Abstract
Polyclonal horse antilymphocyte and rabbit antithymocyte globulins (ATGs) are currently used in severe aplastic anemia and for the treatment of organ allograft acute rejection and graft-versus-host disease. ATG treatment induces a major depletion of peripheral blood lymphocytes, which contributes to its overall immunosuppressive effects. Several mechanisms that may account for lymphocyte lysis were investigated in vitro. At high concentrations (.1 to 1 mg/mL) ATGs activate the human classic complement pathway and induce lysis of both resting and phytohemagglutinin (PHA)-activated peripheral blood mononuclear cells. At low, submitogenic, concentration ATGs induce antibody-dependent cell cytotoxicity of PHA-activated cells, but not resting cells. They also trigger surface Fas (Apo-1, CD95) expression in naive T cells and Fas-ligand gene and protein expression in both naive and primed T cells, resulting in Fas/Fas-L interaction-mediated cell death. ATG-induced apoptosis and Fas-L expression were not observed with an ATG preparation lacking CD2 and CD3 antibodies. Susceptibility to ATG-induced apoptosis was restricted to activated cells, dependent on IL-2, and prevented by Cyclosporin A, FK506, and rapamycin. The data suggest that low doses of ATGs could be clinically evaluated in treatments aiming at the selective deletion of in vivo activated T cells in order to avoid massive lymphocyte depletion and subsequent immunodeficiency.
THE POLYCLONAL antilymphocyte or antithymocyte globulins (ATG)* are potent immunosuppressive agents used in organ transplantation since the late 1960s. They have proved effective either as rescue treatment of first rejection episodes and graft-versus-host reaction or as prophylactic treatment of rejection.1 As an alternative to polyclonal ATGs, monoclonal antibody (MoAb) OKT3 has been extensively used in organ transplantation.2,3 However, in clinical studies, polyclonal ATGs compare favorably to OKT3 both for prophylactic use or in rescue therapy.4 The precise mechanism of action of ATGs is undefined, but the profound lymphocytopenia observed throughout the treatment period mainly contributes to the immunosuppressive effect. Various mechanisms have been proposed to explain lymphocyte depletion, including complement-mediated cytolysis or clearance of lymphocytes by opsonization and phagocytosis by macrophages.5 ATGs are a mixture of multiple antibodies to various lymphocyte surface antigens.6-8 It was recently reported that antibodies specific for HLA class I molecules,9-11 and antibodies to CD2,12,13 CD30,14 CD45,15 and CTLA-416 could induce apoptosis of T cells, whereas anti-HLA class II and anti-HLA class I antibodies can also trigger apoptosis of activated B cells.17 Antibodies to CD2, CD3, CD45, and HLA molecules were identified in ATGs; it may therefore be hypothesized that their binding either to resting or to activated T cells, or both, may trigger a signal of programmed cell death. Furthermore, ATGs contain antibodies to CD2 and CD3, which account for their mitogenic properties.7 Repeated activation of mature T cells through CD2 or CD3 results in apoptosis of activated T cells.18 The major pathway of this activation-induced cell death (AICD) uses the interaction between Fas (Apo-1, CD95) expressed by activated T and B cells and Fas-ligand (Fas-L, CD95-L) produced by a subset of activated T cells.19-21 The present study was designed to investigate in vitro the different mechanisms whereby ATGs can induce peripheral lymphocyte depletion. To this end, we measured the capacity of ATGs bound to peripheral blood lymphocytes (PBL) to bind human C1q and to induce complement-dependent lysis. We determined their activity in antibody-dependent cell-mediated cytotoxicity (ADCC) and their capacity to induce Fas and Fas-L expression. In all those assays, we compared the sensitivity of naive versus mitogen-activated PBL to ATG-induced lysis, in order to identify those mechanisms that could display some specificity toward preactivated PBL. The dose responses were analyzed according to serum concentrations achieved during treatments. Finally, we evaluated the effect of immunosuppressive drugs that interfere with the interleukin-2 (IL-2) pathway (Cyclosporin A, [CsA], FK506, rapamycin) on the development of the sensitivity to ATG-induced lysis.
MATERIALS AND METHODS
Antibodies and reagents.
Rabbit ATG, batch no. 95-07, and horse antilymphocyte globulins, batches no. 1141 and no. 5, were provided by Dr J. Carcagne (Pasteur Merieux serums & vaccins, Lyon, France). Characteristics of each batch have been previously reported.7 F(ab′)2fragments of ATG no. 95-07 were prepared by pepsin digestion and purified by exclusion chromatography on protein A, following standard procedures. Normal rabbit IgG (Zymed, San Francisco, CA) and horse anti-rabies globulins purified according to the same procedure used for ATGs (Pasteur Merieux serums & vaccins) were used as controls. The anti-CD52 MoAb CAMPATH-1M (IgM) was a gift from Prof H. Waldmann (Sir Dunn School of Pathology, University of Oxford, Oxford, UK). The three anti-Fas MoAbs were used in this study, UB2 for cytofluorometry assays; CH11 (IgM), ZB4 (IgG1), and phycoerythrine streptavidin were obtained from Immunotech (Marseille, France). Fluorescein-isothiocyanate (FITC)-conjugated CD25 and CD69 MoAbs were obtained from Becton Dickinson (Mountain View, CA) and two biotinylated anti-Fas-L one from Pharmingen (San Diego, CA) and the other from Alexis Corporation (Coger S.A., Paris, France). CD3 MoAb OKT3 was from Cilag Laboratories (Levallois-Perret, France).
The lectin phytohemagglutinin (PHA), phorbol myristate acetate (PMA), ionomycin, and cycloheximide (CHX) were obtained from Sigma Chemical Co. (St Louis, MO). Rapamycin (RPM) and FK506 were gifts from Dr A. Altmann (La Jolla Institute for Allergy and Immunology, San Diego, CA), and CsA was kindly supplied by Sandoz (Novartis, Paris, France). Human IL-2 and rIFN-γ were kindly provided by Dr J. Banchereau (Schering-Plough, Dardilly, France).
Cell preparation.
Peripheral blood was collected from healthy donors in the presence of sodium citrate. After the addition of a calcium chloride solution, blood was defibrinated by gentle rotation of the flask; mononuclear cells were then isolated by centrifugation on a layer of Histopaque (Sigma). Cells were washed three times in Hank's balanced salt solution (HBSS) before culture. Those cell suspensions referred to as PBL were shown to contain 3.8% ± 0.4% monocytes, as defined by expression of CD14. For complement-mediated lysis and ADCC experiments, peripheral blood mononuclear cells (PBMC) were obtained by centrifugation of heparinized blood on a layer of Histopaque.
Culture medium and cell proliferation.
PBL were resuspended in RPMI 1640 (Sigma) supplemented with 10% fetal calf serum (FCS), 2 mmol/L l-glutamine, and antibiotics (penicillin 100 U/mL, streptomycin 100 μg/mL). For the proliferation assay, cells (106/mL) were incubated in 96-well microplates (Costar, Cambridge, MA) in the presence of PHA (5 μg/mL) or with ATGs at the indicated concentrations. Cultures were maintained in a humid atmosphere at 37°C containing 5% CO2 for the indicate time.
Immunofluorescence assays.
Cells were washed with isotonic NaCl/Pi buffer containing 1% bovine serum albumin (BSA) and 0.2% NaN3 (phosphate-buffered saline [PBS]/BSA/azide). Cells (5 × 105) were incubated with 10 μL labeled MoAbs for 30 minutes at 4°C. Then, after two washes in PBS/BSA/azide buffer, cells were fixed with 1% formaldehyde in PBS/BSA/azide buffer and analyzed by flow cytometry with a FACScan (Becton Dickinson, Pont de Claix, France). For intracellular analysis of Fas-L expression, cells were fixed with freshly prepared 2% paraformaldehyde in PBS and permeabilized by saponin (0.33%) (Sigma).
Measurement of apoptosis.
After 3 days of culture, unstimulated or PHA-activated PBL were harvested. Dead cells were removed by centrifugation on a layer of Histopaque (Sigma), and viable cells were washed in HBSS. Viable cells (106/mL) were incubated in 96-well microplates in the presence of ATG or CH11 MoAb. After incubation, cell death was evaluated by three different techniques. Measurement of mitochondrial transmembrane potential by flow cytometry after 3,3′-dihexyloxacarbocyanine (DiOC6) staining22and detection of phosphatidylserine expression by flow cytometry after addition of FITC-conjugated annexin V23 were performed on the same suspensions at the indicated time. Nuclear apoptosis was assessed by fluorescence microscopy after staining with Hoechst 33342 (Sigma) at 10 μg/mL, following previously described methods.24 Nuclear fragmentation or marked condensation of the chromatin with reduction of nuclear size, or both, were considered typical features of apoptotic cells. On the basis of these measurements, results were expressed either as percentage of apoptotic cells or as percentage of specific apoptosis according to the formula
RNA isolation, reverse transcription, PCR amplification of Fas-ligand mRNA, and quantification.
Total cellular RNA was isolated from 5 × 106 cells, following the method of Chomczynski and Sacchi.25 Reverse transcription of 1 μg RNA was performed using the first-stand cDNA synthesis kit (Pharmacia Biotech, Orsay, France) in a total reaction volume of 15 μL. After 90 minutes at 37°C, the reaction was terminated by heating for 4 minutes at 95°C. PCR was performed in mixtures containing 1 μL cDNA derived from 10 ng total RNA, primers (100 ng of each; Eurogentech, Seraing, Belgium), 2.5 μL 10 × PCR buffer (Promega, Charbonnieres, France) containing 1.5 mmol/L MgCl2, 0.05 mmol/L of each dNTP, and 0.5 U of Taq polymerase (Promega). Primers for Fas-L and Actin included Fas-L sense primer 5′CCATTT-AAC-AGG-CAA-GTC-CAA-CTC-3′, Fas-L anti-sense primer 5′CAA-CAT-TCT-CGG-TGC-CTG-TAA-C-3′, actin sense primer 5′GGG-TCA-GAA-GGA-TTC-CTA-TG 3′, and actin anti-sense primer 5′GGTCTCAAACATGSATCTGGG-3′. These primers were designed to discriminate between the amplification of cDNA (low size PCR products) and contaminating genomic cDNA (high size PCR products). For each amplicon, 23 to 35 amplification cycles (1 minute at 94°C, 1 minute at 58°C, and 1 minute at 72°C) were performed with the PCR system 9600 (Perkin Elmer, Montigny-le-Bretonneux, France). Semiquantitative evaluation of amplification products was performed as described by Morgan et al.26 Briefly, each PCR product (15 μL) was electrophoresed on agarose gel (2%) stained with ethidium bromide and photographed using polaroid type 665 positive/negative film. The specificity of PCR reaction was confirmed by the expected size of the amplification products. The PCR signal intensities were quantitated by scanning the negative film using a Desktop Scanning Densitometer (PDI/Pharmacia Biotech, Saint-Quentin-Yvelines, France) and by evaluating the integrated trace optical density (OD) for each band using Quantity One Software (PDI/Pharmacia Biotech). The point for samples comparison in the exponential amplification range was selected by inspection from semi-logarithmic plots of OD versus cycle numbers. To correct for variations in the amount of input cDNA, results were expressed as the ratio Fas-L OD/actin OD at the point previously determined.
Complement-mediated lysis.
Resting or PHA-activated PBMC were labeled with Na251 CrO4 for 2 hours at room temperature and washed twice. They were resuspended in medium at 2 × 106 cells/mL, and 100 μL of the suspension was added to round-bottomed microtiter plates containing 50 μL of an appropriate dilution of the antibody. After incubation for 10 minutes at room temperature, 50 μL of 40% fresh or heat-inactivated (56°C, 30 minutes) autologous serum (obtained from defibrinated blood) was added. The cell suspensions were incubated at 37°C for 30 minutes, then centrifuged at 100g for 2 minutes, and 100 μL of the supernatant was collected for measurement of released radioactivity. Controls without antibody were used to measure the spontaneous radioactivity release. The percentage of specific 51Cr release was calculated using the formula
C1q binding.
A total of 20 μL of ATGs or control Ig in PBS/BSA/azide was added to PBMC pellets (4 × 105) and incubated at 37°C for 30 minutes. After two washes in PBS, samples were separated in two and incubated at room temperature for 30 minutes in the presence of 50 μL of autologous serum or heat-inactivated (56°C, 30 minutes) serum as a control. After two washes, cells were incubated with 10 μL of polyclonal goat anti-C1q FITC antibody (1/50 Cappel, Durham, NC) at 4°C for 30 minutes. After two washes, cells were fixed with 1% formaldehyde in PBS/BSA/azide buffer and analysis performed on a FACScan flow cytometer.
Antibody-dependent cell cytotoxicity.
Resting and PHA-activated PBMC were labeled with Na251 CrO4 for 2 hours at room temperature and washed twice. They were resuspended in medium at 1 × 106 cells/mL, and 50 μL of the suspension was added to round-bottomed microtiter plates containing 50 μL of an appropriate dilution of the antibody. After incubation for 10 minutes at room temperature, 100 μL of effector cells (25 × 106cells/mL) was added. The cell suspensions were incubated at 37°C for 6 hours, then centrifuged at 100g for 2 minutes and 100 μL of the supernatant collected for measurement of released radioactivity as for complement-mediated lysis.
RESULTS
ATGs induce apoptosis of activated lymphoblasts.
Knowing that ATGs could induce apoptosis of B-cell lines and to a lesser extent, T-cell lines,27 we examined whether such mechanism could also take part in the elimination of peripheral T lymphocytes. Three-day PHA-activated PBL, as well as nonactivated PBL, were treated with ATG no. 95-07, F(ab′)2 fragments of ATG, anti-Fas MoAb CH11 as positive control, and normal rabbit IgG as negative control. Apoptosis was evaluated by DiOC6[3] and annexin V staining (Fig 1) and by fluorescence microscopy after staining with Hoechst 33342 (Fig2). The results showed that ATG no. 95-07 at nonmitogenic concentrations (10 μg/mL), their F(ab′)2fragments, and the anti-Fas MoAb CH11 induced apoptosis of 30% to 40% of PHA-activated PBL, whereas resting PBL were not sensitive (Figs 1and 2). Similar results were observed with ATG no. 1141 obtained from horse (data not shown). Interestingly ATG no. 5 containing CD18, CD11a, anti-β2m, and anti-HLA DR antibodies, but no CD3, CD2, and CD5 specificities, and which is not mitogenic at concentrations ranging from 1 to 1,000 μg/mL, did not induce apoptosis at 10 and 100 μg/mL (Fig 2; data not shown). Normal rabbit did not induce cell death of resting or activated PBL (Fig 2). Similar experiments were repeated with PBL activated by a 3-day culture period with PMA (10 ng/mL) plus ionomycin (500 ng/mL), PMA (10 ng/mL) plus OKT3 (100 ng/mL), or a mitogenic concentration of ATG no. 95-07 or no. 1141 (100 μg/mL). Whatever the activator used, the addition of ATGs (10 μg/mL) or F(ab′)2 fragments thereof resulted in specific apoptosis ranging from 20% to 50% (data not shown).
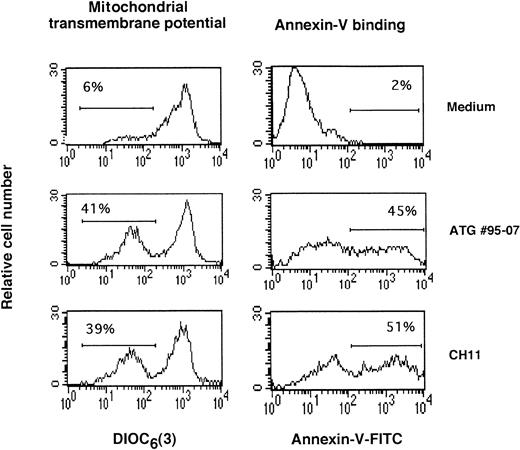
Effect of ATGs on mitochondrial transmembrane potential and on phosphatidylserine expression. PBL were activated for 3 days in presence of PHA (5 μg/mL). After removal of dead cells, medium alone, ATG no. 95-07 (10 μg/mL), or CH11 anti-Fas MoAb (1 μg/mL) was added. After 12 hours, ▵Ψm modifications were evaluated by staining with DiOC6 (3). The expression of phosphatidylserine at the surface membrane was evaluated after 15 hours by measuring annexin-V binding. The percentage of cells with decreased mitochondrial potential membrane or increased expression of phosphatidylserine are indicated for each histogram. Results from one typical experiment among four showing similar percentages.
Effect of ATGs on mitochondrial transmembrane potential and on phosphatidylserine expression. PBL were activated for 3 days in presence of PHA (5 μg/mL). After removal of dead cells, medium alone, ATG no. 95-07 (10 μg/mL), or CH11 anti-Fas MoAb (1 μg/mL) was added. After 12 hours, ▵Ψm modifications were evaluated by staining with DiOC6 (3). The expression of phosphatidylserine at the surface membrane was evaluated after 15 hours by measuring annexin-V binding. The percentage of cells with decreased mitochondrial potential membrane or increased expression of phosphatidylserine are indicated for each histogram. Results from one typical experiment among four showing similar percentages.
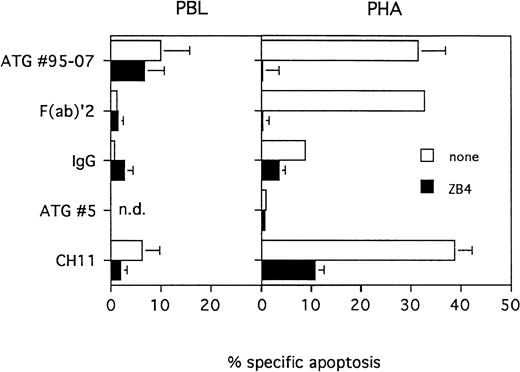
ATGs induce apoptosis of activated T lymphocytes. PBL were cultured in presence of medium alone or PHA (5 μg/mL) for 3 days. Dead cells were removed and viable cells were treated for 20 hours with ATG no. 95-07, F(ab)′2 fragments of ATG no. 95-07, ATG no. 5 or normal rabbit IgG at 10 μg/mL or with the agonist anti-Fas MoAb CH11 at 1 μg/mL. Protection by the antagonist anti-Fas MoAb, was tested by pre-incubating PBL or PHA-activated cells for 1 hour with ZB4 MoAb at 2 μg/mL. The percentage of apoptotic cells was determined by fluorescent microscopy after staining with Hoechst 33342. Results are expressed as mean ± SEM of five different experiments or as mean of two experiments for ATG no. 5.
ATGs induce apoptosis of activated T lymphocytes. PBL were cultured in presence of medium alone or PHA (5 μg/mL) for 3 days. Dead cells were removed and viable cells were treated for 20 hours with ATG no. 95-07, F(ab)′2 fragments of ATG no. 95-07, ATG no. 5 or normal rabbit IgG at 10 μg/mL or with the agonist anti-Fas MoAb CH11 at 1 μg/mL. Protection by the antagonist anti-Fas MoAb, was tested by pre-incubating PBL or PHA-activated cells for 1 hour with ZB4 MoAb at 2 μg/mL. The percentage of apoptotic cells was determined by fluorescent microscopy after staining with Hoechst 33342. Results are expressed as mean ± SEM of five different experiments or as mean of two experiments for ATG no. 5.
ATG-induced apoptosis is fully inhibited by an antagonist anti-Fas antibody.
The apoptotic activity of ATGs was effective only on activated T cells, which express Fas and which are sensitive to Fas-mediated apoptosis28; we therefore studied whether ATG-induced apoptosis was dependent on Fas/Fas-L interaction. To this end, PHA-activated PBL were incubated for 1 hour with the antagonist anti-Fas MoAb ZB4, which blocks the interaction between Fas and Fas-L, before addition of ATG no. 95-07, ATG F(ab′)2 fragments or CH11 MoAb. As shown in Fig 2, ATG-induced apoptosis was completely blocked by ZB4, indicating that ATG-induced apoptosis of activated T cells required Fas/Fas-L interaction. This idea was re-enforced by the observation that simultaneous addition of ATG no. 95-07 (10 μg/mL) and CH11 resulted in the same percentage of apoptotic cells as with each antibody tested alone (data not shown). This result suggests that the same subset of activated T cells is the target of ATGs and anti-Fas antibodies. Furthermore, it shows that ATGs do not contain anti-Fas blocking antibodies, at least in sufficient amount to be detected in this assay.
ATGs induce Fas and Fas-L expression.
In an effort to obtain further evidence for a possible role of Fas/Fas-L interaction in ATG-induced apoptosis, we examined whether ATGs would induce Fas-L expression in both resting and activated-PBL. To this end, PBL were first cultured in presence of a mitogenic concentration of ATG no. 95-07 (100 μg/mL) or PHA or medium alone for 3 days. After elimination of dead cells, preactivated PBL were then incubated for 6 hours with medium alone, ATG no. 95-07 at nonmitogenic (10 μg/mL) and mitogenic (100 μg/mL) concentrations or PHA, and induction of Fas-L mRNA was analyzed by RT-PCR. ATG no. 95-07 at either 10 or 100 μg/mL induced Fas-L mRNA expression by nonactivated and by preactivated-PBL (Fig 3). Similar experiments performed with freshly isolated PBL showed that ATG no. 95-07 (10 and 100 μg/mL), but not control rabbit IgG, strongly induced Fas-L mRNA expression (Fig 3).
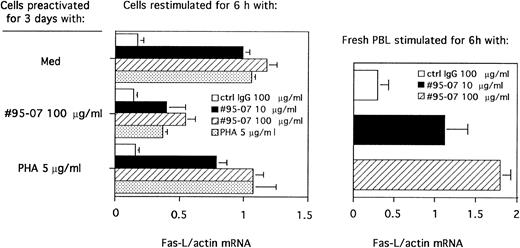
Expression of Fas-L mRNA induced by ATGs. (Left) PBL were cultured in presence of medium alone, ATG no. 95-07 (100 μg/mL) or PHA (5 μg/mL) for 3 days. Dead cells were removed, and viable cells were stimulated with normal rabbit IgG at 100 μg/mL, ATG no. 95-07 at 10 μg/mL and 100 μg/mL or PHA at 5 μg/mL for 6 hours. (Right) Freshly isolated PBL were stimulated with normal rabbit IgG at 100 μg/mL or ATG no. 95-07 at 10 μg/mL and 100 μg/mL for 6 hours. mRNA of each sample was amplified by RT-PCR as described in Materials and Methods with primers specific for actin or Fas-L. The number of amplification cycles selected within the exponential phase of PCR was 29 for actin and 32 for Fas-L. The PCR products were separated on 2% agarose gel and the PCR signal intensities were quantified by scanning the negative film. Results are expressed as the ratio of absorbance of Fas-L/absorbance of actin (mean ± SEM of three experiments).
Expression of Fas-L mRNA induced by ATGs. (Left) PBL were cultured in presence of medium alone, ATG no. 95-07 (100 μg/mL) or PHA (5 μg/mL) for 3 days. Dead cells were removed, and viable cells were stimulated with normal rabbit IgG at 100 μg/mL, ATG no. 95-07 at 10 μg/mL and 100 μg/mL or PHA at 5 μg/mL for 6 hours. (Right) Freshly isolated PBL were stimulated with normal rabbit IgG at 100 μg/mL or ATG no. 95-07 at 10 μg/mL and 100 μg/mL for 6 hours. mRNA of each sample was amplified by RT-PCR as described in Materials and Methods with primers specific for actin or Fas-L. The number of amplification cycles selected within the exponential phase of PCR was 29 for actin and 32 for Fas-L. The PCR products were separated on 2% agarose gel and the PCR signal intensities were quantified by scanning the negative film. Results are expressed as the ratio of absorbance of Fas-L/absorbance of actin (mean ± SEM of three experiments).
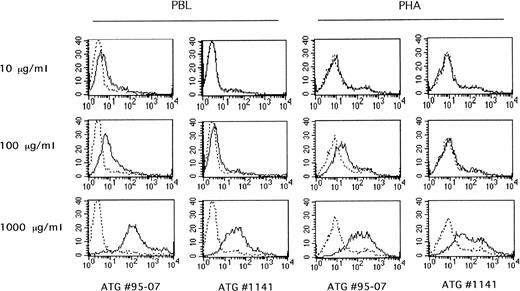
In parallel, surface expression of Fas and Fas-L molecules, but CD25 and CD69 activation markers as well, was analyzed by flow cytometry on PBL cultured in the presence of ATG no. 95-07 at 10 and 100 μg/mL for 1 to 3 days. At mitogenic concentrations (100 μg/mL), ATG no. 95-07 induced CD69, CD25, Fas, and Fas-L expression (Fig4). Surface expression of Fas, CD69, and CD25 reached a maximum at day 2, and that of Fas-L at day 1. At nonmitogenic concentrations (ie, 10 μg/mL), ATG no. 95-07 still induced expression of CD69, Fas, and Fas-L, but not that of the CD25 molecule, suggesting that, at low concentrations, ATGs drive lymphocytes into the G1 phase of the cell cycle but did not allow them to progress to S phase because of the absence of CD25 expression. Interestingly ATG no. 5 at 100 μg/mL did not induce CD69 Fas and Fas-L expression (Fig 4), nor did it trigger apoptosis (Fig 2). Finally, these experiments were completed by intracellular staining of Fas-L in paraformaldehyde-fixed and saponin-permeabilized cells. The results indicate that ATG no. 95-07 (10 and 100 μg/mL) increased intracellular Fas-L in both resting and preactivated PBL, with a maximum on days 1 to 2 (Fig 4; data not shown). Histograms of fluorescence (Fig 4) show that a small subset of PBL is positive before activation, whereas after stimulation by ATG, most of the lymphocyte population becomes Fas-L positive.
Effect of ATGs on CD69, CD25, Fas, and Fas-L surface expression. PBL were cultured in presence of medium alone or ATG no. 95-07 and ATG no. 5 at 10 μg/mL and 100 μg/mL for 3 days. At days 0, 1, 2, and 3, surface expression of CD69, CD25, Fas, and Fas-L was determined by cytofluorometry. In parallel, incorporation of [3H]TdR uptake during the last 8 hours of culture was measured (med 367 ± 41 cpm, ATG no. 5 10 μg/mL 391 ± 23 cpm, ATG no. 5 100 μg/mL 252 ± 26 cpm, ATG no. 95-07 10 μg/mL 532 ± 53 cpm, and ATG no. 95-07 100 μg/mL 11,500 ± 103 cpm). Histograms of Fas-L expression at day 1 are shown. Representative of four experiments with ATG no. 95-07 and of two with ATG no. 5.
Effect of ATGs on CD69, CD25, Fas, and Fas-L surface expression. PBL were cultured in presence of medium alone or ATG no. 95-07 and ATG no. 5 at 10 μg/mL and 100 μg/mL for 3 days. At days 0, 1, 2, and 3, surface expression of CD69, CD25, Fas, and Fas-L was determined by cytofluorometry. In parallel, incorporation of [3H]TdR uptake during the last 8 hours of culture was measured (med 367 ± 41 cpm, ATG no. 5 10 μg/mL 391 ± 23 cpm, ATG no. 5 100 μg/mL 252 ± 26 cpm, ATG no. 95-07 10 μg/mL 532 ± 53 cpm, and ATG no. 95-07 100 μg/mL 11,500 ± 103 cpm). Histograms of Fas-L expression at day 1 are shown. Representative of four experiments with ATG no. 95-07 and of two with ATG no. 5.
Interference with the IL-2 pathway reduces ATGs-induced apoptosis.
Knowing that IL-2 is required for acquisition of susceptibility to Fas-mediated apoptosis,29,30 we analyzed the effect of immunosuppressive agents that interfere with the IL-2 pathway on ATG-induced cell death. PBL were cultured with PHA in the presence of CsA or FK506, which block IL-2 expression at a transcriptional level, or with RPM, which blocks IL-2 signaling. After 3 days, cells were treated with ATGs or F(ab′)2 fragments. The presence of CsA, FK506, or RPM, during T-cell activation, markedly decreased apoptosis mediated by ATG no. 95-07 or their F(ab′)2fragments (Fig 5). In keeping with these results, we observed that addition of rIL-2 during the last 24 hours of cell culture, to PBL activated by PHA in the presence of CsA restored the sensitivity to ATG and F(ab′)2-induced apoptosis (Fig5B). Conversely, the addition of interferon-γ (IFN-γ) restored T-cell proliferation,29 but not the sensitivity to ATG-induced apoptosis. In agreement with previous reports,29 30 similar effects were observed as regards sensitivity to Fas-mediated apoptosis (Fig 5B).
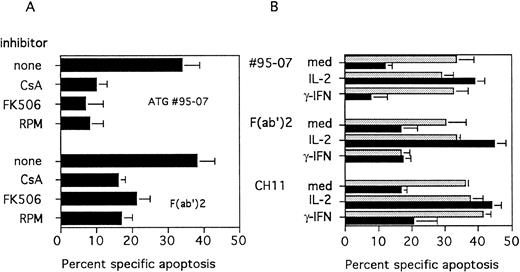
(A) Effect of immunosuppressive agents on ATG-mediated apoptosis. PBL were cultured for 3 days with PHA (5 μg/mL) and CsA (250 ng/mL), FK506 (10 nmol/L) or RPM (60 nmol/L) were added at the onset of the culture. Apoptosis was determined by fluorescence microscopy after staining with Hoechst 33342, 20 hours after treatment with ATG no. 95-07 or their F(ab′)2 fragments at 10 μg/mL. (B) Effect of addition of exogenous IL-2 or IFN-γ. PBL were cultured for 3 days with PHA (5 μg/mL); medium alone (gray bars) or CsA (250 ng/mL) (black bars) were added at the onset of the culture. Recombinant IL-2 (25 U/mL) or rIFN-γ (500 U/mL) was added during the last 24 hours of activation. Apoptosis was determined by fluorescence microscopy after staining with Hoechst 33342, 20 hours after treatment with ATG no. 95-07, their F(ab′)2 fragments at 10 μg/mL or the CH11 (1 μg/mL) MoAb. (Results are expressed as mean ± SEM of three different experiments).
(A) Effect of immunosuppressive agents on ATG-mediated apoptosis. PBL were cultured for 3 days with PHA (5 μg/mL) and CsA (250 ng/mL), FK506 (10 nmol/L) or RPM (60 nmol/L) were added at the onset of the culture. Apoptosis was determined by fluorescence microscopy after staining with Hoechst 33342, 20 hours after treatment with ATG no. 95-07 or their F(ab′)2 fragments at 10 μg/mL. (B) Effect of addition of exogenous IL-2 or IFN-γ. PBL were cultured for 3 days with PHA (5 μg/mL); medium alone (gray bars) or CsA (250 ng/mL) (black bars) were added at the onset of the culture. Recombinant IL-2 (25 U/mL) or rIFN-γ (500 U/mL) was added during the last 24 hours of activation. Apoptosis was determined by fluorescence microscopy after staining with Hoechst 33342, 20 hours after treatment with ATG no. 95-07, their F(ab′)2 fragments at 10 μg/mL or the CH11 (1 μg/mL) MoAb. (Results are expressed as mean ± SEM of three different experiments).
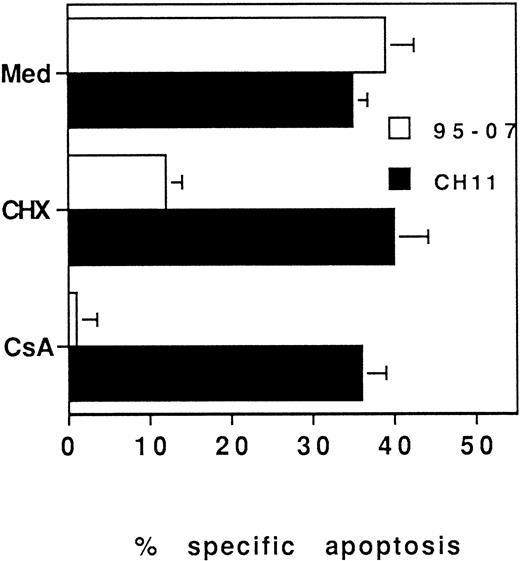
Furthermore, CsA and FK506 were described as strongly inhibiting Fas-L expression in murine T-cell hybridomas.31 Thus, we have tested whether incubation of 3-day PHA-activated PBL with CsA, just before ATG treatment would interfere with ATG-induced apoptosis. A 3-hour preincubation of PHA-blasts with CsA or CHX inhibited ATG-induced cell death but did not interfere with apoptosis induced by the anti-Fas MoAb (Fig 6). These data suggest that immunosuppressive agents that interfere with the IL-2 pathway can prevent ATG-induced apoptosis by inhibiting either Fas-L synthesis or the acquisition of sensitivity to Fas-L–mediated cell death by activated T cells.
ATG-induced apoptosis is inhibited by CsA and requires protein synthesis. PBL were incubated for 3 days in the presence of PHA (5 μg/mL). Dead cells were removed and viable cells were incubated for 3 hours with CsA (250 ng/mL) or CHX (0.5 μg/mL) before treatment with ATG no. 95-07 (10 μg/mL) or CH11 (1 μg/mL). Apoptosis was determined by fluorescence microscopy after staining with Hoechst 33342. Results are expressed as mean ± SD of three different experiments.
ATG-induced apoptosis is inhibited by CsA and requires protein synthesis. PBL were incubated for 3 days in the presence of PHA (5 μg/mL). Dead cells were removed and viable cells were incubated for 3 hours with CsA (250 ng/mL) or CHX (0.5 μg/mL) before treatment with ATG no. 95-07 (10 μg/mL) or CH11 (1 μg/mL). Apoptosis was determined by fluorescence microscopy after staining with Hoechst 33342. Results are expressed as mean ± SD of three different experiments.
ATGs induce complement-mediated cytolysis at supramitogenic concentrations.
Binding of human C1q was measured by incubation of PBL in the presence of ATGs and fresh human serum, followed by flow cytometry assessment of the amount of bound C1q per cell. Heat-inactivated human serum was used as control. Maximal binding was achieved at 1 mg/mL. At lower ATG concentrations, only rabbit, but not equine, ATG bound C1q (Fig7). C1q binding was comparable between resting PBL and preactivated cells.
C1q binding to PBL or PHA-blasts sensitized with ATGs. PBL or PHA blasts were labeled with increasing amount of rabbit ATG (no. 95-07) or horse ATG (no. 1141) and then with autologous serum (solid line) or heat-inactivated serum as control (dashed line). C1q binding was detected by using FITC-goat anti-C1q polyclonal antibody and cell analyzed by flow cytometry as described in Materials and Methods. Representative of three independent experiments.
C1q binding to PBL or PHA-blasts sensitized with ATGs. PBL or PHA blasts were labeled with increasing amount of rabbit ATG (no. 95-07) or horse ATG (no. 1141) and then with autologous serum (solid line) or heat-inactivated serum as control (dashed line). C1q binding was detected by using FITC-goat anti-C1q polyclonal antibody and cell analyzed by flow cytometry as described in Materials and Methods. Representative of three independent experiments.
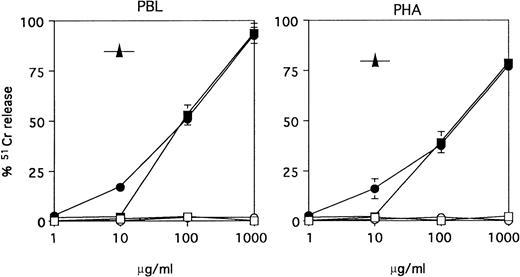
The ability of ATGs to induce resting or PHA-activated PBMC lysis was evaluated in the presence of an exogenous source of human complement. Minimal cytolysis was observed at 10 μg/mL with equine ATG, whereas maximal cytolysis was only achieved at very high concentrations (1 mg/mL) of ATGs. As a positive control of complement-mediated cytolysis, we used the CAMPATH-1M MoAb, which, in agreement with a previous report,32 induced about 80% lysis at 10 μg/mL. Of note, no difference was observed, whether ATGs were obtained from horse (no. 1141) or rabbit (no. 95-07), and whether resting or PHA-activated PBMC were used as target cells in the complement-dependent lysis assay (Fig8).
Complement-mediated lysis of PBMC versus PHA-Blasts. PBMC or 3-day PHA-activated PBMC were labeled with 51Cr and incubated with rabbit ATG (no. 95-07) (▪), horse ATG (no. 1141) (•), control horse (○) or rabbit IgG (□) or the anti-CD52 MoAb CAMPATH-1M (IgM) (▴) at the indicated concentrations, for 30 minutes at 37°C, in the presence of 10% autologous serum. Results are expressed as specific release as defined in Materials and Methods (mean ± SEM of three different experiments).
Complement-mediated lysis of PBMC versus PHA-Blasts. PBMC or 3-day PHA-activated PBMC were labeled with 51Cr and incubated with rabbit ATG (no. 95-07) (▪), horse ATG (no. 1141) (•), control horse (○) or rabbit IgG (□) or the anti-CD52 MoAb CAMPATH-1M (IgM) (▴) at the indicated concentrations, for 30 minutes at 37°C, in the presence of 10% autologous serum. Results are expressed as specific release as defined in Materials and Methods (mean ± SEM of three different experiments).
ATGs induce antibody-dependent cell cytotoxicity at low concentrations.
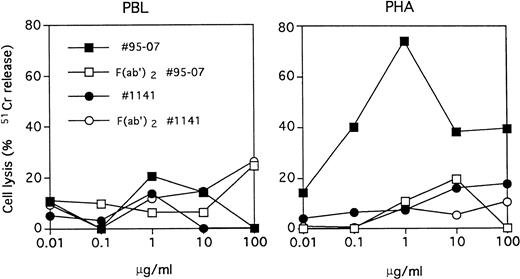
ATGs no. 95-07 and no. 1141 were tested for their ability to induce ADCC of both resting and PHA-activated PBMC. We observed that this effect was concentration dependent, with a maximal cytotoxicity at 1 μg/mL of ATG no. 95-07 and effective only when PHA-activated PBMC were used as target cells (Fig 9). As expected, the ADCC phenomenon was not observed with F(ab′)2fragments of ATG no. 95-07 and was restricted to ATG from rabbit origin, because ATG no. 1141 did not induce cell lysis at concentrations ranging from 0.01 to 100 μg/mL.
Antibody-dependent cell cytotoxicity of PBMC versus PHA-blasts. PBMC or 3-day PHA-activated PBMC were labeled with51Cr and incubated with rabbit ATG (no. 95-07), horse ATG (no. 1141), or their F(ab′)2 fragments at the indicated concentrations in presence of effector cells for 6 hours at 37°C. Results are expressed as specific release as in Fig 8. Representative of two independent experiments.
Antibody-dependent cell cytotoxicity of PBMC versus PHA-blasts. PBMC or 3-day PHA-activated PBMC were labeled with51Cr and incubated with rabbit ATG (no. 95-07), horse ATG (no. 1141), or their F(ab′)2 fragments at the indicated concentrations in presence of effector cells for 6 hours at 37°C. Results are expressed as specific release as in Fig 8. Representative of two independent experiments.
DISCUSSION
Both horse antilymphocyte globulins and rabbit ATGs are still used in the treatment of severe aplastic anemia, organ allograft rejection, and graft-versus-host disease (GVHD), but their mechanisms of action remain largely unknown. A major common feature of ATG treatment is peripheral lymphocyte depletion,1,4,5,33 which usually persists throughout the administration period and slowly reverses thereafter. Although not formally demonstrated in clinical studies, lymphocyte depletion is likely to account for the immunosuppressive activity of ATGs.34 The present study addressed the mechanisms of peripheral lymphocytopenia, with special emphasis on the differential susceptibility of preactivated T cells (PHA blasts) versus nonactivated T cells to ATG-induced cell death. ATGs contain multiple antibody specificities with little batch-to-batch variability despite the use of different cell sources (thymocytes, T-cell lines, or B-cell lines) and different immunization protocols.6-8 We therefore tested two ATG preparations of horse anti-human lymphocyte globulins (no. 1141) and rabbit anti-thymocyte globulins (no. 95-07) currently used in organ and bone marrow transplantation, as well as one horse ATG preparation (no. 5) previously used in kidney transplantation (selected because of its highly unusual lack of mitogenic activity related to the absence of demonstrable CD2 and CD3 specificities).7 Horse anti-lymphocyte globulins are administered at 10 to 15 mg/kg/d,33 and rabbit ATGs at 1.0 to 1.2 mg/kg/d, resulting in average serum levels of 0.5 mg/mL and 80 to 200 μg/mL, respectively.5 These dosages have been selected mostly on empiric grounds, but individual dosage adjustment to maintain absolute T-cell numbers of 50 to 100 cells/μL did not result in a major decrease in daily doses.33 It is worth noting that the 10-fold dosage difference between equine and rabbit ATGs is not paralleled by differences in either specific antibody titers (eg, CD2, CD3, CD4, CD8)7 or in vitro functional properties such as T-cell activation5,6,35,36 or B-cell apoptosis.27
Complement-dependent lysis is initiated by the binding of human C1q to ATG-coated cells. At low and intermediate ATG concentrations, C1q binding was demonstrable with rabbit ATG on both PBL and PHA blasts but remained borderline or not detectable with equine ATG (Fig 7). As shown with chimeric monoclonal antibodies of different isotypes, C1q binding may not be correlated with cell lysis.37 Therefore, we used the highly sensitive chromium release assay to measure complement-dependent lysis. The data (Fig 8) indicate that equine and rabbit ATGs are equally effective on PBMC and PHA blasts, but only at high concentrations. In keeping with our observation, complement consumption, as measured by decreased serum CH50 activity, was recorded in some patients during equine ATG treatment, but never with rabbit ATG (Y. Lebranchu, personal communication, January 1997).
ADCC has been suggested as a possible mechanism of lymphocyte depletion by ATG.1 5 NK cells present in peripheral blood are potent effectors of Fc receptor–dependent cell lysis. Our results indicate that only PHA blasts, but not PBMC, can be lysed through an ADCC mechanism, suggesting that rabbit ATGs could display some selectivity toward preactivated lymphocytes, should a similar mechanism operate in vivo. Equine ATG on the other hand was completely ineffective in this assay.
The major homeostatic mechanism that prevents lymphoid tissue hyperplasia despite repeated antigenic stimulations and T- or B-cell clonal expansion is activation-induced cell death (AICD) mediated by Fas/L-Fas interaction.38 We therefore investigated the possible contribution of the Fas pathway in ATG-induced lympholysis. Fas-L is constitutively expressed in a variety of tissues, including immunologically privileged sites (eg, eye, Sertoli cells), some tumors,39,40 and monocytes41 and produced by a subset of T cells after repeated activation through the TCR/CD3 or CD2 pathways, or both.42 Knowing the T-cell mitogenic properties of ATGs,35,36 we were not surprised to observe that restimulation by ATGs of PBL preactivated by various mitogens, including ATGs themselves, triggered Fas-L gene expression (Fig 3). However, quite unexpectedly, ATGs were also found to induce Fas-L mRNA and protein expression in nonpreactivated PBL, even at low concentrations (10 μg/mL) sufficient to trigger CD69, but not CD25, expression and therefore remain below the mitogenic threshold (Fig 4). Although they express Fas receptors, these CD25 negative cells do not respond to IL-2 and therefore cannot become sensitive to Fas-dependent apoptosis, as discussed below. The fact that blocking Fas/Fas-L interaction completely suppressed ATGs-induced apoptosis (Fig 2) provides unequivocal evidence for a role of the Fas pathway in ATG-mediated lymphocyte cell death. Target cells for Fas-L should not only express Fas receptors that are rapidly induced upon activation, but should also become sensitive to Fas-mediated apoptosis, a property that is strictly dependent on an IL-2 signal.29,30 Hence pharmacological interference with the IL-2 pathway in activated T cells, by the addition of CsA, FK506, or rapamycin, prevents Fas-positive cells from becoming sensitive to ATG- and to Fas-L- (or agonist anti-Fas antibody)–dependent apoptosis. CsA also inhibits Fas-L expression.31 43 Therefore, concomitant administration of ATGs with any immunosuppressive agent that interferes with the IL-2 pathway (eg, CsA, FK506, rapamycin, CTLA-4-Ig, or CD25 antibodies) is likely to prevent Fas-dependent ATG-induced lymphocyte depletion. Furthermore, this mechanism of lymphocyte apoptosis may be impaired in clinical situations associated with high plasma levels of soluble Fas.
In conclusion, this in vitro study describes some of the mechanisms that may account for lymphocyte depletion during ATG therapy. However, one should keep in mind that opsonization and subsequent phagocytosis by spleen, liver, and lung macrophages is likely to account for the massive and rapid lymphocytopenia observed with the current protocols. Nevertheless, other mechanisms should be considered, some of which could represent a therapeutic objective in the design of future protocols aimed at a more selective immunosuppression. Complement-dependent lysis does not discriminate between resting and preactivated T cells. Because it is achieved at high ATG concentrations, it may occur in treatment with horse ATG, but this is less likely with rabbit ATG. In this respect, the relevance of complement-dependent lymphocytotoxicity for the standardization of ATG preparations is questionable. Serum ATG concentrations achieved with current dosages are mitogenic for peripheral T cells. Hence, they could trigger Fas-L expression and induce sensitivity to Fas-L in the vast majority of T cells, unless CsA or FK506 that block these processes is administered concomitantly. An important finding of this study is that some ATG at low, submitogenic concentrations may trigger Fas-L expression, resulting in the selective death of preactivated, but not resting, lymphocytes. An ATG preparation (no. 5) lacking mitogenic activity, and with no demonstrable CD2 and CD3 specificities, was devoid of this property, suggesting that “lymphocyte activating” antibodies (eg, CD2, CD3) may be critical in achieving Fas-dependent apoptosis. Similarly, ADCC that also occurs at low rabbit ATG concentration selectively targets activated, but not resting, T cells. These properties could be used in protocols aiming at the selective elimination of in vivo activated T cells (eg, donor-specific alloreactive T cells in organ transplantation, recipient-specific T cells in GVHD), while sparing nonactivated T cells. Such protocols would require much lower doses than those currently used, in order to maintain serum ATG concentrations within a 10- to 20-μg/mL range, instead of 100 μg/mL. Their feasibility will be evaluated in the cynomolgus monkey and, depending on the outcome of these experiments, clinical trials may be considered.
ACKNOWLEDGMENT
Prof Y. Lebranchu (Tours) is thanked for sharing unpublished observations.
Within the context of this report, ATG is used to refer to either antithymocyte or antilymphocyte globulins.
Supported by Institut National de la Santé et de la Recherche Médicale and by Région Rhône Alpes Grant No. H098730000.
The first two authors contributed equally to this work and therefore share the first authorship.
Address reprint requests to Nathalie Bonnefoy-Berard, PhD, INSERM U80, Hôpital E. Herriot, 69437 Lyon Cedex 03, France.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

![Fig. 4. Effect of ATGs on CD69, CD25, Fas, and Fas-L surface expression. PBL were cultured in presence of medium alone or ATG no. 95-07 and ATG no. 5 at 10 μg/mL and 100 μg/mL for 3 days. At days 0, 1, 2, and 3, surface expression of CD69, CD25, Fas, and Fas-L was determined by cytofluorometry. In parallel, incorporation of [3H]TdR uptake during the last 8 hours of culture was measured (med 367 ± 41 cpm, ATG no. 5 10 μg/mL 391 ± 23 cpm, ATG no. 5 100 μg/mL 252 ± 26 cpm, ATG no. 95-07 10 μg/mL 532 ± 53 cpm, and ATG no. 95-07 100 μg/mL 11,500 ± 103 cpm). Histograms of Fas-L expression at day 1 are shown. Representative of four experiments with ATG no. 95-07 and of two with ATG no. 5.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/7/10.1182_blood.v91.7.2360/3/m_blod4071904.jpeg?Expires=1769778005&Signature=vWe~w72ePIHDiO5g9HjYCPgY43acwkgqM1hPbnK3oxoDTwJWvQj0~gRdJsTjI8nzPIYXbXmHbusq8lVlJOkllITq6eBvNU8PYeA~Q8TfB0Oge9-OMY4BU8v8ce9BuSIZdbezulSCdMue7-mRxm7SwWp7DDgRzb6U2GJ9Zn5aAAJNBxI2cqvrhFuuaG5qVQDHJyVUNCyjOFaAu1-Xu~rgPJXEQ9nKMh23-qZZZhbnNcyumKpgh8vucex2hbRqhWtahvKA45wpFjE8icI9fKu-CRhLzd3XnP5mfYfau1uiGFukVaEarrUXbbFIuLyiIlj5FX7oKxx5AcWNzX4UzNK0KA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal