Abstract
The α4 integrins, which are constitutively expressed on all human leukocyte subtypes except neutrophils, interact with vascular cell adhesion molecule-1 (VCAM-1) and mucosal addressin cell adhesion molecule (MAdCAM-1) on endothelium to mediate selective recruitment of leukocyte subpopulations, other than neutrophils, to sites of inflammation. However, here we report that a different paradigm of leukocyte recruitment may exist in the rat. Flow cytometric analysis of rat neutrophils using a panel of monoclonal antibodies which recognize rat α4 and β1 integrins showed consistent, low levels of expression. Although α4 was expressed at lower levels on neutrophils than all other rat leukocytes, this level of expression was sufficient to mediate significant levels of α4- and β1-dependent neutrophil adhesion to rat and human VCAM-1, and α4-dependent, but β1-independent, adhesion to human MAdCAM-1. These data suggest that rat neutrophils, unlike other species, may use α4 integrins to traffic to sites of inflammation in vivo.
THE INTEGRINS, WHICH mediate leukocyte-endothelial and leukocyte-matrix interactions, are a complex family of heterodimeric glycoproteins consisting of α and β subunit pairs. To date, at least 16 α and eight β subunits have been described and combined to generate over 20 integrin molecules on human cells.1,2 The α4 integrin subunit, first described by Hemler et al3 on T-lymphoblastoid cell lines, has been shown to pair with both the β1 and β7 subunits. By virtue of their ability to interact with endothelial expressed ligands, the α4 integrins α4β1 (very late antigen-4 [VLA-4, CD49d/CD29]) and α4β7 (lymphocyte-Peyer's patch adhesion molecule-1 [LPAM-1, CD49d/CD103]), are believed to play a major role in the recruitment of leukocytes during inflammation. In humans, both α4β1, which binds to endothelial vascular cell adhesion molecule-1 (VCAM-1), and α4β7, which interacts with both VCAM-1 and mucosal addressin cell adhesion molecule-1 (MAdCAM-1), are constitutively expressed on the surface of eosinophils, basophils, and lymphocytes, but are not detected on neutrophils, whereas monocytes only express α4β1.4,5 This limited pattern of α4 integrin expression has been theorized to contribute to the selective recruitment of leukocyte subtypes other than neutrophils to sites of inflammation. In addition, the interactions of α4β1 and α4β7 with their endothelial ligands are unique in that unlike the β2 integrins, α4 integrins can mediate both leukocyte rolling and firm adherence to the endothelial surface.6-9
Based on the use of blocking antibodies, numerous in vivo studies suggest that α4 integrins can play a role in selective leukocyte recruitment in inflammatory disease processes such as allergic inflammation,10-12 arthritis,13 and delayed-type hypersensitivity.14 Although antibodies to both α4 integrins and VCAM-1 have been used to study selective leukocyte recruitment in various animal models, there has been no thorough analysis performed to establish whether α4 integrin expression on leukocytes in various species is the same as that observed in humans. Though guinea pig and sheep neutrophils do not express α4 integrins,12,15,16 some studies in rats and mice have unexpectedly found that antibodies to α4 integrins can affect neutrophil recruitment responses and neutrophil-dependent inflammation in vivo.17,18 Previously, these findings have been attributed to α4 integrin antibody effects on other leukocytes, which in turn may affect neutrophil recruitment. However, Issekutz et al19 have recently shown that, unlike human neutrophils, rat neutrophils constitutively express low levels of α4 integrins, and that administration of an anti-α4 monoclonal antibody (MoAb), in conjunction with an anti-β2 integrin MoAb, inhibits neutrophil migration into arthritic joints in the rat. Although these findings strongly suggest a role for neutrophil-expressed α4 integrins, the investigators did not confirm neutrophil interaction with the endothelial ligands VCAM-1 or MAdCAM-1.19 In the present study, we confirm and extend the findings of Issekutz et al19 by showing that rat neutrophils consistently express α4β1 integrins and use α4β1 integrins to bind VCAM-1, whereas only α4 integrins are used to bind MAdCAM-1 in vitro.
MATERIALS AND METHODS
Rat leukocyte isolation.
Whole blood leukocytes and enriched neutrophil populations were isolated from pentobarbital-anesthetized male Sprague-Dawley rats (Charles River Labs Inc, Wilmington, MA and Harlan Sprague Dawley, Indianapolis, IN) weighing 275 to 300 g. EDTA-anticoagulated arterial blood was obtained via cannulation of the right carotid artery. For whole blood leukocytes, a leukocyte-rich buffy coat was obtained by centrifugation at 400g for 20 minutes at 22°C. Contaminating red blood cells (RBC) were removed via hypotonic lysis performed at 4°C. Cell differentials were determined by Diff-Quick staining (Baxter Scientific Products, McGaw, IL) and viability was confirmed by erythrosin B dye exclusion.
Enriched neutrophils populations (polymorph-nuclear leukocyte [PMN]) were obtained via density gradient centrifugation methods, in a manner similar to that described for human neutrophil isolation.20In brief, EDTA-anticoagulated whole blood was layered over Percoll (specific gravity, 1.085 g/L) and centrifuged for 20 minutes at 22°C, followed by hypotonic lysis of RBC at 4°C. In preparations in which contaminating lymphocytes made up more than 5% of the cell population, the cells underwent a second centrifugation step over Percoll (specific gravity, 1.085 g/L) to remove these cells. Neutrophil populations were 93.1% ± 0.6% pure with 5.5% ± 0.5% contaminating eosinophils and 1.5% ± 0.3% contaminating lymphocytes (n < 20). Cell viability for all flow cytometry and adhesion experiments was greater than 97%.
In addition to isolating neutrophils, mixed populations of rat mononuclear cells (MNC), consisting of lymphocytes and monocytes, were obtained by harvesting the upper layer from the Percoll gradient. These cells were washed twice and subjected to hypotonic lysis to remove any contaminating platelets or RBC. This mononuclear cell population was used as a positive control for analysis of α4 and β1 expression and in VCAM-1 and MAdCAM-1 adhesion assays (see below), and consisted of 7.8% ± 1.5% monocytes and 92.3% ± 1.5% lymphocytes (n = 6).
Flow cytometric analysis of leukocyte adhesion molecules.
The following α4 integrin MoAbs were purchased and used at the indicated saturating concentrations: TA-2 (immunoglobulin [Ig]G1, mouse anti-rat, 1 μg/mL; Seikagaku America, Inc, Rockville, MD), MRα4 (IgG2b, mouse anti-rat, 5 μg/mL; Pharmingen, San Diego, CA), and L25 (IgG1, mouse anti-human, found to cross-react with rat, 5.8 μg/mL; Becton-Dickinson, Mountain View, CA). β1 integrin staining on rat leukocytes was examined using the hamster anti-mouse MoAb Ha2/5 (IgM, 3 μg/mL; Pharmingen) and the hamster anti-mouse MoAb HMβ1-1 (IgG, 3 μg/mL; Pharmingen) found to cross-react with rat. Staining was also attempted with the murine anti-human β7 integrin MoAb ACT-1 (IgG, 1 μg/mL) generously provided by David J. Erle (University of California, San Francisco). Murine anti-rat MoAbs recognizing CD11a (WT.1, IgG2a, 5 μg/mL), CD11b/c (OX-42, IgG2a, 1 μg/mL), CD18 (WT.3, IgG1, 5 μg/mL), and CD3 (G4.18, IgG3, 3.1 μg/mL) were also purchased from Pharmingen, and control, nonbinding, isotype-matched mouse IgG1 and hamster IgM were obtained from Coulter (Hialeah, FL) and Pharmingen, respectively. A mouse anti-human L-selectin MoAb LAM1-116 (IgG2a, 3 μg/mL), cross-reactive with rat, was generously provided by Drs Thomas Tedder and Douglas Steeber (Duke University, Durham, NC).
Labeling of cells for indirect immunofluorescence was performed as described4 using saturating concentrations of fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse secondary antibody (BioSource International, Camarillo, CA) for all preparations except those in which Ha2/5 or HMβ1-1 was the primary MoAb, in which case an FITC-conjugated goat anti-hamster IgG (H + L) antibody (Jackson Immunoresearch Laboratories, Inc, West Grove, PA) was used. Cells were immediately analyzed unfixed using an EPICS Profile flow cytometer (Coulter Corporation, Hialeah, FL). Monocyte and lymphocyte populations were distinguished via their scatter and CD3:CD11b/c staining characteristics. Neutrophil and eosinophil populations were easily distinguished from each other via their light scatter and α4 integrin staining characteristics (ie, eosinophils have higher forward scatter and higher α4 integrin expression than neutrophils). To examine whether α4 integrin expression could be upregulated by neutrophil activation, enriched neutrophil populations were incubated with either phorbol myristate acetate (PMA; 10 ng/mL), fMLP (10−6 mol/L), or C5a (100 ng/mL) for 20 minutes at 37°C before incubation with primary antibodies. Irrelevant isotype-matched control staining with murine IgG1, IgG2a, or IgG3, or hamster IgM, typically yielded mean fluorescence values of 2 to 4. Data are presented as fold mean fluorescence above the respective control to facilitate comparisons among various cell types.
Neutrophil labeling with 51Cr and static adhesion assays.
For adhesion assays, rat neutrophils, mononuclear cells, and human Jurkat cells were labeled with 51Cr as described for human leukocytes.20 The Jurkat human T-lymphocytic cell line, a generous gift of Dr Vincenzo Casolaro (Johns Hopkins Asthma and Allergy Center, Baltimore, MD), was used as a control for adhesion assays because these cells are known to constitutively express high levels of α4β1, but they do not express α4β7 (21 and data not shown). The Jurkat T cells were passaged every 3 to 5 days in RPMI 1640 medium (GIBCO-BRL, Grand Island, NY) supplemented with 10% fetal bovine serum (FBS; Hyclone Laboratories, Inc, Logan, UT), 100 U/mL penicillin G, 100 μg/mL streptomycin, and 0.25 μg/mL amphotericin B (GIBCO-BRL). Chinese hamster ovary (CHO) cells and CHO cells stably transfected with rat or human VCAM-1 (known to bind to both human and rat α422; generously supplied by Dr Roy Lobb, Biogen Inc, Cambridge, MA) were grown to confluence as previously described23 using MEM alpha medium (GIBCO-BRL) supplemented with 10% FBS and methotrexate (500 nmol/L), in 24-well plates for use in static adhesion assays. CHO cells stably transfected with human MAdCAM-1, generously provided by Dr Michael Briskin (LeukoSite, Inc, Cambridge, MA), were grown in a manner identical to VCAM-1–transfected CHO cells except that methotrexate was omitted.
Rat leukocytes and Jurkat T cells were incubated for 30 minutes at 4°C in PAG-Mn buffer (PIPES buffer; 25 mmol/L Piperazine-N, N′-bis-[2-ethanesulfonic acid], 110 mmol/L NaCl, 5 mmol/L KCl [containing 0.003% human serum albumin], 0.1% D-glucose, and 1 mmol/L MnCl2 [Sigma Chemical Co, St Louis, MO]) to enhance α4 avidity.24 Leukocyte aliquots (100 μL, 2.5 × 105 cells/well) were added in duplicate to each well and allowed to adhere for 10 minutes. All adhesion assays were performed at 4°C to diminish β2 integrin interactions. Nonadherent cells were removed by washing with PAG-Mn. Adherent cells were then lysed with 1 mol/L NH4OH for 30 minutes, the supernatant removed, and radioactivity counted on a gamma counter. Total counts (ie, total radioactivity) added per well were determined by counting separate aliquots of 2.5 × 105labeled cells. Percent adhesion was obtained by dividing counts for bound cells by the total counts. In some experiments, cells were preincubated for 30 minutes with blocking antibody to rat α4 (TA-2, 1 μg/mL), β1 (Ha2/5 or HMβ1-1, 3 μg/mL),4 or CD18 (WT.3, 3 μg/mL) to show the specificity of the adhesion interaction. Preincubation with the human VCAM-1 MoAb 2G7 (F(ab′)2, 10 μg/mL)4 was also used to show adhesion specificity in assays using human VCAM-1–transfected CHO cells. All experiments were performed in duplicate and data are presented as mean adhesion for four to seven individual experiments.
Because enriched neutrophil populations contained approximately 7% contaminating cells, we performed additional experiments to determine if adherent cells were neutrophils or contaminating eosinophils or lymphocytes. In some experiments, non-51Cr–labeled neutrophil preparations were allowed to adhere to rat or human VCAM-1–transfected CHO cells as described. After removal of nonadherent cells, PAG-EDTA (5 mmol/L) was added to the wells for 2 minutes to remove adherent cells. These cells were collected and cell differentials were determined by Diff-Quick staining.
Statistical analysis.
All leukocyte adhesion data are presented as mean ± SEM. Data were compared by analysis of variance (ANOVA) using post hoc analysis with Fischer's corrected t-test. Probabilities of .05 or less were considered statistically significant.
RESULTS
Rat neutrophils express α4 and β1 integrins.
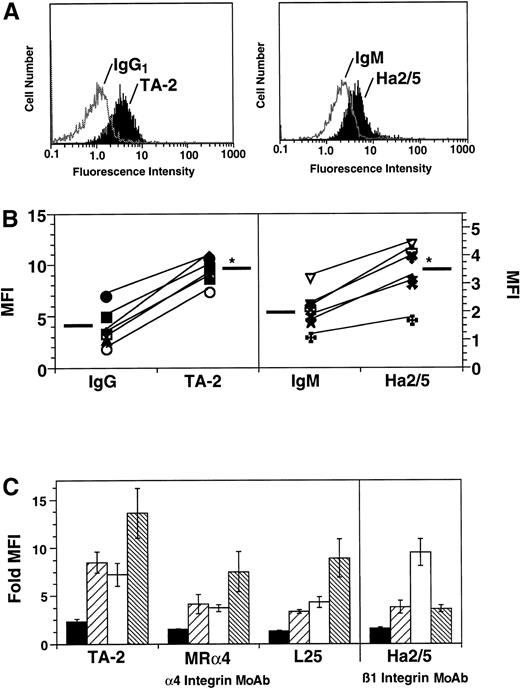
Immunofluorescent staining and flow cytometric analysis were performed on rat whole blood leukocytes and enriched neutrophil populations using a panel of murine anti-α4 MoAbs. Expression of α4 was examined on neutrophils, lymphocytes, monocytes, and eosinophils. Differences in neutrophil and eosinophil scatter in the rat were confirmed in experiments using enriched neutrophil populations in which neutrophils made up approximately 94% of cells, with the remainder being eosinophils. In these experiments two distinct populations, with percentage values corresponding to neutrophils and eosinophils, respectively, could be visualized based on light scatter, and these populations were found to have distinct staining characteristics for α4. These differing scatter characteristics made it possible to independently gate on neutrophils or eosinophils without additional antibody labeling.
Contrary to findings with human neutrophils, rat neutrophils constitutively expressed α4 integrins as confirmed by staining with MoAb TA-2, MRα4, and L25 (Fig 1A and C).Expression of α4 integrins on rat neutrophils was relatively low compared with levels on other cell types, but expression was detectable in all animals examined (Fig 1B). The brightest staining for rat α4 on all cell types was observed with the MoAb TA-2 (Fig 1C). The anti-rat α4 MoAb MRα4 and the anti-human MoAb L25 provided similar levels of staining. Incubation of enriched neutrophil populations with PMA, fMLP, or C5a, at concentrations sufficient to upregulate β2 integrin expression, did not increase expression of α4 as determined by staining with MoAb TA-2 (data not shown).
Indirect immunofluorescence and flow cytometric analysis of the surface expression of α4 and β1 integrins on rat leukocytes. (A) Representative histograms of rat neutrophil staining with TA-2 and Ha2/5 as compared with IgG1 or IgM control, respectively. (B) Actual fluorescence intensity (FI) values for α4 and β1 staining on neutrophils with MoAb TA-2 and Ha2/5 for six rats. *Mean FI values are significantly (P < .05) increased over mean FI values for IgG1 or IgM controls. (C) α4 and β1 expression was examined on neutrophils (PMN, ▪), lymphocytes (LYMPH, ▨), monocytes (MONO, □) and eosinophils (EOS, ▧) using the murine anti-rat α4 MoAb TA-2, MRα4, the murine anti-human α4 MoAb L25, and the hamster anti-murine β1 MoAb Ha2/5. Data are presented as fold increase in mean fluorescence intensity (MFI) over IgG or IgM control (n = 6).
Indirect immunofluorescence and flow cytometric analysis of the surface expression of α4 and β1 integrins on rat leukocytes. (A) Representative histograms of rat neutrophil staining with TA-2 and Ha2/5 as compared with IgG1 or IgM control, respectively. (B) Actual fluorescence intensity (FI) values for α4 and β1 staining on neutrophils with MoAb TA-2 and Ha2/5 for six rats. *Mean FI values are significantly (P < .05) increased over mean FI values for IgG1 or IgM controls. (C) α4 and β1 expression was examined on neutrophils (PMN, ▪), lymphocytes (LYMPH, ▨), monocytes (MONO, □) and eosinophils (EOS, ▧) using the murine anti-rat α4 MoAb TA-2, MRα4, the murine anti-human α4 MoAb L25, and the hamster anti-murine β1 MoAb Ha2/5. Data are presented as fold increase in mean fluorescence intensity (MFI) over IgG or IgM control (n = 6).
To determine if neutrophils also expressed β1 integrins, rat neutrophils, and other leukocyte types were first labeled with the anti-β1 MoAb Ha2/5 (Fig 1A). Rat neutrophils consistently showed low, but significant levels of β1 staining (n = 6), as did other leukocyte types (Fig 1A and B). Levels and patterns of staining for β1 on all cell types were similar when the hamster anti-mouse MoAb HMβ1-1 was used (data not shown). Staining for rat β7 was attempted using the murine anti-human β7 MoAb ACT-1, but staining on all rat cell types was negative, implying a lack of MoAb cross-reactivity with rat. As seen in Fig 2, the relative amounts of α4 expressed on all rat leukocyte subtypes, as compared with the β2 integrins and L-selectin, was low, even for eosinophils, which showed the strongest α4 integrin staining.
Rat α4 expression as compared with the β2 integrins, CD3, and L-selectin. Indirect immunofluorescence and flow cytometric analysis of the surface expression of α4 (TA-2), CD11a, CD11b/c, CD18, CD3, and L-selectin on rat neutrophils (PMN, ▪), lymphocytes (LYMPH, ▨), monocytes (MONO, □), and eosinophils (EOS, ▧). Data are presented as in Fig 1 (n = 5).
Rat α4 expression as compared with the β2 integrins, CD3, and L-selectin. Indirect immunofluorescence and flow cytometric analysis of the surface expression of α4 (TA-2), CD11a, CD11b/c, CD18, CD3, and L-selectin on rat neutrophils (PMN, ▪), lymphocytes (LYMPH, ▨), monocytes (MONO, □), and eosinophils (EOS, ▧). Data are presented as in Fig 1 (n = 5).
Rat neutrophils adhere to VCAM-1 and MAdCAM-1.
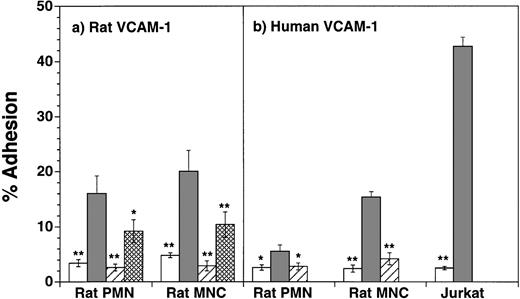
To determine if the levels of α4 integrins on neutrophils were sufficient to mediate neutrophil adhesion to the known ligands for α4β1 and α4β7, we first examined rat neutrophil adhesion to rat and human VCAM-1–transfected CHO cells. As shown in Fig3a, both neutrophils and MNC exhibited significant adherence to rat VCAM-1 CHO cells, as compared with nontransfected CHO cells (eg, for rat neutrophils, 15.8% ± 3.2%v 3.4% ± 0.7% adhesion respectively, P < .01, n = 7). Affinity of binding was relatively low, because neutrophil adhesion to VCAM-1 was not consistently seen in the absence of Mn2+ (data not shown). Adhesion of both cell populations to rat VCAM-1 was completely inhibited by incubation of the cells with the mouse anti-rat α4 blocking antibody TA-2 (1 μg/mL) (PMN, 2.6% ± 0.6%; MNC, 2.9% ± 0.8%). Adhesion was only partially inhibited by incubation of the leukocytes with the anti-β1 MoAb Ha2/5 (PMN, 9.2% ± 2.1%; MNC, 10.4% ± 1.7%). Similar results were obtained with the β1 MoAb HMβ1-1 (n = 2, data not shown). The anti-CD18 MoAb WT.3 did not significantly block neutrophil or MNC adhesion to rat VCAM-1 (n = 2, data not shown).
Adhesion of rat neutrophils (PMN) and mononuclear cells (MNC, lymphocytes and monocytes) to untransfected and rat (panel a, n = 7) or human (panel b, n = 6) VCAM-1–transfected CHO cells. Adhesion was tested to nontransfected CHO cells, or CHO cells transfected with VCAM-1 in the presence or absence of α4 MoAb TA-2. Adhesion of rat neutrophils to rat VCAM-1 was also tested in the presence of the β1 antibody Ha2/5. β1 blocking studies were not performed for neutrophil adhesion to human VCAM-1. Data are presented as mean percent adherence ± SEM. *(P < .05) and **(P < .01) indicate values significantly different from percent adhesion to VCAM-1–transfected CHO-cells. (□), CHO; (▪), VCAM-1 CHO; (▨), VCAM-1 CHO + anti-α4 MoAb; (▩), VCAM-1 CHO + anti-β1 MoAb.
Adhesion of rat neutrophils (PMN) and mononuclear cells (MNC, lymphocytes and monocytes) to untransfected and rat (panel a, n = 7) or human (panel b, n = 6) VCAM-1–transfected CHO cells. Adhesion was tested to nontransfected CHO cells, or CHO cells transfected with VCAM-1 in the presence or absence of α4 MoAb TA-2. Adhesion of rat neutrophils to rat VCAM-1 was also tested in the presence of the β1 antibody Ha2/5. β1 blocking studies were not performed for neutrophil adhesion to human VCAM-1. Data are presented as mean percent adherence ± SEM. *(P < .05) and **(P < .01) indicate values significantly different from percent adhesion to VCAM-1–transfected CHO-cells. (□), CHO; (▪), VCAM-1 CHO; (▨), VCAM-1 CHO + anti-α4 MoAb; (▩), VCAM-1 CHO + anti-β1 MoAb.
Rat neutrophils and MNC also adhered to human VCAM-1–transfected CHO cells (Fig 3b). Although adhesion was less than that observed with rat VCAM-1 CHO cells (eg, for rat neutrophils, 5.5% ± 1.1% adhesion, n = 6), binding was shown to be α4 specific as MoAb TA-2 completely inhibited adhesion. Adhesion of rat neutrophils and MNC was also completely inhibited by pretreatment of VCAM-1 CHO cells with the mouse anti-human VCAM-1 MoAb 2G7 (n = 6, data not shown). Although rat neutrophils showed consistent adherence to rat and human VCAM-1, neutrophil adherence in both cases was less than that observed for mononuclear cells (Fig 3a and b), consistent with the higher levels of α4 expression on rat MNC. Jurkat cells, which were used as a control cell population, adhered avidly to human VCAM-1 (Fig 3b), consistent with their high levels of α4β1 expression. Because enriched neutrophil populations contained approximately 7% contaminating cells, we performed additional experiments to determine whether the cells adhering to VCAM-1 were neutrophils or contaminating eosinophils or lymphocytes. For both rat and human VCAM-1, neutrophils were found to make up greater than 86% of the adherent cells, with eosinophils making up 10.0% ± 2.5% and lymphocytes 3.0% ± 0.8% (n = 5).
Because β1 integrin blockade only partially inhibited adhesion to VCAM-1, and because we were unable to directly identify β7 integrin expression by flow cytometry, we determined if rat leukocytes could adhere to MAdCAM-1, an α4β7 ligand. As shown in Fig4, both rat neutrophils and MNC exhibited significant adherence to human MAdCAM-1–transfected CHO cells (eg, neutrophil adhesion 15.9% ± 4.3%), as compared with untransfected CHO cells (4.3% ± 1.2% adhesion; Fig 4). Again, the mouse anti-rat α4 MoAb TA-2 was used to show the α4 specificity of rat neutrophil and MNC adhesion to MAdCAM-1. Adhesion of rat neutrophils and MNC to MAdCAM-1 CHO cells was completely blocked by the addition of MoAb TA-2 to cell preparations. The β1 MoAb Ha2/5 did not have any significant effect on neutrophil adhesion to MAdCAM-1 CHO cells, although it did significantly inhibit MNC adhesion to MAdCAM-1. Similar results were observed for both cell types with MoAb HMβ1-1 (n = 2, data not shown).
Adhesion of rat neutrophils (PMN, n = 5), mononuclear cells (MNC, n = 5), or Jurkat cells (n = 4) to untransfected and human MAdCAM-1–transfected CHO cells. Adhesion was tested to nontransfected CHO cells, or CHO cells transfected with MAdCAM-1 in the presence or absence of α4 MoAb TA-2 or β1 MoAb Ha2/5. Data are presented as mean percent adherence ± SEM. **(P < .01) indicate values significantly different from percent adhesion to MAdCAM-1–transfected CHO cells. (□), CHO; (▪), MAdCAM-1 CHO; (▨), MAdCAM-1 CHO + anti-α4 MoAb; (▩), MAdCAM-1 CHO + anti-β1 MoAb.
Adhesion of rat neutrophils (PMN, n = 5), mononuclear cells (MNC, n = 5), or Jurkat cells (n = 4) to untransfected and human MAdCAM-1–transfected CHO cells. Adhesion was tested to nontransfected CHO cells, or CHO cells transfected with MAdCAM-1 in the presence or absence of α4 MoAb TA-2 or β1 MoAb Ha2/5. Data are presented as mean percent adherence ± SEM. **(P < .01) indicate values significantly different from percent adhesion to MAdCAM-1–transfected CHO cells. (□), CHO; (▪), MAdCAM-1 CHO; (▨), MAdCAM-1 CHO + anti-α4 MoAb; (▩), MAdCAM-1 CHO + anti-β1 MoAb.
DISCUSSION
Previous studies have shown that normal human, guinea pig, and sheep neutrophils do not constitutively express α4 integrins.4,12,15,16 Although α4 integrins are not constitutively present on human neutrophils, Kubes et al25have shown that under certain experimental conditions such as treatment with dihydrocytochalasin B or after in vitro transendothelial migration, human neutrophils can be induced to express α4 integrins and can adhere to stimulated endothelial cells under static and flow conditions.9,25 However, previous data19 and data presented here indicate that rat neutrophils, unlike other species, constitutively express α4 integrins. Flow cytometric analysis of rat neutrophils using the mouse anti-rat α4 MoAbs TA-2 and MRα4, as well as the mouse anti-human α4 MoAb L25, showed a consistent, low level of α4 expression (eg, 2.3 ± 0.3 mean fold fluorescence above background IgG with TA-2). These data confirm and expand the findings of Issekutz et al19 in which low levels of α4 expression were shown on the surface of rat neutrophils using a single MoAb TA-2. We show a similar level of α4 expression on neutrophils using MoAb TA-2, as well as with the other MoAbs which bind rat and human α4 integrins. Direct comparison of α4 expressed on neutrophils to that on other rat leukocytes via flow cytometry shows that although they are consistently present, neutrophils express the lowest levels of α4 integrins, with rat eosinophils expressing the highest levels.
In addition, we show low levels of β1 integrin expression on rat neutrophils. Flow cytometric analysis of rat β1 expression using MoAb Ha2/5 revealed consistent, low-level β1 expression on neutrophils, with greater expression on lymphocytes and eosinophils, and the greatest expression on monocytes. However, the partial to minimal inhibitory activity seen with β1 integrin blockade in the VCAM-1 and MAdCAM-1 adhesion assays may suggest the presence of an additional α4 integrin subunit, such as β7. It is possible that neutrophil-expressed α4β7 could account in part for neutrophil adherence to both VCAM-1 and MAdCAM-1, because α4β7 is a ligand for both molecules. Unfortunately, the lack of antibodies which cross-react with rat β7, and the inability to obtain eosinophil-free preparations of neutrophils for immunoprecipitation experiments makes it impossible to determine the exact heterodimeric composition of rat neutrophil α4 integrins at this time. Interestingly, the ability of the anti-β1 MoAb to significantly block MNC adhesion to MAdCAM-1 may suggest that MNC-expressed α4β1 integrins can interact with MAdCAM-1 in the rat. In mouse and human cell systems, α4 integrin interactions with MAdCAM-1 have been seen only with α4β7, not α4β1.
Beyond showing the expression of α4 and β1 on the neutrophil surface, we also showed that neutrophil-expressed α4 integrins can mediate neutrophil, as well as mononuclear cell, adhesion to VCAM-1 and MAdCAM-1 expressed on transfected CHO-cells. Isolated rat neutrophils incubated in Mn2+-containing buffer specifically adhered to both rat and human VCAM-1–transfected CHO cells and MAdCAM-1–transfected CHO cells at 4°C. Neutrophils did not consistently adhere to VCAM-1 or MAdCAM-1 in the absence of Mn2+, suggesting that these cells expressed low levels of activated α4 integrins. This may in part explain differences between our findings and those of Andrew et al26 in which they were unable to show α4β7-mediated adhesion to VCAM-1 at 4°C. Adhesion to both VCAM-1 and MAdCAM-1 was completely blocked by anti-α4 MoAb TA-2. Our findings that rat neutrophils bind VCAM-1 and MAdCAM-1 in an α4-dependent manner support in vivo data from Issekutz et al19 which indicate that the MoAb TA-2 may effect neutrophil recruitment in the rat. Although these findings19 strongly suggest a role for neutrophil-expressed α4, the investigators did not confirm that neutrophil α4 integrins were expressed at sufficient levels to mediate interaction with the endothelial ligands VCAM-1 or MAdCAM-1. However, adhesion data from our studies clearly indicate that the in vivo effect of diminished neutrophil recruitment observed with the administration of MoAb TA-2 is likely the result of antibody blockade of neutrophil interaction with VCAM-1. Furthermore, the ability of α4 integrin MoAb to block rat neutrophil adhesion to MAdCAM-1 suggests that α4 integrin MoAb may also be capable of blocking neutrophil trafficking to the gut. Blocking rat α4 integrins may also inhibit neutrophil interaction with the matrix protein fibronectin, because emigrated rat neutrophil binding to cardiac myocytes has been shown to be α4 integrin and fibronectin dependent.27
Studies by Issekutz et al19 provide the most direct evidence that neutrophil-expressed α4 integrins may be important for neutrophil recruitment in the rat, but earlier data from Mulligan et al,17 published before α4 integrin identification on rat neutrophils, also support this. In these experiments, the rat α4 antibody TA-2 was found to significantly reduce neutrophil infiltration, changes in lung permeability, and hemorrhage in a model of intrapulmonary IgG deposition. These investigators have previously shown this model of lung injury to be almost exclusively neutrophil mediated, with some role for alveolar macrophages.28,29 In their discussion of the data the investigators speculate that the effects observed in this model, with the antibody TA-2, may be attributed to a role for α4 in macrophage cytokine release.17 Although the potential effects of MoAb TA-2 on macrophage function can not be discounted, our findings would suggest that the inhibition of neutrophil infiltration is more likely a direct effect of the antibody on neutrophil interaction with α4 integrin ligands. Examples of α4 MoAb reduction of neutrophil recruitment also exist in the mouse. Chisholm et al18 found reduced neutrophil-dependent edema with an α4 MoAb treatment in a mouse model of T-cell–dependent contact hypersensitivity. Here the investigators again speculate that the decreased neutrophil recruitment is the result of decreased T-cell infiltration and thus decreased mediator release. It is likely that these observations are in part correct, but the presence of α4 on mouse neutrophils has not been examined, and therefore a direct effect of the α4 MoAb on neutrophil recruitment can not be ruled out.
In conclusion, we have shown that rat neutrophils, unlike neutrophils from most other species, constitutively express low levels of functional α4 and β1 integrins. The low level expression of α4 integrins can mediate neutrophil binding to both rat and human VCAM-1 as well as human MAdCAM-1. These data show a novel role for α4 integrins in rat neutrophil recruitment and suggest that MoAbs reacting with α4, β1, VCAM-1, MAdCAM-1, or perhaps β7 administered in vivo in rat models of cell recruitment may directly affect neutrophil recruitment.
ACKNOWLEDGMENT
We gratefully acknowledge Drs Thomas Tedder, Douglas Steeber, David J. Erle, and Walter Newman for providing MoAbs; Dr Roy Lobb for the VCAM-1–transfected CHO cells; Dr Michael J. Briskin for the MAdCAM-1–transfected CHO cells; and Dr Vincenzo Casolaro for the Jurkat T-cell line.
Supported by National Institutes of Health (NIH) Grants No. HL-49545 and AI-07056, the Burroughs Wellcome Fund, and with funds from the Office of Naval Research through the Asthma and Allergy Foundation of America.
Address reprint requests to Bruce S. Bochner, MD, Johns Hopkins Asthma and Allergy Center, 5501 Hopkins Bayview Circle, Baltimore, MD 21224.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal