Abstract
Inhibitory antibodies directed against factor VIII develop in a substantial number of patients with hemophilia A as a consequence of factor VIII replacement therapy. These antibodies usually recognize discrete epitopes within the A2 and/or the C2 domains of factor VIII. Here, we have characterized the antibodies present in the plasma of a patient affected by severe hemophilia A. The antibodies reacted readily with the metabolically labeled factor VIII light chain and fragments thereof when analyzed by immunoprecipitation. The inhibitory activity could be neutralized by the complete light chain, whereas only slight neutralization occurred with a fragment comprising the isolated C2 domain. Binding of the majority of antibodies to in vitro synthesized factor VIII fragments was dependent on the presence of amino acid residues Gln1778-Met1823, a region known to contain a factor IXa binding site. Functional characterization showed that purified IgG from the patient's serum inhibited binding of factor IXa to immobilized factor VIII light chain in a dose-dependent manner. These data indicate that human alloantibodies may inhibit factor VIII activity by interfering with factor IXa–factor VIIIa complex assembly.
BLOOD COAGULATION factor VIII serves as a cofactor of factor IXa in the intrinsic pathway of factor X activation. Factor VIII circulates in plasma as a heterodimer, composed of a heavy chain (A1-A2-B) and a light chain (A3-C1-C2).1,2 Before activation, factor VIII is associated with von Willebrand factor (vWF). Limited proteolysis by thrombin coincides with release of the active cofactor from the factor VIII-vWF complex.3
Deficiency or disfunction of factor VIII is associated with the hereditary bleeding disorder hemophilia A.4 Bleeding episodes can be prevented or arrested by administration of factor VIII concentrate. In response to multiple treatments, inhibitory antibodies directed against factor VIII may develop. This serious complication occurs in approximately 25% of patients affected by the severe form of hemophilia A.5-7 The regions to which most human anti-factor VIII antibodies bind have been localized to the A2 and C2 domain of factor VIII by immunoblotting and immunoprecipitation assays.8-11 Detailed analysis has established that amino acid sequence Arg484-Ile508 in the A2 domain and residues Val2248 to Ser2312 in the C2 domain constitute major epitopes of factor VIII inhibiting antibodies.12 13
Several mechanisms by which inhibitors interfere with factor VIII procoagulant activity have been identified. Human anti-A2 antibodies act as noncompetitive inhibitors of the factor X activating complex by impeding its catalytic activity.14 Antibodies directed against the C2 domain interfere with binding of factor VIII to phospholipids and vWF.15-17 Disruption of factor VIII-vWF interaction leads to factor VIII elimination in vivo because binding to vWF is essential to stabilize factor VIII in the circulation. Recently, a novel inhibitory mechanism with a converse effect on the interaction between factor VIII and vWF was disclosed. A human antibody that requires the presence of vWF to display inhibitory characteristics has been shown to retard the release of thrombin-cleaved factor VIII from vWF. This antibody, directed towards amino acid sequence Val2248-Gly2285 of the C2 domain, does not interfere with phospholipid binding of factor VIII.18
Studies using inhibitor neutralization assays have provided evidence for the presence of additional inhibitor epitopes on the factor VIII light chain located outside the C2 domain.10,13 19 The inhibitory mechanism of those antibodies has remained unresolved so far. Here, we have characterized human inhibitory antibodies directed towards the light chain of factor VIII by performing neutralization assays and immunoprecipitation analysis. Functional studies showed that the antibodies inhibit factor VIII activity by interfering with the interaction between factor IXa and the light chain of factor VIII.
MATERIALS AND METHODS
Materials.
DNA-modifying enzymes, restriction enzymes, Grace's insect medium, fetal calf serum (FCS), and antibiotics were obtained from GIBCO (Breda, The Netherlands). The serum-free medium EX-CELL 401 was purchased from JRH Biosciences (Sera Lab Ltd, Crawley, UK). Oligonucleotide primers, protein G Sepharose-4FF, CNBr-activated Sepharose-4B, and protein A Sepharose CL-4B were obtained from Pharmacia-LKB (Woerden, The Netherlands). Pfu-polymerase was purchased from Stratagene (Cambridge, UK). Radioactive chemicals were obtained from Amersham (Bucks, UK). The “Taq track” sequencing system, the plasmid pSP64 and the in vitro transcription and translation system using the SP6-expression system were obtained from Promega (Madison, WI). The plasmid pAcGP67B and the Baculogold Baculovirus Autographa californica DNA were purchased from Pharmingen (San Diego, CA). Spodoptera frugiperda (Sf-9) insect cells and Trichoplusia ni High Five insect cells were obtained from Invitrogen (Leek, The Netherlands).
Proteins.
Human factor VIII light chain and factor IXa were obtained from human plasma as previously described.20 Monoclonal antibodies (MoAbs) CLB-CAg 9, CLB-CAg 69, CLB-CAg 117,21,22 and CLB-FIX 1423 have been described previously. MoAb ESH4 was obtained from American Diagnostics Inc (Greenwich, CT) and has been characterized before.13 Murine MoAbs directed against human IgG1, IgG2, IgG3, and IgG4 were obtained from the Central Laboratory of the Netherlands Red Cross Blood Transfusion Service.
Coagulation assays.
Blood was collected by venepuncture in one-tenth final volume of 3.2% (wt/vol) trisodium citrate. After centrifugation for 10 minutes at 12,000g, plasma was collected and immediately stored at −70°C in 0.5 to 1.0 mL aliquots. Factor VIII procoagulant activity was assayed by a one-stage clotting assay.24 Inhibitor titers were measured in plasma using the Bethesda assay essentially as described previously and expressed as Bethesda units per milliliter (BU/mL).25
Plasmid constructions.
The construction of plasmids encoding the factor VIII heavy chain, A2 domain, and factor VIII light chain has been described previously.26 The plasmid pCLB-GP67-C2, encoding the C2 domain (amino acid residues 2172 to 2332), was constructed by polymerase chain reaction (PCR) using oligonucleotide primer C2-1 (5′ GTGCCATGGGTAGTTGCAGCATGCCATTG 3′; nucleotide position 6574-6591 of factor VIII, sense) and primer C2-2 (5′CCATAGGTTGGAATCTAA 3′; nucleotide position 1222-1239 of pBPV, antisense), using plasmid pCLB-BPVdB695 as a template.27 Reaction conditions for PCR were: 5 minutes, 95°C; 2 minutes, 50°C; 3 minutes, 72°C; 37 cycles of 45 seconds, 95°C; 90 seconds, 50°C; 3 minutes, 72°C; 5 minutes, 65°C in the presence of 1 mmol/L dNTPs,Pfu-polymerase reaction buffer, 50 pmol of each oligonucleotide primer, and 2.5 U Pfu-polymerase. After amplification the fragment was digested with NcoI and NotI and cloned into plasmid pAcGP67B. Truncated fragments corresponding to parts of the A3 domain of factor VIII were constructed with the use of plasmid pSP/F8-80K1 as a template, as described previously.21 23
Expression and metabolic labeling of selective domains of factor VIII in insect cells.
Recombinant baculovirus expressing the recombinant factor VIII heavy chain, A2 domain, light chain, and C2 domain were obtained after transfection of Sf-9 cells according to the instructions of the supplier. The production of [35S] methionine-labeled factor VIII fragments has been described previously.26 In short, High Five cells were infected with recombinant baculovirus and pulse-labeled with [35S] methionine. Medium of metabolically labeled cells was then collected in immunoprecipitation buffer. In vitro translation of the truncated fragments of the A3 domain of factor VIII was performed with the use of the SP6-expression system, in the presence of [35S]methionine, as described previously.23
Immunoprecipitation.
Immunoprecipitation was performed essentially as described previously.26 Briefly, conditioned media were incubated overnight at 4°C with preformed complexes of Protein G Sepharose-4FF and 30 μL patient plasma. The expression of the factor VIII fragments was monitored using MoAbs CLB-CAg 9 (1 μg/mL) directed against the A2 domain of the heavy chain of factor VIII, CLB-CAg 117 (1 μg/mL) directed against the C2 domain of the light chain of factor VIII, and MoAb CLB-CAg 69 (1 μg/mL) directed against amino acid sequence Lys1673-Arg1689 of the factor VIII light chain.22 The specificity of the adsorption was addressed using plasma of several healthy donors. Antibody subclass typing was performed using subclass specific MoAbs (αIgG1, αIgG2, αIgG3, and αIgG4) covalently linked to CNBr-activated Sepharose-4B. Bound proteins were eluted by boiling in sodium dodecyl sulfate (SDS) sample buffer and analyzed under reducing conditions on a 10% (wt/vol) SDS-polyacrylamide gel and visualized by autoradiography.
Inhibitor neutralization assay.
Fragments corresponding to the factor VIII light chain and the C2 domain were derived from High Five cells infected with recombinant baculovirus encoding the factor VIII light chain and the C2 domain. Recombinant factor VIII fragments present in 75% (vol/vol) serum free medium and 25% (vol/vol) Grace's insect medium containing 2.5% (vol/vol) FCS were dialyzed against 50 mmol/L Tris-HCl, pH 7.4; and 150 mmol/L NaCl. The amount of factor VIII fragments in the conditioned medium was determined by an enzyme-linked immunosorbent assay method using MoAbs ESH413 and CLB-CAg 117 (Fig1), both directed against the C2 domain of factor VIII. Samples were incubated with immobilized ESH4 (0.5 μg/well) in 50 mmol/L Tris-HCl, pH 7.4; 1 mol/L NaCl; and 2% human serum albumin (HSA). Bound factor VIII fragments were detected using peroxidase-labeled CLB-CAg 117 in 50 mmol/L Tris-HCl, pH 7.4; 1 mol/L NaCl; and 0.1% (vol/vol) Tween-20. Pooled normal human plasma served as a standard. Inhibitor neutralization was performed essentially as described previously.19 After measurement of the inhibitor titer, the inhibitor plasma was diluted to 2 BU/mL in a buffer consisting of 50 mmol/L Tris-HCl, pH 7.3; and 0.2% HSA. Diluted inhibitor plasma was incubated for 2 hours at 37°C with an equal volume of pooled normal plasma and an equal volume of conditioned medium. The conditioned medium contained increasing concentrations of either factor VIII light chain or C2 domain, diluted in the same buffer as the inhibitor plasma. Residual factor VIII activity in the incubation mixture was determined relative to a control sample that was incubated in the absence of inhibitor plasma. Functional integrity of the fragment corresponding to the C2 domain was determined by neutralization experiments with the factor VIII inhibiting, conformation-dependent MoAb CLB-CAg 117. On a molar basis, factor VIII fragments corresponding to the light chain and the C2 domain could alleviate the inhibitory activity of CLB-CAg 117 to the same extent (data not shown). This observation indicates that the conformation of the recombinant C2 domain fragment is very similar to that of the C2 domain within the factor VIII light chain.
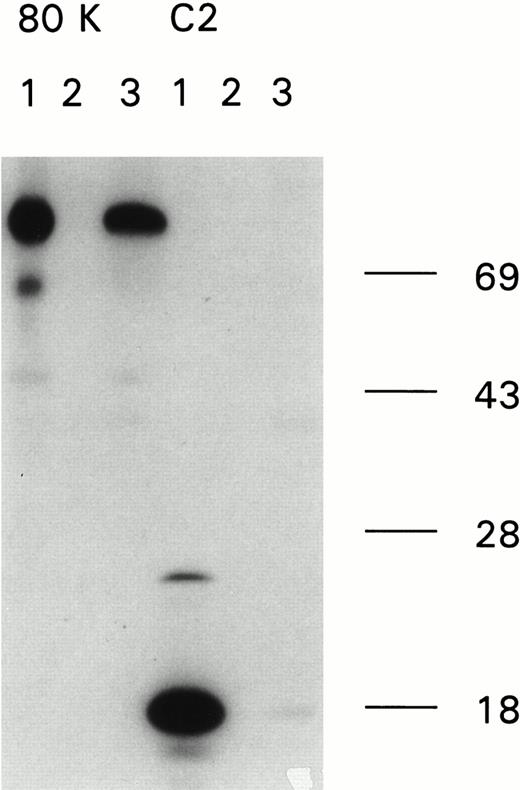
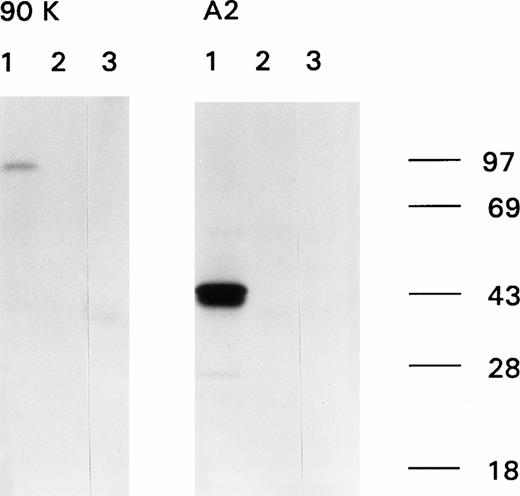
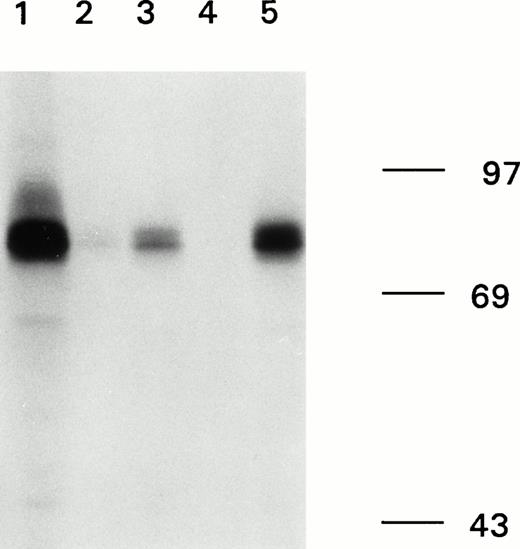
Immunoprecipitation of recombinant factor VIII fragments with antibodies present in the inhibitor plasma. Recombinant factor VIII fragments corresponding to the factor VIII light chain (80K), the C2 domain (C2), the factor VIII heavy chain (90K), and the A2 domain (A2) were expressed in High Five cells and metabolically labeled as described.26 Binding of antibodies to the metabolically labeled factor VIII fragments was assessed by immunoprecipitation. Bound proteins were eluted and analyzed under reducing conditions on a 10% (wt/vol) SDS-polyacrylamide gel. (A) Reactivity of anti-factor VIII antibodies with factor VIII light chain fragments. Lane 1, CLB-CAg 117; lane 2, control plasma; lane 3, antibodies present in patient's plasma. (B) Reactivity of antibodies with factor VIII heavy chain fragments. Lane 1, CLB-CAg 9; lane 2, control plasma; lane 3, antibodies present in patient's plasma. (C) Subclass determination of anti-factor VIII antibodies. Binding of anti-factor VIII antibodies to metabolically labeled factor VIII light chain was performed using subclass specific antibodies. Lane 1, total IgG; lane 2, IgG1; lane 3, IgG2; lane 4, IgG3; lane 5, IgG4. Molecular weight markers are given at the right of the figure.
Immunoprecipitation of recombinant factor VIII fragments with antibodies present in the inhibitor plasma. Recombinant factor VIII fragments corresponding to the factor VIII light chain (80K), the C2 domain (C2), the factor VIII heavy chain (90K), and the A2 domain (A2) were expressed in High Five cells and metabolically labeled as described.26 Binding of antibodies to the metabolically labeled factor VIII fragments was assessed by immunoprecipitation. Bound proteins were eluted and analyzed under reducing conditions on a 10% (wt/vol) SDS-polyacrylamide gel. (A) Reactivity of anti-factor VIII antibodies with factor VIII light chain fragments. Lane 1, CLB-CAg 117; lane 2, control plasma; lane 3, antibodies present in patient's plasma. (B) Reactivity of antibodies with factor VIII heavy chain fragments. Lane 1, CLB-CAg 9; lane 2, control plasma; lane 3, antibodies present in patient's plasma. (C) Subclass determination of anti-factor VIII antibodies. Binding of anti-factor VIII antibodies to metabolically labeled factor VIII light chain was performed using subclass specific antibodies. Lane 1, total IgG; lane 2, IgG1; lane 3, IgG2; lane 4, IgG3; lane 5, IgG4. Molecular weight markers are given at the right of the figure.
Binding assays.
IgG was purified from the patient's serum using protein A Sepharose CL-4B. The final preparations of total IgG had an inhibitor titer of 4 BU/mg IgG. IgG derived from a healthy donor was used as a control. Binding of factor IXa to immobilized factor VIII light chain was assessed by using a modification of a previously described method.20 Briefly, MoAb CLB-CAg 12 (10 μg/mL) was immobilized to microtiter wells and incubated with 62.5 nmol/L of plasma-derived factor VIII light chain. Subsequently, varying concentrations of purified IgG (0 to 1.7 mg/mL, corresponding to approximately 0 to 10.7 μmol/L) were incubated for 2 hours at 37°C in a buffer containing 20 mmol/L Histidine, pH 6.2; 100 mmol/L NaCl; 5 mmol/L CaCl2; and 0.1% Tween-20. Wells were washed three times with the same buffer before 40 nmol/L factor IXa was added and incubated for 4 hours at 37°C. Bound factor IXa was detected by incubating for 15 minutes at room temperature with peroxidase-labeled MoAb CLB-FIX 14.23 Residual factor IXa bound was calculated relative to a control experiment in which no IgG was added.
Patient.
The patient, born in Turkey in 1952, was a sporadic case of severe hemophilia A. He was treated with whole blood and plasma transfusions for various bleeding episodes before he came to the Netherlands in 1974. In 1977 a lack of recovery was noted after administration of cryoprecipitate (2,000 IU factor VIII) for gastric bleeding and hemarthros. It was concluded that he had developed antibodies directed towards factor VIII. No data on the inhibitor titer from the period 1977 to 1982 are available. Between 1982 and 1995 the inhibitor titer varied between 40 to 80 BU/mL. He was successfully treated with FEIBA (Baxter IMMUNO, Vienna, Austria) for hemarthros and muscle bleedings. The patient did not undergo an immune tolerance regimen. The experiments in this study were all performed on a plasma sample from 1995 in which the inhibitor titer was 40 BU/mL.
RESULTS
Epitope mapping of anti-factor VIII antibodies.
We characterized the anti-factor VIII antibodies present in the plasma of a patient severely affected by hemophilia A. Immunoprecipitation analysis, using metabolically labeled factor VIII fragments expressed in insect cells, clearly showed binding to the light chain of factor VIII (Fig 1A, left). Immunoprecipitation using IgG subclass specific antibodies showed that predominantly IgG4 and IgG2 antibodies accounted for binding to the radio-labeled factor VIII light chain, whereas some IgG1 antibodies could be detected as well (Fig 1C). A faint signal was observed for binding of the antibodies to a radio-labeled fragment corresponding to the C2 domain (Fig 1A, right). No binding to either the heavy chain of factor VIII or the A2 domain could be detected (Fig1B). These findings suggest that the patient's plasma contains antibodies against at least one epitope on the factor VIII light chain located outside the C2 domain. To address this issue from a functional perspective, an inhibitor neutralization assay using recombinant factor VIII light chain and C2 domain was performed. By virtue of their distinctive neutralization of the inhibitory activity, these factor VIII fragments provide an estimate of the relative contributions of the epitope in the C2 domain and the unknown light chain epitope to the entire inhibitory activity present in the patient's plasma. Complete neutralization occurred by addition of the light chain fragment, whereas only 5% neutralization was obtained when the same concentration of a fragment corresponding to the C2 domain was added (Fig 2). This indicates that the factor VIII inhibitory activity present in the patient's plasma is predominantly accounted for by antibodies that bind to a light chain epitope located outside the C2 domain.
Neutralization of inhibitor activity by recombinant factor VIII fragments. Neutralization of the inhibitory activity by factor VIII light chain (•) and factor VIII C2 domain (○). The patient's plasma was diluted to 2 BU/mL and incubated for 2 hours at 37°C with the indicated concentrations of recombinant factor VIII light chain or C2 domain. The residual factor VIII activity in the incubation mixture was determined relative to a control sample that was incubated in the absence of inhibitor plasma. Each datapoint represents the mean (±SD) of three experiments.
Neutralization of inhibitor activity by recombinant factor VIII fragments. Neutralization of the inhibitory activity by factor VIII light chain (•) and factor VIII C2 domain (○). The patient's plasma was diluted to 2 BU/mL and incubated for 2 hours at 37°C with the indicated concentrations of recombinant factor VIII light chain or C2 domain. The residual factor VIII activity in the incubation mixture was determined relative to a control sample that was incubated in the absence of inhibitor plasma. Each datapoint represents the mean (±SD) of three experiments.
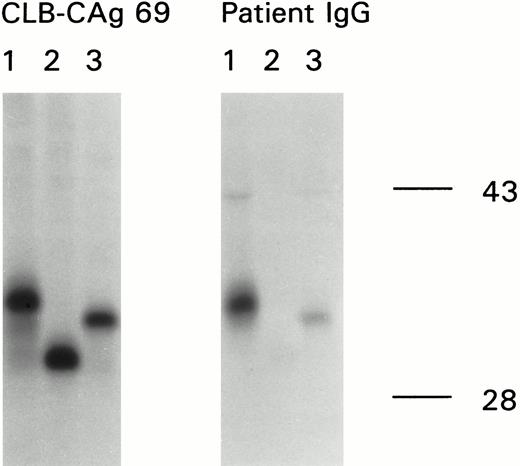
An important functional region located outside the C2 domain on the factor VIII light chain, consisting of amino acid residues Glu1811-Lys1818, has previously been identified as a binding site for factor IXa.23 To address the question of whether the antibodies recognize an epitope in the vicinity of the factor IXa binding site on the factor VIII light chain, we performed immunoprecipitation experiments using in vitro synthesized factor VIII fragments. These recombinant factor VIII fragments, comprising carboxyterminal truncations of the factor VIII light chain were in vitro transcribed and then translated in the presence of [35S]-methionine21 (Fig3A). Binding of the in vitro synthesized factor VIII fragments using CLB-CAg 69, directed against amino acid sequence Lys1673-Arg1689, showed that all fragments had the expected size (Fig 3B, left). The antibodies present in the patient's plasma bound to the larger two fragments. Binding to the fragment comprising amino acid sequence Asp1563-Gln1778 was strongly reduced (Fig 3B, right). With reference to Fig 3A these data show that amino acid sequence Gln1778-Met1823 comprises a major epitope for the antibodies present in the patient's plasma.
Epitope mapping of antibodies present in the inhibitor plasma by immunoprecipitation. (A) Structure of the factor VIII light chain and the carboxyterminal truncated polypeptides synthesized in vitro and used for immunoprecipitation. The bar at the top of the figure represents the domain structure of the factor VIII light chain with the neighboring B domain. The amino acid residue numbers below the bar indicate the boundaries between the domains. The epitope for MoAb CLB-CAg 69, consisting of the amino acid residues Lys1673-Arg1689 within the amino-terminal region of the A3 domain (22), is indicated by a black bar. The three lines at the bottom of the figure represent three carboxyterminal truncated polypeptides. 1, Asp1563-Asp1840; 2, Asp1563-Gln1778; 3, Asp1563-Met1823. (B) Reactivity of MoAb CLB-CAg 69 (left) and patient's plasma (right) with truncated recombinant fragments of the A3 domain of factor VIII. Binding of anti-factor VIII antibodies to the metabolically labeled factor VIII fragments was assessed by immunoprecipitation and analyzed under reducing conditions on a 10% (wt/vol) SDS-polyacrylamide gel. Lane 1, Asp1563-Asp1840; lane 2, Asp1563-Gln1778; lane 3, Asp1563-Met1823.
Epitope mapping of antibodies present in the inhibitor plasma by immunoprecipitation. (A) Structure of the factor VIII light chain and the carboxyterminal truncated polypeptides synthesized in vitro and used for immunoprecipitation. The bar at the top of the figure represents the domain structure of the factor VIII light chain with the neighboring B domain. The amino acid residue numbers below the bar indicate the boundaries between the domains. The epitope for MoAb CLB-CAg 69, consisting of the amino acid residues Lys1673-Arg1689 within the amino-terminal region of the A3 domain (22), is indicated by a black bar. The three lines at the bottom of the figure represent three carboxyterminal truncated polypeptides. 1, Asp1563-Asp1840; 2, Asp1563-Gln1778; 3, Asp1563-Met1823. (B) Reactivity of MoAb CLB-CAg 69 (left) and patient's plasma (right) with truncated recombinant fragments of the A3 domain of factor VIII. Binding of anti-factor VIII antibodies to the metabolically labeled factor VIII fragments was assessed by immunoprecipitation and analyzed under reducing conditions on a 10% (wt/vol) SDS-polyacrylamide gel. Lane 1, Asp1563-Asp1840; lane 2, Asp1563-Gln1778; lane 3, Asp1563-Met1823.
Effect of human anti-factor VIII antibodies on the interaction of factor IXa with the factor VIII light chain.
Binding to the factor VIII light chain epitope Gln1778-Met1823 suggests that the antibodies inhibit factor VIII activity by interfering with complex assembly of factor VIII and factor IXa.23 To test this, we evaluated the effect of patient's IgG in a binding assay of factor IXa and immobilized factor VIII light chain. Increasing concentrations of patient's IgG inhibited the binding of factor IXa to the factor VIII light chain in a dose-dependent manner (Fig4). The concentration of IgG required to achieve 50% inhibition was 0.5 mg/mL, corresponding to 2 BU/mL. In contrast, IgG from a healthy individual did not compete for binding of factor IXa. These data provide evidence that the antibodies present in the plasma of a patient with hemophilia A inhibit factor VIII activity by interfering with assembly of the factor VIIIa–factor IXa complex.
Effect of IgG on the interaction between factor IXa and factor VIII light chain. Various concentrations of purified IgG from the inhibitor patient (•) and a healthy control (○) were incubated with factor VIII light chain immobilized by MoAb CLB-CAg 12 on a microtiter plate. After 2 hours incubation at 37°C the IgG was removed and 40 nmol/L factor IXa was added. Residual factor IXa bound was determined after 4 hours of incubation at 37°C and calculated relative to a control experiment in which no IgG was added. Each datapoint represents the mean (±SD) of at least three experiments.
Effect of IgG on the interaction between factor IXa and factor VIII light chain. Various concentrations of purified IgG from the inhibitor patient (•) and a healthy control (○) were incubated with factor VIII light chain immobilized by MoAb CLB-CAg 12 on a microtiter plate. After 2 hours incubation at 37°C the IgG was removed and 40 nmol/L factor IXa was added. Residual factor IXa bound was determined after 4 hours of incubation at 37°C and calculated relative to a control experiment in which no IgG was added. Each datapoint represents the mean (±SD) of at least three experiments.
DISCUSSION
Inhibitory antibodies that arise after factor VIII replacement therapy constitute a serious complication of hemophilia treatment. Insight into the epitope specificity and mechanism of action of these inhibitory antibodies has evolved over the past few years.12-17 At present two major inhibitor epitopes have been localized at amino acid sequence Arg484-Ile508 and Val2248-Ser2312 of factor VIII.12,13 Here, we describe an additional inhibitor epitope for human anti-factor VIII antibodies, consisting of amino acid residues Gln1778-Met1823, located within the A3 domain of factor VIII. Previous studies from our laboratory have provided evidence for the presence of a factor IXa interactive site at amino acid position Glu1811-Lys1818 of the factor VIII light chain.20 23 In agreement with the observed epitope specificity, purified IgG derived from patient's plasma interferes with binding of factor IXa to the factor VIII light chain. It should be noted that a large amount of purified IgG is needed to inhibit binding of factor IXa to the factor VIII light chain. This is most likely caused by the low amount of factor VIII–specific antibodies present in the purified IgG fraction that was used in this study.
Attempts to further narrow the epitope of the human alloantibody described above using synthetic peptides corresponding to Tyr1786-Ala1801, Gly1799-Lys1813, and Thr1815-Ala1834 have been unsuccessful. Purified patient's IgG did not bind to the three synthetic peptides when immobilized on microtiter wells. Furthermore, none of the three peptides could alleviate the inhibitory activity of the antibody in inhibitor neutralization assays when added in concentrations up to 1 mmol/L (data not shown). The above findings suggest that binding of the human antibody described in this study may depend on the conformation of this part of the factor VIII light chain.
Several inhibitors both in congenital and acquired hemophilia have been described which can be almost completely neutralized by the factor VIII light chain, whereas only partial neutralization occurs in the presence of the C2-domain.13,19 Recently, an inhibitor patient was identified whose antibodies bound to a fragment comprising the A3 and C1 domain of factor VIII.28 Binding of the antibody to this fragment could be completely abolished by the addition of the synthetic peptide Lys1804-Val1819 which overlaps the inhibitor epitope described in this study.28 Together with our data these findings suggest that a subset of human inhibitors interacts with the factor IXa binding site on the light chain of factor VIII.
It should be noted that the heterogeneity of the antibody preparation used in this study may complicate interpretation of the results obtained. For instance, we are unable to exclude that an additional antibody binding site is present between amino acid residues Lys1818 and Cys2174 on the factor VIII light chain. Furthermore, our analysis may be affected by the small amounts of anti-C2 antibodies present in the patient's plasma. The above considerations warrant careful interpretation of the results obtained but do not seem to limit our conclusion with regard to the biological activity of the inhibitory antibodies described in this study.
The interaction between factor IXa and the factor VIII light chain is modulated by vWF.20 Thus, amino acid sequence Gln1778-Met1823 which contains the factor IXa binding site may not be exposed when factor VIII is bound to vWF. Shielding of antigenic sites by vWF has been observed for the extensively studied epitope in the C2 domain of factor VIII.29 In the latter study the presence of additional epitopes on the factor VIII light chain located outside the C2 domain was not taken into account. Reduced binding of inhibitors to Gln1778-Met1823 may in part explain the modulating effect of vWF on the inhibitory activity described in that study.29 Whether shielding of antigenic sites on the factor VIII light chain by vWF constitutes an etiologic factor in inhibitor formation remains to be established.
ACKNOWLEDGMENT
We thank Dr P.J. Lenting for his advice concerning the factor IXa binding assay and competition experiments and H. ter Maat for providing the purified factor IXa. We gratefully acknowledge Dr O.D. Christophe and Dr E.N. van den Brink for critical reading of the manuscript.
Address reprint requests to Jan Voorberg, PhD, Dept of Blood Coagulation, Central Laboratory of the Netherlands Red Cross Blood Transfusion Service, Plesmanlaan 125, 1066 CX Amsterdam, The Netherlands.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal