Abstract
A new transgenic mouse has been generated in which the proto-oncogene BCL-2 is ubiquitously overexpressed. H2K-BCL-2 transgenic mice overexpress BCL-2 in all cells of the hematolymphoid system and have been used to assess the role of BCL-2 in protecting cells of the hematolymphoid system from the consequences of ionizing radiation. We have expanded on previous studies that have demonstrated protection for specific (lymphoid) cell populations and show that systemic overexpression of BCL-2 can protect the hematopoietic system as a whole, including hematopoietic stem cells (HSC), thus increasing the radioresistance of the animal. The increase in radioresistance in H2K-BCL-2 transgenic mice has two components: an increase in the radioresistance of individual cells and, to a lesser extent, an increase in the size of certain critically important cell populations, such as HSC. Bone marrow transplantation experiments show that the increased radioresistance of the transgenic animals is provided by cells of the hematopoietic system. Protection against the consequences of irradiation is not limited to the increased expression levels of BCL-2 in transgenic mice; levels of endogenous BCL-2 are higher in lymphocyte populations that survive irradiation in wild-type mice. We show that ubiquitous overexpression of BCL-2 in the hematopoietic system can be used to increase the resistance of animals to lethal challenges such as irradiation.
Bcl-2 IS THE FIRST characterized member of a family of genes that play a role in the regulation of apoptosis. Genes from this family fall into two distinct groups; they prevent or induce apoptosis. The biochemical basis for their actions remains unclear, despite recent advances in understanding the way in which the proteins involved in apoptosis act1,2and interact.3,4 The various members of the Bcl-2 family can form (hetero)dimers and thus can neutralize each others actions.5 Overexpression of either an apoptosis-preventing or apoptosis-promoting family member will increase or decrease the resistance to apoptosis by binding to endogenously expressedBcl-2 family members. More recently, it has become clear that these proteins are also subjected to regulation by posttranslational modifications, most notably serine phosphorylation.6 7
Overexpression of Bcl-2 in transgenic mice has been studied extensively in the lymphoid system,8-10 and to a lesser extent in the myeloid system.11 In addition, Bcl-2 has been overexpressed in several nonhematopoietic tissues in transgenic mice, including liver,12 neuronal tissue,13 the olfactory system,14 and the gonads.15 Although it has been overexpressed in early hematopoietic progenitor cells in vitro,16Bcl-2 overexpression has not been targeted to HSCs in vivo. To achieve this, we used the well-characterized H-2Kbpromoter,17,18 expressed at high levels on HSCs.19 20 H2K-BCL-2 transgenic mice overexpress BCL-2 in HSCs, but also in all other hematolymphoid cells, and presumably in all tissues expressing MHC class I.
Overexpression of Bcl-2 family members can block many forms of apoptosis, both in transfected cell lines and in transgenic mice. Irradiation is one of the apoptosis-inducing stimuli that can be blocked by Bcl-2 or Bcl-XL overexpression. This has been shown in lymphoid-targeted overexpression in transgenic mice, both in vitro and in vivo.9,21-23Bcl-2 null mutant mice show an increased susceptibility to the effects of irradiation on lymphoid populations in vitro.24,25Hematopoietic recovery after radiation requires proliferation and differentiation of HSC into the various depleted compartments. Even though HSC seem to be less sensitive to the effects of ionizing irradiation than more mature progenitors26 and lymphoid cells,27 it is the sensitivity of HSC that limits the long-term survival, thereby limiting the application of radiation as a therapeutic tool.28
We have used the ubiquitous expression pattern of the H2K-BCL-2 transgene to address the role of BCL-2 systematically in the response to ionizing irradiation of hematopoietic cells in vivo. We have found that not only transgenic lymphoid cells, but also erythroid and myeloid cells, can tolerate increased levels of irradiation before undergoing apoptosis, and that this is true both for mature cells and for progenitor and stem cells. This leads to a dramatically increased tolerance to radiation for the organism as a whole. In addition we examined the role of endogenous BCL-2 in radioresistance by comparing expression levels of lymphoid populations before and after irradiation. Wild-type (WT) cells that survive irradiation expressed significantly higher levels of BCL-2 than the pre-irradiated populations.
MATERIALS AND METHODS
Molecular biology.
The H2K-i-LTR cassette vector expresses cDNAs under control of the H2K-promoter/enhancer and Moloney MuLV enhancer/poly(A) site and consists of the HindIII–Nru I H2Kbpromoter fragment. The Nru I site was converted with a linker into a Not I site, and the fragment was attached to theNot I–Kpn I fragment of the H2K gene. TheNot I–Kpn I fragment contains intron and exon sequences (the sites are in exons 1 and 3, respectively). This deletes the Nru I–Not I fragment of H2K (most of exon 1, including the ATG). The HindIII–Kpn I H2K sequence was linked to the Eag I–Sal I-fragment from plasmid pTDK90,29 which contains the Moloney LTR. The humanBCL-2 cDNA-fragment11 was excised from its vector by digesting with Kpn I and Cla I. The resulting fragment was blunted, Not I linkers were ligated onto it and it was introduced as an Not I fragment into the H2K-i-LTR cassette resulting in the pH2K-BCL-2. Plasmids were constructed and tested according to established procedures.30 RNA was isolated and analyzed as described.31 The probe used was the BCL-2 fragment contained in the transgene.
Mice.
The HindIII fragment of pH2K-BCL-2 that was used for microinjection was isolated by agarose gel electrophoresis, electroeluted, and treated with Geneclean (BIO101, Vista, CA) according to the manufacturer's instructions. The DNA was injected into zygotes from crosses between F1(C57BL/6 × C3H) mice. Mice that were positive by Southern blot analysis were back-crossed with C57BlKa, Thy-1.1, Ly-5.1 (H-2b). The transgene was followed in later generations by flow cytometric screening for expression of human BCL-2 protein in peripheral blood cells.
Irradiation.
Mice, and purified HSCs, were irradiated with a 200-kV x-ray machine. The dose rate is 63 cGy/min. Fractionated irradiation was done at 3-hour intervals. Cells were kept on ice during irradiation. Mice were given acid water before irradiation and antibiotic water (1.1 g/L neomycin sulfate and 106 U/L polymyxin B sulfate) for at least 8 weeks after irradiation to reduce the chance of infection from opportunistic pathogens. Mice used for irradiation were 8 to 12 weeks old.
Tissue culture.
Bone marrow–derived mast cells (BMMC) were cultured as described.32 Briefly, bone marrow cells were plated in RPMI 1640 with 15% fetal calf serum (FCS), 5 × 10−5 mol/L β-mercaptoethanol, penicillin, streptomycin, and 20% WEHI-3b conditioned medium as a source of IL-3. Nonadherent cells were subcultured weekly. The histology of the mast cells in culture after 4 weeks was checked on May-Grünwald/Giemsa–stained cytospins. For interleukin-3 (IL-3) deprivation experiments, cells were washed several times in medium without IL-3 and then incubated in medium without IL-3. Cells were counted using an improved Neubauer hemocytometer, viable cells are defined as trypan-blue excluding cells. Splenocytes (1 to 2 × 107) were plated in 10 mL RPMI 1640 with 5% FCS, 5 × 10−5 mol/L β-mercaptoethanol, penicillin, streptomycin in T25 flasks. At regular intervals, samples were taken and the viability of the cells determined, or cells were analyzed by flow cytometry as described below. AC6-2.1 murine bone marrow stroma cells33 were maintained in the same medium as splenocytes. Cells were subcultured using collagenase/dispase (Boehringer, Mannheim, Germany). Cells were irradiated before plating of bone marrow cells (3,000 rads, Cs source). Bone marrow from irradiated mice was plated (102 to 107 cells/well on 12-well dishes, depending on irradiation dose). Medium was changed weekly (biweekly initially for cells plated at high densities).
Flow cytometry.
Single-cell suspensions were obtained in staining medium consisting of phosphate-buffered saline (PBS) plus 3% FCS and 10 mmol/L Hepes, pH 7.0, after ammonium chloride lysis of the red blood cells,34 if necessary, and straining through a nylon mesh. Cells were preincubated with mouse-IgG (Sigma, St Louis, MO) and stained for 20 minutes with the various antibodies at the appropriate dilutions and, if necessary, incubated with secondary antibodies. BCL-2 stainings were modified from Veis et al.35 Cells were stained with antibodies against surface markers, followed, when staining for the human BCL-2 protein, by fixation (5 minutes 0.8% formaldehyde in PBS) and permeabilization (5 minutes 0.3% saponin in staining medium). Cells were then incubated for 3 to 16 hours with anti-BCL-2 antibody in 0.3% saponin in staining medium, followed, if necessary, by incubation with the appropriate secondary antibody for 20 minutes. For staining of the mouse BCL-2 protein, the fixation step was omitted, and cells were incubated with the BCL-2 antibody for 2 hours in 0.03% saponin in staining medium. Sorting of HSC was done as described by Morrison and Weissman.34 Briefly, bone marrow cells are enriched for Sca-1+ cells, using MACS-columns (Milteny Biotech, Auburn, CA). The stained (Thy1.1-FITC, Lineage-cocktail, Sca-1-biotin and c-KIT-APC, followed by the secondary antibodies anti-rat-PE and streptavidin–texas red) and enriched cells are sorted as described. The lineage cocktail consists of antibodies against CD3, CD4, CD5, CD8, B220, TER119, Mac-1, and Gr-1. Labeled cells were analyzed and sorted with a dual laser fluorescence-activated cell sorter (FACS) (Becton Dickinson Immunocytometry Systems, San Jose, CA), modified as described by Parks et al,36 and made available through the FACS shared user group at Stanford University. Dead cells were excluded from analysis by their propidium iodide staining characteristics. Two parameter data are presented as 5% probability plots.
The rat antibodies 53-7.3 (anti-CD5), 53-6.7 (anti-CD8), TER-119 (anti-erythro), GK 1.5 (anti-CD4), KT 31.1 (anti-CD3), 6B2 (anti-B220), M1/70 (anti-Mac-1), 8C5 (anti-GR-1), and E13-161 (anti-Sca-1) were prepared from the respective hybridoma clones as were the conjugates KT 31.1-PE, 6B2-FITC, M1/70-FITC, 8C5-PE, 19XES-FITC (anti-Thy-1.1), 53-2.1-FITC (anti-Thy-1.2), A201.7-APC (anti-Ly5.1) and 2B8-APC (anti-c-kit). Secondary antibodies were obtained from Caltag (Burlingame, CA). Avidin Texas red was obtained from Cappel, anti-human BCL-2 (clone 124) from Dako (Glostrup, Denmark). Anti-mouse BCL-2 (clone 3F11), anti H-2Kb-PE (clone AF6-88.5), anti H-2Dd-biotin (clone 34-5-8S) and anti-NK-1.1-PE (clone PK136) were purchased from Pharmingen (San Diego, CA).
Western blot analysis.
Organs were lysed in KLB-buffer (1% Triton X100, 0.05% sodium dodecyl sulfate [SDS] 150 mmol/L NaCl, 5 mmol/L EDTA, and 10 mmol/L Na-phosphate, pH 7.2). The lysates were cleared by centrifugation (10 minutes, microfuge) and the protein content was determined using the Bio-Rad protein assay, according to the manufacturer's instructions (Bio-Rad Laboratories, Richmond, CA). The proteins were separated by SDS-polyacrylamide gel electrophoresis (PAGE) and blotted onto nitrocellulose as described.37 The dried blots were prehybridized with 5% milk powder in PBS, followed by incubation with a mouse–anti-human BCL-2 antibody (Dako). Staining was visualized using an ECL-kit (Amersham, Arlington Heights, IL) according to the manufacturer's instructions.
RESULTS
H2K-BCL-2 transgenic mice express functional protein.
After introduction of the H2K-BCL-2 construct (Fig1A) into zygotes, 64 mice were obtained, 10 of which carried the transgene. Analysis has concentrated on founderlines 1038, 1043, and 1053. Southern blot analysis showed low to intermediate copy numbers for these founderlines. The percentage of positive founder animals with high expression levels (see below) indicates that there is no selection against ubiquitous overexpression of this transgene during development.
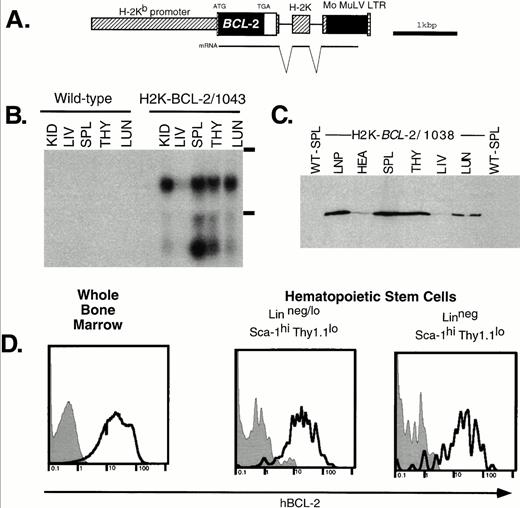
Expression of the H2K-BCL-2 transgene. (A) The H2K-BCL-2 transgenic construct (B) Northern blot analysis of organs from an H2K-BCL-2/1043 transgenic mouse and a wild-type littermate. The blot is probed with the human BCL-2 cDNA insert. Kid, kidney; Liv, liver; Spl, spleen; Thy, thymus; Lun, lung; LNP, peripheral lymph nodes; Hea, heart. The position of the ribosomal bands is indicated on the right. (C) Western blot analysis of organs from an H2K-BCL-2 transgenic mouse. The blot is probed with a human BCL-2–specific antibody. All lanes contain the same amount of protein except lymph nodes, which contains one half the amount of the other lanes. (D) Analysis of BCL-2 expression in hematopoietic stem cells. Bone marrow was stained with antibodies against Thy1.1, Sca-1, Lin, and BCL-2. Data collected from 200,000 bone marrow cells was used to show the BCL-2 expression in Linneg/lo and Linneg hematopoietic stem cell populations. Gray histograms show staining for the human BCL-2 protein in wild-type cells, the bold lines depict staining in H2K-BCL-2 transgenic cells. A representative experiment out of three is shown.
Expression of the H2K-BCL-2 transgene. (A) The H2K-BCL-2 transgenic construct (B) Northern blot analysis of organs from an H2K-BCL-2/1043 transgenic mouse and a wild-type littermate. The blot is probed with the human BCL-2 cDNA insert. Kid, kidney; Liv, liver; Spl, spleen; Thy, thymus; Lun, lung; LNP, peripheral lymph nodes; Hea, heart. The position of the ribosomal bands is indicated on the right. (C) Western blot analysis of organs from an H2K-BCL-2 transgenic mouse. The blot is probed with a human BCL-2–specific antibody. All lanes contain the same amount of protein except lymph nodes, which contains one half the amount of the other lanes. (D) Analysis of BCL-2 expression in hematopoietic stem cells. Bone marrow was stained with antibodies against Thy1.1, Sca-1, Lin, and BCL-2. Data collected from 200,000 bone marrow cells was used to show the BCL-2 expression in Linneg/lo and Linneg hematopoietic stem cell populations. Gray histograms show staining for the human BCL-2 protein in wild-type cells, the bold lines depict staining in H2K-BCL-2 transgenic cells. A representative experiment out of three is shown.
RNA analysis of founderline 1043 (Fig 1B) shows abundant RNA expression in spleen thymus, lung and kidneys; the lowest expression levels are in liver. The lower band in Fig 1B most likely represents a shortened RNA transcript attributable to cryptic polyadenylation sites in the 3′ untranslated region of BCL-2. Figure 1C shows BCL-2 protein levels in different organs. These results are consistent with the RNA data; BCL-2 is weakly expressed in the liver, whereas it is highly expressed in hematopoietic organs such as thymus and spleen. The highest expression level is in the lymph nodes. There are no major differences in the expression pattern of the BCL-2 protein in the four lines tested by Western blot analysis: 1038, 1039, 1043, and 1053. Minor differences in expression levels were found in thymus (highest level detected in 1043) and heart (highest level detected in 1053). BCL-2, as detected by flow cytometry, was expressed at similar levels in all nucleated blood cells in the founderlines used for analysis.
As expected, all nucleated bone marrow cells stain positive for the transgene-derived human BCL-2 protein. Expression in HSCs was determined in two ways. Flow cytometric analysis of bone marrow stained for Sca-1, Lin, Thy-1.1, and hBCL-2 (Fig 1D) shows that the Thy-1.1lo Sca-1hi Linneg/lo HSC populations express the transgene at high levels, similar to unfractionated bone marrow. This is true for both the population that contains many short-term multilineage-reconstituting cells (Linneg/lo, .05% of bone marrow) and the population that is highly enriched for long-term multilineage reconstituting potential (Linneg, .01% of bone marrow).34 In addition, sorted WT and transgenic HSCs characterized by the staining profile that we routinely employ for HSC isolation (Sca-1hic-KIThi Linneg/loThy1.1lo),34 were restained with a human BCL-2 specific antibody and analyzed by flow cytometry and immunohistochemistry. We found again that all transgenic HSCs express high levels of human BCL-2 (not shown).
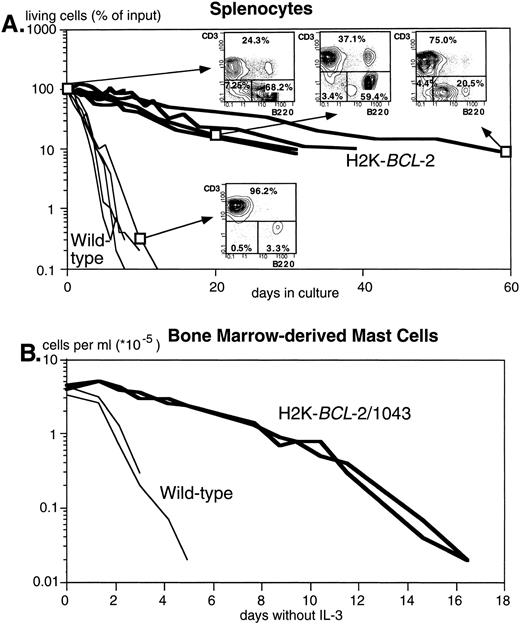
Figure 2A shows the dramatically increased survival in cultures without added growth factors of splenocytes from three different founderlines, compared with their WT littermates. Both B and T lymphocytes are protected. T cells, however, are protected to a higher degree, as illustrated by the reversed ratio of T versus B cells after 2 months in culture (FACS plots in Fig 2A). Figure 2B illustrates survival of bone marrow–derived mast cells after IL-3 withdrawal. Protection against apoptosis induced by growth factor deprivation has also been shown in NK-1.1+ splenocytes, lipopolysaccharide (LPS)-stimulated splenocytes, thymocytes, monocytes, and macrophages (data not shown).
The H2K-BCL-2 transgene protects cells from apoptosis. (A) Survival of unstimulated splenocytes from H2K-BCL-2 transgenic mice (thick lines) and wild-type littermates (thin lines) in vitro. The transgenic mice were from founderlines 1038, 1039, and 1053. Data from five animals per group, cultured in four separate experiments. The FACS plots show the B (B220+) and T (CD3+) cell populations in transgenic spleen at day 0, 20, and 62 of culture and in the wild-type spleen at day 9. (B) Survival of bone marrow-derived mast cells in vitro from H2K-BCL-2/1043 transgenic mice (thick lines) and wild-type littermates (thin lines) after IL-3 withdrawal. The cell culture results from two animals per group are depicted.
The H2K-BCL-2 transgene protects cells from apoptosis. (A) Survival of unstimulated splenocytes from H2K-BCL-2 transgenic mice (thick lines) and wild-type littermates (thin lines) in vitro. The transgenic mice were from founderlines 1038, 1039, and 1053. Data from five animals per group, cultured in four separate experiments. The FACS plots show the B (B220+) and T (CD3+) cell populations in transgenic spleen at day 0, 20, and 62 of culture and in the wild-type spleen at day 9. (B) Survival of bone marrow-derived mast cells in vitro from H2K-BCL-2/1043 transgenic mice (thick lines) and wild-type littermates (thin lines) after IL-3 withdrawal. The cell culture results from two animals per group are depicted.
H2K-BCL-2 transgenic mice develop normally, except for a twofold to fivefold increase in size of the lymphoid organs (thymus, spleen, lymph nodes), in the number of circulating white blood cells, and in the number of HSCs. Other organs, and the body weight of the mice, do not significantly differ from WT littermates. Table1 shows the combined data for different founder lines. The increase in thymus size and cellularity seen in H2K-BCL-2 transgenic mice has not been reported in other transgenic lines that overexpress BCL-2 in the thymus.9,10,38 Table 2 shows the sizes of the various major subpopulations in the thymus, as defined by CD4/CD8 and CD3/c-KIT. Although all have expanded, the largest relative expansion is seen in the c-KIT+ cells that are undergoing positive selection.39 The CD3+c-KIT+ cells, which are making the transition from double positive to single positive cells,39have expanded the most, approximately 10-fold, in these transgenic mice. In addition, there is a relative increase in CD4 and CD8 single positive cells. Thymus expansion (weight) is more pronounced in founderline 1043 (235% of WT, n = 4) than in founderline 1038 (142% of WT, n = 5). In addition to the increase in number of lymphoid cells, there are more HSCs. The number of cells with the surface phenotype Sca-1hi c-KIThi Thy1.1loLinneg/lo in bone marrow is increased twofold to threefold in transgenic mice.
Organ Weights and Cell Counts in H2K-BCL-2 Mice
| . | Wild-Type . | H2K-BCL-2 . | P . | ||
|---|---|---|---|---|---|
| wt . | n . | wt . | n . | ||
| Mouse (g) | 22.2 ± 2.0 | 10 | 23.3 ± 3.6 | 10 | .4094 |
| Spleen (mg) | 83 ± 29 | 22 | 256 ± 86 | 22 | <.0001-150 |
| Thymus (mg) | 58 ± 23 | 13 | 90 ± 28 | 14 | .0035-150 |
| Lymph nodes (mg) | 12 ± 4 | 5 | 39 ± 13 | 5 | .0022-150 |
| Heart (mg) | 100 ± 20 | 3 | 120 ± 20 | 3 | .2879 |
| Kidney (mg) | 250 ± 40 | 3 | 280 ± 90 | 3 | .6257 |
| Spleen (106) | 87 ± 38 | 10 | 295 ± 122 | 10 | <.0001-150 |
| Thymus (106) | 146 ± 41 | 5 | 310 ± 34 | 5 | .0001-150 |
| Bone marrow (106) | 125 ± 38 | 11 | 126 ± 57 | 11 | .9619 |
| WBC (103/μL) | 8.5 ± 3.1 | 11 | 39.2 ± 19.2 | 19 | <.0001-150 |
| . | Wild-Type . | H2K-BCL-2 . | P . | ||
|---|---|---|---|---|---|
| wt . | n . | wt . | n . | ||
| Mouse (g) | 22.2 ± 2.0 | 10 | 23.3 ± 3.6 | 10 | .4094 |
| Spleen (mg) | 83 ± 29 | 22 | 256 ± 86 | 22 | <.0001-150 |
| Thymus (mg) | 58 ± 23 | 13 | 90 ± 28 | 14 | .0035-150 |
| Lymph nodes (mg) | 12 ± 4 | 5 | 39 ± 13 | 5 | .0022-150 |
| Heart (mg) | 100 ± 20 | 3 | 120 ± 20 | 3 | .2879 |
| Kidney (mg) | 250 ± 40 | 3 | 280 ± 90 | 3 | .6257 |
| Spleen (106) | 87 ± 38 | 10 | 295 ± 122 | 10 | <.0001-150 |
| Thymus (106) | 146 ± 41 | 5 | 310 ± 34 | 5 | .0001-150 |
| Bone marrow (106) | 125 ± 38 | 11 | 126 ± 57 | 11 | .9619 |
| WBC (103/μL) | 8.5 ± 3.1 | 11 | 39.2 ± 19.2 | 19 | <.0001-150 |
Mean ± SD. The transgenic group contains data from founderlines 1038, 1039, 1043, and 1053. Data are for 4- to 12-week-old mice; transgenic and wild-type mice are age-matched. Body weights were determined for 7- to 9-week-old mice from founderlines 1038 and 1043, and their wild-type littermates. The data are for five males and five females per genotype.
Values that show a significant difference (t-test).
Thymic Populations in H2K-BCL-2 Transgenic Mice
| . | Wild-Type . | H2K-BCL-2 . | P . |
|---|---|---|---|
| CD4−CD8− | 9.1 ± 2.4 | 16.5 ± 5.7 | .1069 |
| CD4+CD8+ | 121.0 ± 27.2 | 222.3 ± 30.9 | .0130* |
| CD4+CD8− | 15.9 ± 2.4 | 41.8 ± 8.9 | .0082* |
| CD4−CD8+ | 8.1 ± 1.0 | 22.2 ± 4.3 | .0052* |
| CD3−c-KIT+ | 1.1 ± 0.4 | 2.6 ± 0.7 | .0322* |
| CD3+c-KIT+ | 0.4 ± 0.1 | 4.0 ± 0.4 | .0001* |
| CD3+c-KIT− | 20.3 ± 3.2 | 62.5 ± 10.5 | .0026* |
| CD3−c-KIT− | 133.1 ± 27.2 | 235.1 ± 30.9 | .0127* |
| . | Wild-Type . | H2K-BCL-2 . | P . |
|---|---|---|---|
| CD4−CD8− | 9.1 ± 2.4 | 16.5 ± 5.7 | .1069 |
| CD4+CD8+ | 121.0 ± 27.2 | 222.3 ± 30.9 | .0130* |
| CD4+CD8− | 15.9 ± 2.4 | 41.8 ± 8.9 | .0082* |
| CD4−CD8+ | 8.1 ± 1.0 | 22.2 ± 4.3 | .0052* |
| CD3−c-KIT+ | 1.1 ± 0.4 | 2.6 ± 0.7 | .0322* |
| CD3+c-KIT+ | 0.4 ± 0.1 | 4.0 ± 0.4 | .0001* |
| CD3+c-KIT− | 20.3 ± 3.2 | 62.5 ± 10.5 | .0026* |
| CD3−c-KIT− | 133.1 ± 27.2 | 235.1 ± 30.9 | .0127* |
Means ± SD, n = 3, for the various populations (millions/thymus). The data are for H2K-BCL-2 transgenic mice from founderline 1043 and wild-type littermates. The mice were 8 weeks old at the time of analysis.
Values considered to show a significant difference (t-test).
Radioresistance of H2K-BCL-2 transgenic mice.
Overexpression of Bcl-2 protects cells from death induced by irradiation. In view of the ubiquitous expression of this transgene, which includes expression in progenitor and stem cells, we tested whether H2K-BCL-2 transgenic mice show an increased resistance to the lethal effect of irradiation. As shown in Fig3A, H2K-BCL-2 transgenic mice had an increased resistance to total body irradiation (TBI); the LD50/30 (dose at which 50% of the animals survive for at least 30 days) for single-dose irradiation has increased from approximately 6.5 Gy (WT) to 8.5 Gy (H2K-BCL-2). Whereas all animals, transgenic and WT, die after receiving doses higher than 9.5 Gy, death is delayed in transgenic animals. WT animals die at day 9 or 10 after receiving 12 Gy TBI; H2K-BCL-2 mice die 3 to 4 days later. After receiving 15 Gy total body irradiation (TBI), WT animals die at days 5 to 6, and transgenic mice 1 to 3 days later. More than 80% of the transgenic mice survive long term (more than 3 months) when subjected to a routine lethal preconditioning regimen for bone marrow or stem cell transplantation, fractionated irradiation of 9.5 Gy (two doses of 4.75 Gy, 3 hours apart; Fig 3B). Radiation-induced death at this dose is caused by failure of the hematopoietic system. This is confirmed by bone marrow transfer experiments: bone marrow cells (5 × 105) from either H2K-BCL-2 transgenic mice (H-2Kb) or WT littermates were transferred into lethally irradiated (split-dose, 8 Gy) allogeneic host mice (Balb/c, H-2Dd). Multilineage, long-term reconstitution was confirmed by analysis of circulating white blood cells, using MHC class I to distinguish host and donor-derived cells. Reconstituted mice were reirradiated (lethal dose, 9.5 Gy, split-dose) 3 to 4 months after reconstitution. As expected, none of the nonreconstituted mice, or mice that were reconstituted with WT bone marrow, survived (Fig 3C). However, four of five mice that were repopulated with H2K-BCL-2 bone marrow from founderlines 1038 or 1039 survived this second TBI. This is significantly different (p = .0238, Fisher's exact test) from the expected outcome of lethal irradiation—no 30-day survivors.
Radioprotective effect of the H2K-BCL-2 transgene. (A) Survival of H2K-BCL-2 transgenic and wild-type mice after single dose irradiation at the doses indicated. Graph is based on survival data from 84 wild-type and 51 transgenic mice, 2 (extreme values) to 17 (mid-range) mice were assayed per irradiation dose. The 30-day survival of wild-type mice differs significantly from transgenic mice at 6.5, 7, and 8 Gy (P values [Fisher's exact test] are .0359, .0002, and .0310, respectively). (B) Long-term survival of H2K-BCL-2 transgenic mice after lethal total body irradiation, 9.5 Gy, split dose. The difference in 90-day survival is highly significant, P < .0001 (Fisher's exact test). (C) Survival of reconstituted Balb/c mice after split dose 9.5-Gy irradiation. These mice were reconstituted with bone marrow from H2K-BCL-2 transgenic mice and wild-type littermates at 3 to 4 months before irradiation.
Radioprotective effect of the H2K-BCL-2 transgene. (A) Survival of H2K-BCL-2 transgenic and wild-type mice after single dose irradiation at the doses indicated. Graph is based on survival data from 84 wild-type and 51 transgenic mice, 2 (extreme values) to 17 (mid-range) mice were assayed per irradiation dose. The 30-day survival of wild-type mice differs significantly from transgenic mice at 6.5, 7, and 8 Gy (P values [Fisher's exact test] are .0359, .0002, and .0310, respectively). (B) Long-term survival of H2K-BCL-2 transgenic mice after lethal total body irradiation, 9.5 Gy, split dose. The difference in 90-day survival is highly significant, P < .0001 (Fisher's exact test). (C) Survival of reconstituted Balb/c mice after split dose 9.5-Gy irradiation. These mice were reconstituted with bone marrow from H2K-BCL-2 transgenic mice and wild-type littermates at 3 to 4 months before irradiation.
Effects of irradiation on the hematopoietic system.
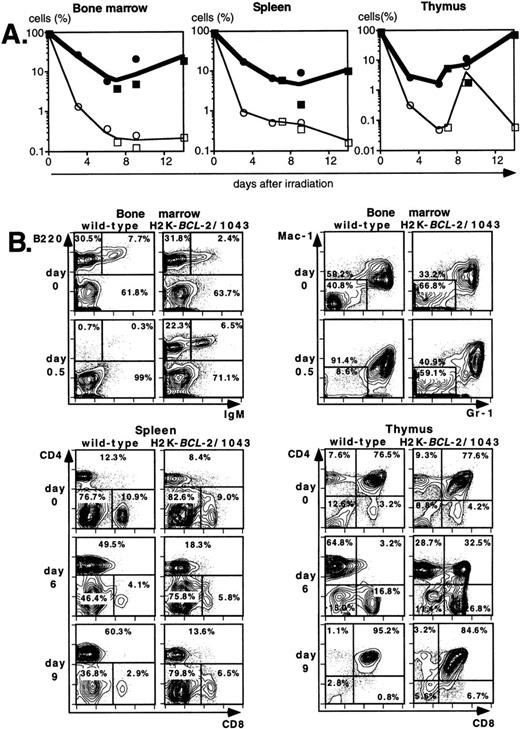
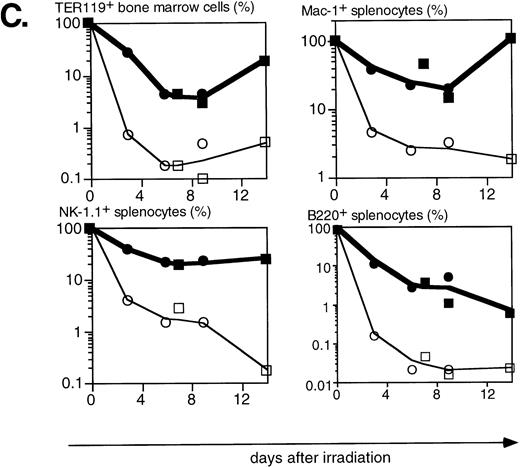
Lethal irradiation has profound effects on the hematopoietic system. In less than a day the number of cells, and composition of the hematopoietic organs, changes dramatically. Total cellularity of the hematopoietic organs in WT mice is reduced by two to three orders of magnitude in a few days (Fig4A). Cellularity continues to decrease over time until the animal dies, usually 12 to 14 days after irradiation. Severe infections, or irradiation doses of 15 Gy or higher that lead to extensive damage to the gastrointestinal tract, can lead to earlier deaths (5 to 8 days after irradiation). The relative reduction in cellularity after irradiation is far less severe in H2K-BCL-2 transgenic mice, one to two orders of magnitude less (Fig 4A). Cellularity is shown as a percentage of the unirradiated organ, to illustrate the difference in radiosensitivity of the cells. It does not take into account the increased size of spleen and thymus in transgenic animals (Table 1). The absolute difference in size of splenic and thymic cell populations in transgenic mice thus is two to three times larger than indicated in (Fig 4A and C). Such a correction is not necessary for bone marrow, for which the cell counts do not differ between transgenic and WT animals. Protection against radiation-induced death is extended to all hematolymphoid cell types (Fig 4B and C). Most apparent is the effect on B and pre-B lymphocytes, which, in WT mice, virtually disappear from bone marrow within a day. In H2K-BCL-2 transgenic mice large numbers of B220+ cells were retained in both bone marrow and spleen, and their numbers begin to increase again after 9 days. Nucleated erythroid precursors (TER119+) and myeloid cells (Mac-1+) also remain in much larger numbers (Fig 4C). The composition of WT bone marrow changes drastically after irradiation, the myeloid cells (Gr-1 and Mac-1+ cells40) (Fig 4B), which are more radioresistant, initially make up the vast majority of cells. At later points, a large percentage of NK-1.1 cells can be found (see below). By contrast, in H2K-BCL-2 transgenic bone marrow, all major populations—myeloid, lymphoid, and erythroid—are retained. The flow cytometry plots shown in Fig 4B illustrate that both CD4 and CD8 positive T cells from the spleen are protected by this transgene. The change in their ratio that is observed in WT mice (CD8+ cells are far more radiosensitive than CD4+ cells27) is virtually absent in transgenic mice. Spleen-derived B lymphocytes from H2K-BCL-2 transgenic mice also show a dramatically increased radioresistance, as do natural killer (NK) cells (Fig 4C). The decrease in the number of NK cells is up to 100-fold less in transgenic mice following irradiation (absolute numbers up to 300-fold higher). In thymus the most dramatic effect is seen with the most radiosensitive population, CD4+CD8+ double-positive cells.27These cells show a 1,000-fold higher survival in transgenic mice compared with WT mice (Fig 4A and B). Cellularity increases transiently 9 days after irradiation in WT thymus (observed in thymi from 4 of 4 WT mice, in two experiments). This very rapid increase consists of cells that are double positive for both CD4 and CD8, presumably derived from surviving thymic progenitor cells (CD4−8−3− or CD4lo8−3−).41 It does not herald stable repopulation, as thymic cellularity collapses again during the time the mice succumb to the effects of irradiation (12 to 14 days after irradiation). This induction of CD4+CD8+ cells after irradiation is reminiscent of what has been reported in RAG-deficient mice.42
Radioprotective effect of H2K-BCL-2 for hematopoietic populations following irradiation. (A) Cellularity in bone marrow, spleen, and thymus of H2K-BCL-2 transgenic mice and wild-type littermates following split-dose, 9.5-Gy total body irradiation. Bold lines, filled symbols depict H2K-BCL-2, thin lines; open symbols wild-type littermates. Circles show data from founderline 1043, rectangles from founderline 1053. Each datapoint represents one to three mice, the results from two separate experiments (circles and rectangles) are shown. (B) Flow cytometric analysis of bone marrow, thymus and spleen of H2K-BCL-2/1043 transgenic mice and wild-type littermates before (day 0) and after split-dose, 9.5-Gy total body irradiation. (Top left) B cells in bone marrow 16 hours after irradiation. This illustrates the extreme radiosensitivity of wild-type B cells. (Top right) Myeloid cells at the same timepoint. These relatively radioresistant cells accumulate in wild-type mice. (Bottom left) CD4 and CD8 staining in spleen 6 and 9 days after irradiation, illustrating the relative radiosensitivity of CD8 cells in wild-type mice. (Bottom right) CD4 and CD8 populations in thymus 6 and 9 days after irradiation. (C) Fate of various hematopoietic populations after irradiation. Symbols as in (A). The top two plots show erythroid cells in bone marrow (left) and myeloid cells in spleen (right). The second row shows NK cells (left) and B cells (right) in spleen. All as percentage of the populations before irradiation.
Radioprotective effect of H2K-BCL-2 for hematopoietic populations following irradiation. (A) Cellularity in bone marrow, spleen, and thymus of H2K-BCL-2 transgenic mice and wild-type littermates following split-dose, 9.5-Gy total body irradiation. Bold lines, filled symbols depict H2K-BCL-2, thin lines; open symbols wild-type littermates. Circles show data from founderline 1043, rectangles from founderline 1053. Each datapoint represents one to three mice, the results from two separate experiments (circles and rectangles) are shown. (B) Flow cytometric analysis of bone marrow, thymus and spleen of H2K-BCL-2/1043 transgenic mice and wild-type littermates before (day 0) and after split-dose, 9.5-Gy total body irradiation. (Top left) B cells in bone marrow 16 hours after irradiation. This illustrates the extreme radiosensitivity of wild-type B cells. (Top right) Myeloid cells at the same timepoint. These relatively radioresistant cells accumulate in wild-type mice. (Bottom left) CD4 and CD8 staining in spleen 6 and 9 days after irradiation, illustrating the relative radiosensitivity of CD8 cells in wild-type mice. (Bottom right) CD4 and CD8 populations in thymus 6 and 9 days after irradiation. (C) Fate of various hematopoietic populations after irradiation. Symbols as in (A). The top two plots show erythroid cells in bone marrow (left) and myeloid cells in spleen (right). The second row shows NK cells (left) and B cells (right) in spleen. All as percentage of the populations before irradiation.
Quantitative effects of irradiation on hematopoietic progenitors and stem cells.
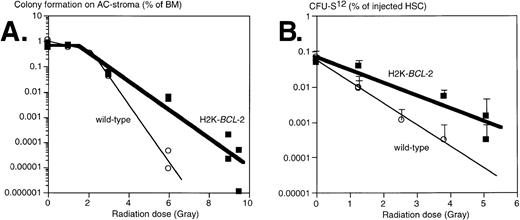
Clonogenic hematopoietic progenitors are sensitive to the effects of irradiation, albeit to different degrees for different cells.26 We have tested the effects of irradiation on progenitors from H2K-BCL-2 transgenic and WT littermate mice by evaluating two different populations: (1) clonogenic precursors, defined by colony formation on AC-6 or AC-11 stroma cells33; and (2) sorted HSC, using colony-forming unit-spleen (CFU-S) day 12 as an assay. Results obtained from plating on AC6-2.1 and AC11 cells did not differ significantly and have been combined. In this assay, comparable to various colony-forming cell (CFC) assays in semisolid media, approximately 1% of nonirradiated bone marrow gave rise to colonies of B-lymphoid, erythroid, and myeloid cells, confirmed by flow cytometry (data not shown). In this experiment, whole bone marrow was plated, isolated either from nonirradiated mice or from mice that had been irradiated immediately before plating. The plating efficiency of nonirradiated bone marrow on AC stroma cells was similar in WT and transgenic cultures (Fig 5A). At low irradiation doses, cells from both WT and transgenic mice were able to repair damage, as indicated by the shoulders in the curves. At higher doses, an exponential killing curve is seen. However, curve-fitting shows that transgenic bone marrow is less susceptible to radiation induced death; the slopes of these curves differ significantly, P = .0026. The D0 value (dose that kills 1 log e, or 63%, of cells at the exponential phase) increases from .55 Gy (WT) to 1.20 Gy (H2K-BCL-2), the extrapolation number n decreases from 100 to 3.3. The corresponding D10 values (dose that kills 1 log10, or 90%, of cells) were 0.92 Gy for WT and 1.77 Gy for H2K-BCL-2. Qualitative shifts occur after irradiation. At a 4-Gy dose, the percentage of B lymphocytes after 8 days on AC6 is 5.7 times higher (41.8% v 7.3%) in H2K-BCL-2 transgenic than in WT cultures. WT cultures contain relatively more myeloid cells, 89.1% versus 44.9% in transgenic cultures (average of two experiments). We also analyzed the radiation sensitivity of purified populations of HSC (Sca-1hi c-KIThiThy-1.1lo Linneg/lo), isolated by FACS. Day 12 CFU-S was used as an assay for sorted HSC after irradiation, because this is a quantitative assay for an activity present in purified populations of HSC. We found that these cells did not have a discernible repair phase (Fig 5B). Purified H2K-BCL-2 transgenic HSCs have an increased resistance to the effects of irradiation, and the slopes of the response curves obtained differ significantly, P = .0018. D0 values were 0.70 Gy for the WT HSCs and 1.18 Gy for H2K-BCL-2 HSCs. The corresponding D10 values are 1.63 Gy for WT and 2.74 Gy for H2K-BCL-2 HSCs. The inherent difference in radiosensitivity between WT and H2K-BCL-2 HSC was confirmed by a mixing experiment. A mixture of HSC (Sca-1hi c-KIThiThy-1.1lo Linneg/lo) was isolated from WT (66%) and H2K-BCL-2/1038 (33%) bone marrow, irradiated at 0, 1.3, or 5.1 Gy, and injected into lethally irradiated hosts, 100 or 200 cells per animal (0 Gy), 500 cells per animal (1.3 Gy) or 2,000 cells per animal (5.1 Gy). Host and donor cells can be distinguished by the congenic Ly5.1/Ly5.2 marker, WT, and H2K-BCL-2 cells by expression of human BCL-2 protein. Reconstituted animals were killed at day 12, and the spleens were pooled and analyzed by flow cytometry. As expected, the spleens contained myeloid and erythroid (Gr-1, Mac-1, TER119), but no lymphoid (B220, CD3, NK-1.1) donor-derived cells (results not shown). However, the percentage of donor-derived myeloid cells derived from transgenic HSC increased from 32% (nonirradiated HSC, 7 animals) to 58% (1.3 Gy irradiation, 5 animals) to 96% (4 Gy irradiation, two animals).
Survival of progenitors of H2K-BCL-2 transgenic and wild-type mice after irradiation. (A) Plating of bone marrow from irradiated mice on AC-6 or AC11 stromal cells. Bone marrow was isolated immediately after irradiation, which was given in a single dose, and plated on preirradiated stroma cells. Plating efficiency of clonogenic cells was determined at day 8 by counting the colonies. Combined data from three experiments, each datapoint represents one irradiated mouse. (B) Radioprotection of CFU-S day 12. HSCs were sorted from bone marrow from H2K-BCL-2 transgenic and wild-type mice, aliquoted, exposed to a single dose of irradiation, and injected into lethally irradiated (9.5 Gy, split dose) C57BI/Ka mice, five mice per group. Spleen colonies were counted on day 12. Error bars indicate standard deviations for each group of five spleens. Combined data from three separate experiments, containing a total of seven datapoints for the transgenic, and six for the wild-type mice. Some of these datapoints overlap and cannot be distinguished in the figure.
Survival of progenitors of H2K-BCL-2 transgenic and wild-type mice after irradiation. (A) Plating of bone marrow from irradiated mice on AC-6 or AC11 stromal cells. Bone marrow was isolated immediately after irradiation, which was given in a single dose, and plated on preirradiated stroma cells. Plating efficiency of clonogenic cells was determined at day 8 by counting the colonies. Combined data from three experiments, each datapoint represents one irradiated mouse. (B) Radioprotection of CFU-S day 12. HSCs were sorted from bone marrow from H2K-BCL-2 transgenic and wild-type mice, aliquoted, exposed to a single dose of irradiation, and injected into lethally irradiated (9.5 Gy, split dose) C57BI/Ka mice, five mice per group. Spleen colonies were counted on day 12. Error bars indicate standard deviations for each group of five spleens. Combined data from three separate experiments, containing a total of seven datapoints for the transgenic, and six for the wild-type mice. Some of these datapoints overlap and cannot be distinguished in the figure.
Endogenous Bcl-2 is expressed at higher levels after irradiation.
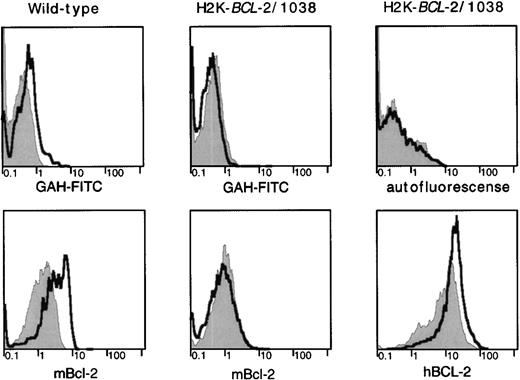
Because overexpression of a BCL-2 transgene confers an advantage to cells that have been irradiated, we investigated expression of endogenous BCL-2 in cell populations before and after irradiation in WT mice. The radioresistant lymphocyte populations that survive irradiation express higher levels of the endogenous BCL-2 protein than phenotypically similar unirradiated populations (Fig6). These higher levels of the endogenous protein after irradiation are not apparent in H2K-BCL-2 transgenic mice. However, in transgenic mice, expression of the transgene is higher in cells surviving irradiation. Higher levels of endogenous BCL-2 after irradiation are not seen in all types of hematopoietic cells. Some myeloid cells (eg, Mac-1–positive cells in bone marrow) do not show altered expression levels in cells that survive irradiation.
BCL-2 expression in radioresistant populations. H2K-BCL-2/1038 transgenic and wild-type mice (littermates) were analyzed for expression of endogenous (mBCL-2) and transgenic (hBCL-2) protein 2 days after lethal irradiation (9.2-Gy split dose). Staining of CD4+ splenocytes is depicted. Filled gray histogram, staining in untreated animals; thick line, staining after lethal irradiation. (Top) Background staining of the secondary antibody (goat–anti-hamster-FITC) used to detect hamster–anti-mouse BCL-2 or autofluorescence (mouse–anti-human BCL-2 was FITC-conjugated). (Left) Mouse BCL-2 expression in a wild-type mouse. (Middle) Mouse BCL-2 expression in a H2K-BCL-2 transgenic mouse. (Right) Expression of the transgene (human BCL-2) in a H2K-BCL-2 transgenic mouse. One representative experiment (out of four) is shown.
BCL-2 expression in radioresistant populations. H2K-BCL-2/1038 transgenic and wild-type mice (littermates) were analyzed for expression of endogenous (mBCL-2) and transgenic (hBCL-2) protein 2 days after lethal irradiation (9.2-Gy split dose). Staining of CD4+ splenocytes is depicted. Filled gray histogram, staining in untreated animals; thick line, staining after lethal irradiation. (Top) Background staining of the secondary antibody (goat–anti-hamster-FITC) used to detect hamster–anti-mouse BCL-2 or autofluorescence (mouse–anti-human BCL-2 was FITC-conjugated). (Left) Mouse BCL-2 expression in a wild-type mouse. (Middle) Mouse BCL-2 expression in a H2K-BCL-2 transgenic mouse. (Right) Expression of the transgene (human BCL-2) in a H2K-BCL-2 transgenic mouse. One representative experiment (out of four) is shown.
DISCUSSION
We have a created a transgenic mouse model in which the humanBCL-2 gene is overexpressed in cells from all hematopoietic lineages, including progenitor and stem cells. Surprisingly, transgenic animals develop normally, except for an expanded lymphoid compartment. Interestingly, the expanded lymphoid compartment in these transgenics includes the thymus, something that has not been observed in other transgenic mice that overexpress BCL-2 in T cells.9,10,38 Reasons for this difference could be that this transgene is expressed earlier during thymic T-cell differentiation, or that overexpression in H2K-BCL-2 transgenic mice extends to the non–T-cell compartments in the thymus, allowing them to expand and support increased T-cell development. In agreement with high expression of functional BCL-2 in T cells is the fact that the H2K-BCL-2 transgene is able to rescue T-cell development in IL-2R γ-chain null mutant mice.43 BCL-2 has been shown to allow lymphoid cells to survive higher levels of irradiation. We have used the ubiquitous overexpression of BCL-2 to investigate how this affects the response to irradiation of multiple hematopoietic cell populations in transgenic mice. We show here that overexpression ofBCL-2 throughout the hematopoietic system protects mice from radiation-induced hematopoietic failure, and consequent death. The hematopoietic populations in H2K-BCL-2 transgenic mice that have an increased resistance to the effects of irradiation include not only mature B- and T-cell populations, on which attention has focused before,9 21-23 but also NK, myeloid, erythroid, progenitor, and stem cells. The increased radioresistance of more mature populations is probably important for the short-term survival of irradiated H2K-BCL-2 transgenic mice, whereas the increased radioresistance of HSC ensures long-term survival. The increase in radioresistance provided by the H2K-BCL-2 transgene in mice has several different components. One of these is an increase in size of some of the critical cell populations, especially lymphocytes and HSCs, which means that more cells will survive irradiation. In addition, and more importantly, there is an increased cellular radioresistance, which enables cells expressing the transgene to survive higher doses of irradiation than WT cells, without undergoing apoptosis. It is not possible to assess directly the contribution of both components to the increase in HSC survival after irradiation, and thus long-term survival of the mice, because we cannot generate mice with comparable numbers of transgenic or WT HSC in a controlled fashion, not even after transplantation. However, a twofold increase in HSC would only increase the LD50/30 (based on HSC-survival) of WT mice by 0.48 Gy, much less than the 2 Gy that is actually observed. It would take approximately an 18-fold increase in HSC to increase the LD50/30 by 2 Gy [D0·ln(18), or D10·log(18)]. Therefore, the increased survival of transgenic animals cannot be explained by the increase in HSC alone but is mainly dependent on the increase in cellular radioresistance. The increased radioresistance of transgenic HSC is demonstrated in an experiment in which a mixture of WT and transgenic HSC was irradiated at different doses and injected into lethally irradiated mice; relative engraftment of H2K-BCL-2 transgenic HSC increases dramatically with increasing levels of irradiation.
The values that we find for D0 and LD50/30 in WT mice are consistent both with published values44,45 and with our own data on HSC in mice. A 2-month-old C57BL/Thy1.1 mouse contains approximately 1 to 2 × 105 long-term and short-term HSC; injection of 40 of these cells radioprotects approximately 50% of the injected mice.34,46 At 6.5-Gy irradiation, the depletion should be 6.5/D10·log10. Since we find a D10 of 1.63 Gy for WT HSC, a 4 log10 depletion is expected. This should leave 10 to 20 HSC, close to the number needed for 50% radioprotection (40 cells), taking injection losses into account. Because of the difference in D10 in H2K-BCL-2 transgenic mice, a 6.5-Gy irradiation dose would only lead to a 2.4 log10 depletion, leaving approximately 40-fold more HSC (not taking into account the increased starting number of HSC). However, at 8.5 Gy, the observed LD50/30 dose for transgenic mice, only a 3.1 log10 depletion would be predicted, not enough to explain the hematopoietic failure observed (death at 10 to 20 days after irradiation). One explanation could be that the CFU-S12 measured does not reflect in vivo radioprotection. More likely, and in line with published observations,26 would be the possibility that survival of HSCs does not closely follow exponential kinetics at higher irradiation doses. The large number of cells that would need to be isolated by sorting prevents a direct testing of survival at these high doses.
Clonogenic precursors that form colonies on AC6 have lower D0 and D10 values than sorted HSC. This is seemingly in contrast to published data,26,47 that show myeloid-CFC to be at least as radioresistant as stem cells. However, AC stroma reads out not only myeloid CFC, but CFC with erythroid and B-lymphoid potential as well. These precursors clearly differ in their radiosensitivity, with B-lymphoid precursors being the most radiosensitive. As shown in Fig 5A, a 5.2 log10 depletion (.000006%, instead of 1% of bone marrow-forming colonies) in precursors is seen in WT bone marrow at the LD50/30 level of irradiation (6.5 Gy). This number is directly derived from the graph shown in Fig 5A and does take into account the repair phase observed at lower irradiation doses. Assuming approximately 3 × 108bone marrow cells per mouse,38 and 1% of the bone marrow cells reading out as CFC on AC stroma cells (Fig 5A), this would indicate a near-total depletion of committed hematopoietic precursor cells. In H2K-BCL-2 transgenic bone marrow only a 2.9 log10 depletion is seen at the same irradiation level, which would leave an estimated 4 × 103 committed progenitor cells, assuming the same number of clonogenic cells per bone marrow initially.
Increased levels of endogenous and transgenic BCL-2 are seen in surviving lymphocyte populations after irradiation. Either cells expressing BCL-2 at high levels are selected following irradiation, or irradiation upregulates BCL-2 expression. Analysis of the effects of irradiation in a primitive human hematopoietic cell line does not support a direct upregulation as a consequence of irradiation.48 The fact that endogenous BCL-2 levels remain the same in transgenic cells after irradiation also suggests that BCL-2 expression is not induced by irradiation. Thus, higher levels of endogenous BCL-2 correlate with increased radioresistance, analogous to the protection provided by the transgene-derived protein. Different expression levels of anti-apoptotic genes such as Bcl-2 might explain the observed biphasic nature of the radiosensitivity of T-cell subsets27 and the PHA-induced radioresistance in radiosensitive thymocyte subsets.49 Another argument for the involvement of BCL-2 in the protection against radiation-induced apoptosis comes from the recent observations that the protein-serine/threonine kinase Raf-1, which is activated by upstream protein-tyrosine kinases after irradiation,50 can directly associate with the Bcl-2 protein and functionally activate it by phosphorylating,7 and thus inactivating,6Bad, a negatively acting member of the Bcl-2 family. While the radioprotective effect ofBcl-2 could reflect only the increased survival time of cells, allowing normal DNA repair to take place before the cell attempts to undergo cell division, recent results suggest a more direct interaction. It has been reported that overexpression of Bcl-2 can prevent downregulation of DNA repair enzymes such as apurinic/apyrimidinic endonuclease.51 This implies that cells overexpressing Bcl-2 might not only survive longer, and thus have more time to repair DNA damage, but might also have an increased repair capacity. The fact that higher BCL-2 levels after irradiation are only seen in lymphoid populations, but not in myeloid cells, indicates that Bcl-2 levels are limiting in the former, but not the latter. This is in line with the observation that lymphoid, but not myeloid cells in Bcl-2 null mutant mice are hypersensitive to apoptotic stimuli.24 25 For cell populations in which higher Bcl-2 levels are not seen in cells that have survived irradiation, other Bcl-2 family members, such as Bcl-XL or A1, may be critical.
We have shown that ubiquitous overexpression of BCL-2 in H2K-BCL-2 transgenic mice increased their resistance to radiation, by protecting the hematopoietic system as a whole. Surprisingly, this overexpression did not disrupt normal developmental processes in which apoptosis is thought to play a critical role. The reason for this is currently under investigation as is the effect ofBCL-2 overexpression on HSCs in vivo and in vitro. Overexpression of an anti-apoptotic protein has obvious advantages for transformed cells. Irradiation, and most chemotherapeutic agents, function through induction of apoptosis.52 The results presented here show that overexpression of Bcl-2 should increase the resistance to therapeutic interventions such as irradiation, not only for tumor, but also for a wide range of bystander cells. It is difficult to assess the contributions of single genes to the increased resistance of tumor cells to apoptosis induced by irradiation or chemotherapeutic agents using tumor derived cell lines that carry many, mostly unknown, mutations. The transgenic mouse model presented here allows the systematic investigation of the role that oncogenes such as Bcl-2 can play in overcoming radiation or chemotherapeutic challenges in both mature and immature hematopoietic cells of all lineages. Crosses between different transgenic mice will allow the evaluation of combinations of genes.
The ability of Bcl-2 to block apoptosis induced by a number of cancer therapeutic agents should lead to a consideration of the clinical use of Bcl-2 as a gene therapy agent enabling normal hematolymphoid cells to survive ordinarily supralethal therapeutic regimens. The risk/benefit ratio may be unacceptably high, sinceBcl-2 is a proto-oncogene, presumably resulting from its anti-apoptotic functions that allow DNA alterations to accumulate. However, if means to purge Bcl-2 overexpressing cells quantitatively in vivo can be achieved, or if its expression can be regulated (especially downregulated) effectively, the risk/benefit ratio for several kinds of cancer patients may become acceptable.
ACKNOWLEDGMENT
We thank Libuse Jerabek for laboratory management, Julie Christensen and Andreea Nicoleau for the generation of transgenic mice, Veronica Braunstein for antibody preparation, Lucino Hidalgo for animal care, Tim Knaak for assistance in fluorescence-activated cell sorting, Eric Lagasse for performing the apoptosis assays on monocytes and macrophages, Annette Schlageter for critically reading the manuscript, and Mark Alkema for providing the pTDK cassette vector.
Supported by grants from the Dutch Cancer Foundation/Koningin Wilhelmina Fonds (J.D.), American Cancer Society-California Division (K.L.G.) and SyStemix/Sandoz (I.L.W.).
Address reprint requests to Jos Domen, PhD, Department of Pathology and Developmental Biology, B263 Beckman Center, Stanford University School of Medicine, Stanford, CA 94305-5428.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

![Fig. 3. Radioprotective effect of the H2K-BCL-2 transgene. (A) Survival of H2K-BCL-2 transgenic and wild-type mice after single dose irradiation at the doses indicated. Graph is based on survival data from 84 wild-type and 51 transgenic mice, 2 (extreme values) to 17 (mid-range) mice were assayed per irradiation dose. The 30-day survival of wild-type mice differs significantly from transgenic mice at 6.5, 7, and 8 Gy (P values [Fisher's exact test] are .0359, .0002, and .0310, respectively). (B) Long-term survival of H2K-BCL-2 transgenic mice after lethal total body irradiation, 9.5 Gy, split dose. The difference in 90-day survival is highly significant, P < .0001 (Fisher's exact test). (C) Survival of reconstituted Balb/c mice after split dose 9.5-Gy irradiation. These mice were reconstituted with bone marrow from H2K-BCL-2 transgenic mice and wild-type littermates at 3 to 4 months before irradiation.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/7/10.1182_blood.v91.7.2272/3/m_blod4072203.jpeg?Expires=1769173334&Signature=egk2JdOm3TH4ct4LSpvqbvpietWra6jM0aEIjurA5LJmy4Zz7GJW~LpZOrHZHry1n43lRj9Ss5YblUBLRepQC-TWcj3Dg5ps41BuOzLT-t1CR7WUxxZbgJ4kWss21Gc0I8zO9rFG~VDI5IFNBMg7xu7mqvkiP1MMzjhSK7cF8erda~VRFs0OiHR-YBFPVGl3jgzwbehTaDXRdeWk8OPWmrEpmwPcGldWoS4I-wskbdHHyFdgDbdDH0vB2lW22x2fTO1lm83ZPwVDHvsP04qP~kTBoV2lifHTWJRWGRznvb739qIKHjUWeIsoKv1B6C3BnPKW7Y-yKIvg-pOYecHK2Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal