEL) is a distinct clinicopathologic entity associated with Kaposi's sarcoma-associated herpes virus (KSHV). Several cytokines, including interleukin-6 (IL-6), basic fibroblast growth factor (bFGF), and platelet-derived growth factor (PDGF) may be important for survival of KS cells. However, little is known about the interaction of cytokines with KSHV-infected lymphocytes from PEL. Therefore, we investigated what cytokines were produced by KSHV-infected PEL cell lines (KS-1, BC-1, BC-2), what cytokine receptors were expressed by these cells, what response these cells had to selected cytokines, and what was the effect of IL-6 antisense phosphorothioated oligonucleotides. Reverse transcriptase-polymerase chain reaction (RT-PCR) and protein studies showed that these three cell lines produced IL-10, IL-6, and the receptors for IL-6. The granulocyte macrophage colony-stimulating factor (GM-CSF), IL-1β, IL-8, IL-12, bFGF, PDGF, and c-kit transcripts were not detected in the cell lines. High levels (0.7 to 5 ng/mL/106cells/48 hours) of IL-6 protein were consistently detected in supernatants of the cell lines by enzyme-linked immunosorbent assay (ELISA) tests. In clonogenic assays, interferon-α (IFN-α) and IFN-γ suppressed the clonal growth of the PEL cells, but GM-CSF, IL-4, IL-6, IL-8, IL-10, and oncostatin M did not change it. We examined for several autocrine loops that have been suggested to occur in KS. Experiments using antisense oligonucleotides showed that the clonal growth of KS-1 and BC-1 was nearly 100% inhibited by IL-6 antisense oligonucleotides (10 μmol/L), but not at all by either oligonucleotides (≤10 μmol/L) to IL-6 sense, IL-6 scrambled, viral IL-6 (vIL-6) antisense, or IL-10 antisense. Furthermore, the IL-6 antisense oligonucleotides had no effect on two B-cell lymphoma cell lines, which were not infected with KSHV. Addition of IL-6 antibody did not inhibit clonal growth of any of the cell lines. Taken together, we have defined the cytokines and their receptors expressed on PEL cells and have found that these cells synthesized IL-6 and IL-6 receptors; interruption of this pathway by IL-6 antisense oligonucleotides specifically prevented the growth of these cells. These findings will offer potential new therapeutic strategies for PEL.
RECENTLY, KAPOSI'S sarcoma-associated herpes virus (KSHV or HHV8) has been associated with Kaposi's sarcoma (KS) and primary effusion lymphomas (PEL).1-4 KSHV is a γ-2 herpes virus (genus Rhadinovirus) and is the first member of this genus known to infect humans.5 KSHV-associated lymphomas arise predominantly, but not exclusively, in human immunodeficiency virus (HIV)-positive patients and are characterized by presentation as malignant effusions without solid tumor masses.6-8 PEL exhibits distinctive clinical and biologic features, including immunoblastic morphology, indeterminate immunophenotype, clonal Ig gene rearrangements indicating a B-cell genotype; and the cells frequently contain the Epstein-Barr virus (EBV) in the absence of c-myc gene rearrangements.6-8 However, the pathogenesis and growth control mechanisms of PEL are not well understood.
Prior studies of KS-derived cell lines have shown that these cells can produce several cytokines including granulocyte macrophage colony-stimulating factor (GM-CSF), tumor necrosis factor (TNF), interleukin-1β (IL-1β),9-16 as well as small inflammatory cytokines known as chemokines.17 Of particular interest, IL-6, oncostatin M, and platelet-derived growth factor (PDGF) have been reported as autocrine growth factors in KS. Recently, investigators have suggested that KSHV may provoke cytokine production in KS.17 Moreover, four virus proteins are of interest: two of them are similar to two human macrophage inflammatory protein (MIP) chemokines, another is partially homologous to IL-6, and a fourth is similar to interferon regulatory factor (IRF).18,19 In addition, KSHV may be present in bone marrow stromal cells in patients with multiple myeloma, and viral stimulation of IL-6 may influence the growth of the myeloma cells.20,21 Furthermore, IL-10 and IL-12 were reported as autocrine growth factors for acquired immunodeficiency syndrome (AIDS)-related B-cell lymphomas.22 23 These facts encouraged us to study the expression of human cytokines and their receptors, as well as their regulation to understand their involvement in KSHV-infected PEL cells. This study suggests that IL-6 is an important cell survival factor for PEL cells; and IL-6 antisense oligonucleotides perhaps can be a therapeutic agent for PEL.
MATERIALS AND METHODS
Cell lines.
Three PEL cell lines (KS-1,BC-1, and BC-2) were used in this study. The KS-1 cell line was established from a lymphomatous pleural effusion collected from a HIV-negative patient.24 The BC-1 and BC-2 cell lines were derived from malignant effusion samples collected from AIDS-related lymphomas.25 All of these cell lines are positive for KSHV. BC-1 and BC-2 cell lines harbor EBV, but the KS-1 cell line is negative for EBV.24,25 EW36 and DS179 are non-AIDS–related B-cell lymphoma cell lines, which are not infected with either KSHV or EBV.22 23 The cell lines were maintained in suspension culture in RPMI 1640 plus 10% fetal bovine serum (FBS) at 37°C in 5% CO2, and subcultured every 3 to 4 days.
Reverse transcriptase-polymerase chain reaction (RT-PCR).
Total RNA was isolated from three PEL cell lines by using Trizol (GIBCO-BRL, Gaithersburg, MD). The cDNA synthesis was performed using Moloney murine leukemia virus RT. The cDNA product was amplified by commercially available primers for cytokines and their receptors (Clontech Laboratories, Palo Alto, CA). Gene expression of GM-CSF, IL-1β, IL-8, IL-10, IL-12, TNFα, PDGFα, basic fibroblast growth factor (bFGF), IL-4 receptor (IL-4R), IL-6R, and c-kitwas analyzed in this study. Efficiency of RT was controlled in each sample by PCR amplification of β-2 microglobulin using amplimers yielding a 135-bp product (sense 5′-GGAAAAG-ATGAGTATGCCTG-3′, antisense 5′-TTCACTCAATCCAAATGC-GG-3′). Thirty cycles of PCR amplification were performed for evaluation of each of the cytokines and their receptors.
Quantification of IL-6 and IL-10.
IL-6 and IL-10 were quantified by using an IL-6 or IL-10-specific enzyme-linked immunosorbent assay (ELISA) (ELISA kits purchased from R & D Systems, Minneapolis, MN). The IL-6 antibody has no cross-reactivity with vIL-6.18 PEL cells (1 × 106 /mL) were treated without or with 12-0-tetradecanoylphorbol 13-acetate (TPA; Sigma, St Louis, MO) at 10 ng/mL for 48 hours; subsequently supernatants of cultured cells were collected and examined for IL-6 production by ELISA.
Expression of IL-6 receptor.
Western blot analysis was performed with monoclonal mouse antihuman IL-6 receptor antibody (R & D Systems), which recognized 55 kD IL-6 receptor protein. Sodium dodecyl sulfate-polyacrylamide gel electropheresis (SDS-PAGE) was performed as previously described.26 Briefly, proteins (40 μg) were size fractionated under denaturing conditions on 12.5 % SDS-PAGE–running gel and transferred to Immobilon polyvinylidine fluoride membrane (Millipore, Bedford, MA) The protein was detected using the enhanced chemiluminescence system from Amersham (Arlington Heights, IL).
Colony formation assay in soft-gel.
Cells were cultured in a two-layer soft-agar system for 14 days as previously described,27 and colonies were counted using an inverted microscope. Lymphoma cell lines were plated at 5,000 to 25,000/mL. Various cytokines (0.1 to 1,000 ng/mL), interferon-α-2b (IFN-α-2b; Schering, Kenilworth, NJ, 1 to 10,000 U/mL), IFNγ-1b (Genentech Inc, San Francisco, CA, 1 to 10,000 U/mL), antihuman IL-6 antibodies (1 to 1,000 ng/mL, R & D Systems), all-trans retinoic acid (ATRA, Sigma, 10−11to 10−7 mol/L), or α-, and β-human chorionic gonadotropin (hCG, Sigma, 0.01 to 0.1 μg) were mixed into the lower agar layer. The GM-CSF, IL-4, IL-6, IL-8, IL-10, and oncostatin M were purchased from Genzyme Corp, Cambridge, MA.
Treatment of cell lines with sense and antisense oligonucleotides.
A 15-base antisense phosphorotioated oligonucleotides (TCCTGGGGGTACTGG) specific for sequences in the second exon of the human IL-6 gene were synthesized (Research Genetics, Huntsville, AL). Two scramble oligonucleotides (C-1, C-2) for the human IL-6 were synthesized as controls (C-1, GCGTTAGCGTCGGT; C-2, CGTTACGTGGGGGCT). Two antisense oligonucleotides were designed for viral IL-6 (CAACTTGAACCAGCACAT, +1 to +18; GATGTGCGTCTTACTCAG, +427 to +444). An IL-10 antisense oligonucleotide (AGCAGTGCTGAGCTGTGCAT) was made as previously reported.28 Sense oligonucletides (control) were synthesized for human IL-6, virus IL-6, and IL-10; and they were used as controls in each of the antisense oligonucleotide experiments. The oligonucleotides were custom-made, containing phosphorothioate, and were purified by reverse-phase high performance liquid chromatography. Various concentrations (0.01 to 10 μmol/L) of both oligonucleotides were tested. In clonogenic assays, oligonucleotides were mixed into the lower agar layer; and the two-layer, soft-agar system was used, as described above.
RESULTS
Expression of cytokines and cytokine receptors in PEL cell lines.
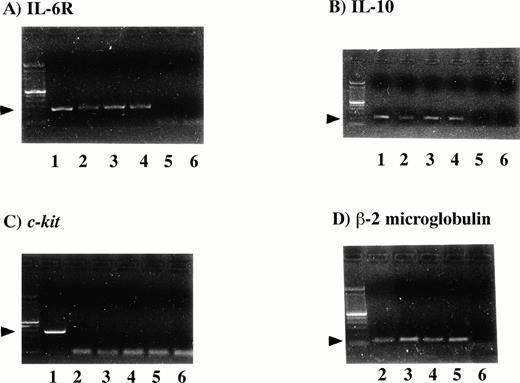
In RT-PCR studies, all three PEL cell lines (KS-1, BC-1, and BC-2)showed the same pattern of cytokine and cytokine receptor expression. IL-10 and IL-6R were expressed in the PEL cell lines (Fig 1). The GM-CSF, IL-1β, IL-8, IL-12, bFGF, PDGFα, IL-4R, and c-kit were not detected in the cell lines (Table 1). Expression of IL-6R protein was detected in the PEL cell lines by Western blot analysis (Fig 2).
Detection of IL-6R mRNA and IL-10 mRNA in the PEL cell lines. One microgram of RNA was used for cDNA synthesis. RT-PCR was performed for IL-6R, IL-10, c-kit and β-2 microglobulin. One fifth of the PCR mixture was analyzed by ethidium bromide staining of the 1.5% agarose gel (Lane 1, positive controls; lane 2, KS-1 cells; lane 3, BC-1 cells; lane 4, BC-2; lane 5, Jurkat cells, as a negative control; lane 6, water control). All three PEL cell lines were positive for IL-6R (A), 251-bp, IL-10 (B), 204-bp, and β-2 microglobulin (D), but negative for c-kit (C), 388-bp.
Detection of IL-6R mRNA and IL-10 mRNA in the PEL cell lines. One microgram of RNA was used for cDNA synthesis. RT-PCR was performed for IL-6R, IL-10, c-kit and β-2 microglobulin. One fifth of the PCR mixture was analyzed by ethidium bromide staining of the 1.5% agarose gel (Lane 1, positive controls; lane 2, KS-1 cells; lane 3, BC-1 cells; lane 4, BC-2; lane 5, Jurkat cells, as a negative control; lane 6, water control). All three PEL cell lines were positive for IL-6R (A), 251-bp, IL-10 (B), 204-bp, and β-2 microglobulin (D), but negative for c-kit (C), 388-bp.
Summary of Expression of Cytokines and Their Receptors in PEL Cell Lines (KS-1, BC-1, and BC-2)
| Cytokines and Their Receptors . | Evaluation of Their Expression in KS-1, BC-1, and BC-2 . | |
|---|---|---|
| RT-PCR . | Protein . | |
| IL-6 | + | + |
| IL-10 | + | + |
| IL-6R | + | + |
| GM-CSF | − | |
| IL-1b | − | |
| IL-12 | − | |
| bFGF | − | |
| PDGF-α | − | |
| IL-4R | − | |
| c-kit | − | |
| Cytokines and Their Receptors . | Evaluation of Their Expression in KS-1, BC-1, and BC-2 . | |
|---|---|---|
| RT-PCR . | Protein . | |
| IL-6 | + | + |
| IL-10 | + | + |
| IL-6R | + | + |
| GM-CSF | − | |
| IL-1b | − | |
| IL-12 | − | |
| bFGF | − | |
| PDGF-α | − | |
| IL-4R | − | |
| c-kit | − | |
Expression of IL-6R protein. Three PEL cell lines (lane 2, KS-1; lane 3, BC-1; lane 4, BC-2) expressed IL-6R protein (55 kD) by Western blot analysis. Myeloma cell line U266 (lane 1) was the positive control.
Expression of IL-6R protein. Three PEL cell lines (lane 2, KS-1; lane 3, BC-1; lane 4, BC-2) expressed IL-6R protein (55 kD) by Western blot analysis. Myeloma cell line U266 (lane 1) was the positive control.
IL-6 and IL-10 production in the PEL cells.
The supernatants of the three PEL cell lines and five KSHV−, EBV− B-cell lymphoma cell lines were analyzed for IL-6 production by ELISA. As shown in Table 2, high levels of IL-6 (0.7 to 5 mg/mL), and IL-10 (0.21 to 17 mg/mL) were detected consistently in the supernatants of the PEL cell lines. Exposure to TPA (10 ng/48 h) resulted in 70% and 40% decrease of synthesis of IL-6 from KS-1 and BC-1 (106 cells/mL), respectively. IL-6 production was decreased in supernatants from the TPA-treated PEL cells (Table 2). Exposure of the cells to vIL-6 antisense oligonucleotides did not effect the IL-6 production from the PEL cells (data not shown).
IL-6 and IL-10 Production in Lymphoma Cells
| Cell Lines . | KSHV . | EBV . | IL-6 (pg/mL)* . | IL-10 (pg/mL)* . |
|---|---|---|---|---|
| KS-1 | + | − | 720 ± 25 | 370 ± 25 |
| KS-1 + TPA | 250 ± 10 | |||
| KS-1 + IL-6S | 200 ± 20† | |||
| KS-1 + IL-6AS | 50 ± 15† | |||
| KS-1 + IL-10S | 170 ± 50† | |||
| KS-1 + IL-10AS | 10 ± 10† | |||
| BC-1 | + | + | 5,000 ± 500 | 210 ± 30 |
| BC-1 + TPA | 3,000 ± 500 | |||
| BC-1 + IL-6S | 5,000 ± 750† | |||
| BC-1 + IL-6AS | 850 ± 60† | |||
| BC-1 + IL-10S | 150 ± 65† | |||
| BC-1 + IL-10AS | 10 ± 10† | |||
| BC-2 | + | + | 700 ± 60 | 17,000 ± 800 |
| EW36 | − | − | 14 ± 10 | |
| DS179 | − | − | 18 ± 10 |
| Cell Lines . | KSHV . | EBV . | IL-6 (pg/mL)* . | IL-10 (pg/mL)* . |
|---|---|---|---|---|
| KS-1 | + | − | 720 ± 25 | 370 ± 25 |
| KS-1 + TPA | 250 ± 10 | |||
| KS-1 + IL-6S | 200 ± 20† | |||
| KS-1 + IL-6AS | 50 ± 15† | |||
| KS-1 + IL-10S | 170 ± 50† | |||
| KS-1 + IL-10AS | 10 ± 10† | |||
| BC-1 | + | + | 5,000 ± 500 | 210 ± 30 |
| BC-1 + TPA | 3,000 ± 500 | |||
| BC-1 + IL-6S | 5,000 ± 750† | |||
| BC-1 + IL-6AS | 850 ± 60† | |||
| BC-1 + IL-10S | 150 ± 65† | |||
| BC-1 + IL-10AS | 10 ± 10† | |||
| BC-2 | + | + | 700 ± 60 | 17,000 ± 800 |
| EW36 | − | − | 14 ± 10 | |
| DS179 | − | − | 18 ± 10 |
*Results represent the mean (± SD) of three independent experiments with 1 × 106 cells/mL cultured for 48 hours; the IL-6 and IL-10 were measured in the conditioned media.
A total of 1 × 105 cells/mL were cultured with sense or antisense oligonucleotides (10 μmol/L) for 72 hours, and the supernatants were used for IL-6 and IL-10 ELISA assays. IL-6S, IL-6 sense oligonucleotides; IL-6AS, IL-6 antisense oligonucleotides; IL-10S, IL-10 sense oligonucleotides; IL-10 antisense oligonucleotides.
The effect of cytokines and other agents on clonogenic growth of PEL cells.
Neither IL-6, GM-CSF, IL-4, IL-8, IL-10, nor oncostatin M stimulated the clonal growth of either the KS-1 or BC-1 cell lines (Table 3). Also, anti–IL-6 antibodies, ATRA, α-hCG, and β-hCG (Table 3) did not suppress the clonal growth of KS-1 and BC-1 cell lines. On the other hand, IFN-α and IFN-γ markedly inhibited clonal growth of KS-1 (Table 3). Effective dose inhibiting 50% clonal growth [ED50] of KS-1 was 500 U/mL and 1,000 U/mL for IFN-α and IFN-γ, respectively; and ED50 for BC-1 was 10,000 U/mL for IFN-γ. Growth of BC-1 was not affected by IFN-α (Table 3).
Effects of Cytokines and Other Agents on Clonogenic Growth of KS-1 and BC-1 Cell Lines
| Cytokines and Other Agents . | Effect on Clonal Growth of PEL Cell Lines . | |
|---|---|---|
| KS-1 . | BC-1 . | |
| Human IL-6 antisense | Inhibition | Inhibition |
| (10 μmol/L) | (95%)* | (98%)* |
| Viral IL-6 antisense | NE | NE |
| (1 to 10 μmol/L) | ||
| Human IL-10† | NE | NE |
| Human IL-10 antisense | NE | NE |
| IFN-α | Inhibition | NE |
| (1,000 U/mL) | (99%)* | |
| IFN-γ | Inhibition | NE |
| (1,000 U/mL) | (48%)* | |
| IL-6† | NE | NE |
| GM-CSF† | NE | NT |
| Oncostatin M† | NE | NT |
| IL-4† | NE | NE |
| IL-8† | NE | NE |
| ATRA† | NE | NT |
| hCG α and β† | NE | NT |
| Cytokines and Other Agents . | Effect on Clonal Growth of PEL Cell Lines . | |
|---|---|---|
| KS-1 . | BC-1 . | |
| Human IL-6 antisense | Inhibition | Inhibition |
| (10 μmol/L) | (95%)* | (98%)* |
| Viral IL-6 antisense | NE | NE |
| (1 to 10 μmol/L) | ||
| Human IL-10† | NE | NE |
| Human IL-10 antisense | NE | NE |
| IFN-α | Inhibition | NE |
| (1,000 U/mL) | (99%)* | |
| IFN-γ | Inhibition | NE |
| (1,000 U/mL) | (48%)* | |
| IL-6† | NE | NE |
| GM-CSF† | NE | NT |
| Oncostatin M† | NE | NT |
| IL-4† | NE | NE |
| IL-8† | NE | NE |
| ATRA† | NE | NT |
| hCG α and β† | NE | NT |
Abbreviations: NE, no effect; NT, not tested.
% of inhibition.
Concentration and exposure duration provided in Materials and Methods.
Effects of IL-6 antisense oligonucleotides on clonal growth of PEL cells.
Exposure to human IL-6 antisense oligonucleotides resulted in a significant decrease in the clonal growth of the PEL cells with colony formation nearly 100% inhibited by 10 μmol/L IL-6 antisense oligonucleotides (Figs 3 and4). IL-6 sense or scramble oligonucleotides (10 μmol/L) did not inhibit clonal growth of PEL cells. When cells (105/mL) were treated with IL-6 antisense oligonucleotides for 3 days in suspension culture, IL-6 production was markedly decreased in supernatants from the antisense-treated PEL cells. Production of IL-6 in the sense and antisense oligonucleotides-treated cultures was 200 pg/mL and 50 pg/mL, respectively in KS-1; 4,500 pg/mL and 750 pg/mL, respectively, in BC-1. The vIL-6 and IL-10 antisense oligonucleotides (10 μmol/L) had no effect on clonal growth of the PEL cells (Fig 5 and Table 1). Furthermore, the clonal growth of two KSHV−,EBV−, B-cell lymphoma lines (EW36, DS179) was not inhibited by the IL-6 antisense oligonucleotides (data not shown).
Effect of human IL-6 antisense oligonucleotides on clonal growth of KSHV-infected lymphoma cell lines. The PEL cell lines (A), KS-1; (B), BC-1, and the KSHV-negative lymphoma cell line (C), EW36 were cultured in soft agar with human IL-6 sense (□), scramble (C-1) (○), or antisense (◊)phosphorotioated oligonucleotides at 1, 5, and 10 μmol/L. Results were expressed as a percentage of control cells not exposed to oligonucleotides; results represent the mean ± SD of three experiments done in triplicate. Results with another scramble oligonucleotide (C-2, ≤ 10 μmol/L) showed similar results as the C-1 scramble oligonucleotides.
Effect of human IL-6 antisense oligonucleotides on clonal growth of KSHV-infected lymphoma cell lines. The PEL cell lines (A), KS-1; (B), BC-1, and the KSHV-negative lymphoma cell line (C), EW36 were cultured in soft agar with human IL-6 sense (□), scramble (C-1) (○), or antisense (◊)phosphorotioated oligonucleotides at 1, 5, and 10 μmol/L. Results were expressed as a percentage of control cells not exposed to oligonucleotides; results represent the mean ± SD of three experiments done in triplicate. Results with another scramble oligonucleotide (C-2, ≤ 10 μmol/L) showed similar results as the C-1 scramble oligonucleotides.
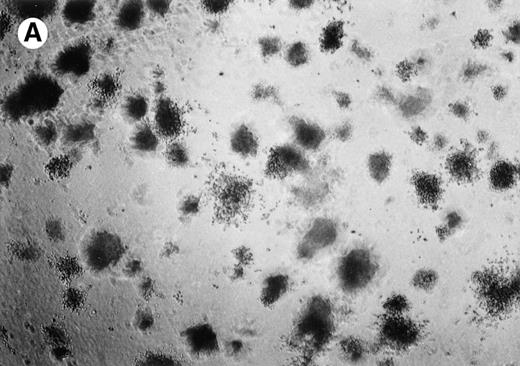
Lymphoma colonies formed by the BC-1 cells treated with either sense or antisense IL-6 oligonucleotides. Cells were plated at 5,000 cells/mL and cultured for 14 days with either IL-6 sense (10 μmol/L) (A) or IL-6 antisense oligonucleotides (10 μmol/L) (B). Colony formation was almost completely inhibited in cultures containing IL-6 antisense oligonucleotides.
Lymphoma colonies formed by the BC-1 cells treated with either sense or antisense IL-6 oligonucleotides. Cells were plated at 5,000 cells/mL and cultured for 14 days with either IL-6 sense (10 μmol/L) (A) or IL-6 antisense oligonucleotides (10 μmol/L) (B). Colony formation was almost completely inhibited in cultures containing IL-6 antisense oligonucleotides.
Effect of viral IL-6 antisense oligonucleotides on clonal growth of KSHV-infected lymphoma cell lines. The PEL cell lines (A) KS-1 and (B) BC-1 were cultured in soft agar with either IL-6 sense (□) or antisense (◊) phosphorotioated oligonucleotides at 1, 5, and 10 μmol/L. Results were expressed as a percentage of control cells not exposed to oligonucleotides; results represent the mean ± standard deviation (SD) of three experiments done in triplicate.
Effect of viral IL-6 antisense oligonucleotides on clonal growth of KSHV-infected lymphoma cell lines. The PEL cell lines (A) KS-1 and (B) BC-1 were cultured in soft agar with either IL-6 sense (□) or antisense (◊) phosphorotioated oligonucleotides at 1, 5, and 10 μmol/L. Results were expressed as a percentage of control cells not exposed to oligonucleotides; results represent the mean ± standard deviation (SD) of three experiments done in triplicate.
DISCUSSION
We detected significant IL-6 production and IL-6R expression in the KSHV-associated PEL cells; and the IL-6 antisense oligonucleotides decreased clonal growth of these PEL cells. IL-6 has been suggested to be involved in three other KSHV-associated diseases, KS,11 Castleman's disease,29 and multiple myeloma.30 Studies have suggested that IL-6 is an autocrine growth factor for AIDS-KS cells.11 Taken together, these data suggest that IL-6 production may have an important role in the pathogenesis of KSHV-associated diseases. In our studies, even though IL-6 antisense oligonucleotides profoundly inhibited the clonal proliferation of the PEL, exogenously added IL-6 did not enhance and IL-6 antibodies did not inhibit clonal growth of the KSHV-infected cells. These results suggest that the IL-6 stimulatory signal may be transmitted via an intracellular interaction between IL-6 and its receptor.31
Our previous data showed that TPA induced lytic viral production in KS-1 cells, and Renne et al32 and Verbeek et al33 have found that TPA-treatment also induced lytic viral production in BC-1 cells. Our present data showed that TPA suppressed human IL-6 production from the PEL cells. Therefore, activation of KSHV does not stimulate production of human IL-6 from the PEL cells.
Of particular interest is how IFN-α and IFN-γ cause growth inhibition of the PEL cells. These IFNs did not suppress production of IL-6 by the PEL cells, and the inhibition of clonal growth of the PEL cells caused by the IFNs could not be reversed by the addition of IL-6 (data not shown). Therefore, the growth inhibitory effect of IFNs does not occur as a result of downregulation of IL-6 production by the PEL cells.
The GM-CSF, IL-1β, TNF-α, bFGF, and PDGF-α cytokines are expressed in KS, but were not detected in the PEL cells. Thus, these cytokines may not be closely associated with KSHV infection, and their production in KS may be due to the KS phenotype. Furthermore, both ATRA and hCGH have been reported to inhibit the growth of KS,34 35 but we found that these compounds did not suppress the growth of the PEL cells; again showing the difference in response of two different KSHV-associated malignancies.
The growth control of PEL cells appears to be different from that of other AIDS-related lymphomas. Both IL-10 and IL-12, which have been reported as autocrine growth factors for AIDS-related lymphomas,22,23 do not appear to be important for the growth of the PEL cells. Also, expression of c-kit was not detected in the PEL cells. This cytokine receptor is usually expressed on CD30+, anaplastic large cell lymphomas (ALCL).36 Some PEL cases expressed CD30 on their surface and showed ALCL-like morphology.4 The expression of c-kit may be a good marker to distinguish PEL from ALCL.
This and the study of Moore et al18 are several of the initial reports describing the expression and regulation of cytokines and their receptors associated with PEL. We provide evidence that IL-6 is associated with the growth of PEL. The analysis of the level of IL-6 production using sera and effusions from individuals with PEL may provide an indirect marker of extent of disease and/or prognosis. Moreover, our results indicate that IL-6 antisense oligonucleotides might be a new therapeutic approach for PEL. In vivo experiments using the IL-6 antisense oligonucleotides are now ongoing in our laboratory.
ACKNOWLEDGMENT
We thank Dr Ethel Cesarman for providing us BC-1 and BC-2 cell lines, Dr David Benjamin for very generously providing the EW36 and DS179 cell lines, and Scholastica Park and Marge Goldberg for technical assistance.
Supported in part by National Institutes of Health Grants No. UO1 CA 66533-02 and CA 42710, the Concern Foundation, and the Parker Hughes Trust. H.P.K. is a member of the Jonsson Comprehensive Cancer Center and holds the Mark Goodson Chair in Oncology Research. D.J.P. is supported in part by the UCLA AIDS Institute grant (UCLA CFAR, NIAID, NIH).
Address reprint requests to H. Phillip Koeffler, MD, Department of Medicine, Division of Hematology/Oncology, Cedars-Sinai Medical Center/UCLA School of Medicine, 8700 Beverly Blvd, B208, Los Angeles, CA 90048.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal