Abstract
The human interleukin-5 receptor (hIL-5R) consists of a unique α subunit (hIL-5Rα) and a common β subunit (βc) that activate two Janus kinases (JAK1 and JAK2) and a signal transducer and activator of transcription (STAT5). The precise stoichiometry of the hIL-5R subunits and the role of JAK kinases used in IL-5 signaling were investigated. We analyzed the interaction between hIL-5Rα and βc by immunoprecipitation using anti–hIL-5Rα and anti-βc monoclonal antibodies. The binding of JAK1 and JAK2 to each hIL-5R subunit was also evaluated in the hIL-5–responsive cell line, TF-h5Rα. It was observed that IL-5 stimulation induced the recruitment of βc to hIL-5Rα, although in the absence of IL-5 the subunits remain independent. In the absence of IL-5, JAK2 and JAK1 were associated with hIL-5Rα and βc, respectively. IL-5 stimulation resulted in tyrosine phosphorylation of JAK2, JAK1, βc, and STAT5. Moreover, IL-5–induced dimerization of IL-5R subunits caused JAK2 activation and βc phosphorylation even in the absence of JAK1 activation. Furthermore, tyrosine phosphorylation of JAK1 was dependent on the activation of JAK2. Detailed study of the C-terminal truncated cytoplasmic domain of hIL-5Rα revealed that the cytoplasmic stretch at position 346-387, containing the proline-rich region, is necessary for JAK2 binding. These observations suggest that activation of hIL-5Rα–associated JAK2 is indispensable for the IL-5 signaling event.
HUMAN INTERLEUKIN-5 (hIL-5) is a cytokine responsible for the proliferation, differentiation, and activation of eosinophils.1-3 Accumulating evidence suggests that eosinophils recruited by hIL-5 play a crucial role in allergic diseases such as bronchial asthma and in parasitic infections.3hIL-5 induces intracellular signals after binding to the functional hIL-5 receptor (hIL-5R) complex consisting of the hIL-5Rα and β subunits.4-6 Binding of hIL-5 occurs specifically through hIL-5Rα, which alone binds hIL-5 (kd, 250 to 590 pmol/L).5 Although the β subunit does not bind hIL-5 by itself, it does form a high-affinity receptor in combination with hIL-5Rα.5-7 The β subunit is the common β subunit (βc) of receptors for IL-3 and granulocyte-macrophage colony-stimulating factor (GM-CSF).6-9 Recent studies of the IL-3R and GM-CSFR have shown that each ligand triggers heterodimerization of the ligand-specific α and βc subunits.10-12 However, the formation of functional hIL-5R complexes has not yet been elucidated clearly.
Tyrosine phosphorylation of several cellular proteins, including βc, occurs within a few minutes in response to IL-5 stimulation.13,14 Because hIL-5Rα and βc do not contain a kinase domain or kinase activity in their structures, signaling via IL-5R must originate in activation of cytoplasmic kinases that are associated with hIL-5R subunits. The Janus kinases ([JAKs] JAK1, JAK2, JAK3, and TYK2),15-17 a family of nonreceptor tyrosine kinases, are likely candidates for regulating IL-5 signaling.18,19 Indeed, IL-5 was found to activate JAK1 and JAK2, resulting in the activation of downstream signal transducers and activators of transcription (STAT5, STAT1, and STAT3).20-24The cytoplasmic domain of βc, particularly the membrane-proximal region containing the box1 motif, has been shown to regulate JAK2 activation in the GM-CSFR system.25 We have previously reported the indispensable role of the cytoplasmic domain of mouse IL-5Rα for IL-5–induced activation of JAK1 and JAK2.13 24 The precise stoichiometry of the molecular interactions between JAKs (JAK1 and JAK2) and each hIL-5R subunit in IL-5–mediated signal transduction remained to be elucidated. To reevaluate the role of the hIL-5Rα cytoplasmic domain in regulating JAK activation, the associations of JAK1 and JAK2 with hIL-5Rα and βc were examined.
We describe the unassociated state of hIL-5Rα and βc subunits in the absence of IL-5. Following hIL-5 stimulation, hIL-5R subunits with their respective associated JAK2 and JAK1 are phosphorylated on their tyrosine residues and heterodimerized. Furthermore, we describe the activation of JAK2 in the absence of JAK1 activation.
MATERIALS AND METHODS
Reagents.
Baculovirus-expressed recombinant hIL-5 was purified to homogeneity from the supernatant of SF9 cells using single-step immunoaffinity chromatography with an anti–IL-5 monoclonal antibody (MoAb) as previously described.7 Mono5 (biologically active monomeric IL-5) was prepared as previously described.26 Human GM-CSF was kindly provided by Kirin Brewery Co (Maebashi, Japan). The MoAbs against hIL-5Rα (KM1266 and KM1074) have been established27 and were purified using Protein G–coupled Sepharose (Pharmacia Biotech, Uppsala, Sweden) from ascites of nude mice transplanted with a hybridoma. Rabbit anti-STAT5 antiserum was kindly provided by H. Wakao (DNAX Research Institute, Palo Alto, CA). MoAbs and polyclonal antibodies (Abs) against βc were obtained from Santa Cruz Biotechnology Inc (Santa Cruz, CA). Antiphosphotyrosine Ab 4G10 and antiserum against JAK1 and JAK2 were obtained from Upstate Biotechnology Inc (Lake Placid, NY).
Cells.
TF-1 cells,28 a human proerythroleukemic cell line, were kindly provided by T. Kitamura (DNAX Research Institute). TF-h5Rα cells,22 TF-1 cells transfected with hIL-5Rα cDNA, were maintained in RPMI 1640 supplemented with 10% fetal calf serum (FCS) containing 5 ng/mL hIL-5. YY-1 cells, an eosinophilic subline of HL-60, were maintained in Iscove's modified Dulbecco's medium supplemented with 10% FCS. To induce expression of hIL-5Rα, YY-1 cells were incubated at 2 × 105 cells/mL with 0.4 mmol/L sodium butyrate (pH 7.8) for 1 week before use, as previously described (YY-Bu).22
Constructs of glutathione S-transferase fusion proteins and kinase-negative JAKs.
cDNA constructs encoding the glutathione S-transferase (GST) fusion protein for the entire hIL-5Rα cytoplasmic domain were obtained by inserting the polymerase chain reaction (PCR) fragments of hIL-5Rα into the EcoRI/Xho I site of the pGEX4T-1 vector (Pharmacia Biotech). The following fusion protein constructs (with corresponding amino acids indicated) were also generated by PCR for this study: GST-5RαCD (346-400), GST-TCD4 (346-387), GST-TCD2 (346-365), GST-TCD1-1 (346-358), GST-TCD1 (346-356), GST-TCD0 (346-351), and GST-dDC1 (346-350/359-400). cDNA encoding GST fusion protein with the cytoplasmic domain of the βc subunit was constructed by ligating the fragment, which was digested byFspI/BglII and followed by a fill-in reaction, into pGEX4T-3 vector (Pharmacia Biotech). The βc fragment corresponds to amino acid 456 to 544 of the βc (GST-BCD). These GST fusion proteins were expressed in Escherichia coli, and were affinity-purified on glutathione-Sepharose (Pharmacia Biotech).
JAK1 cDNA (pBSK-JAK1) was kindly provided by J. Ihle (St Jude Childrens Research Hospital, Memphis, TN). The kinase-negative JAK1 that lacks the C-terminus kinase domain was isolated from the Sal I andBglII sites. A Not I linker was attached theBglII site of the fragment and inserted into the Xho I and Not I sites of pME18S. The expression plasmid for a kinase-negative JAK2 (pME18S ΔJAK2)25 was a kind gift from S. Watanabe (Institute of Medical Science, University of Tokyo, Tokyo, Japan).
Immunoprecipitation and immunoblotting.
Cells were stimulated with 300 ng/mL hIL-5 or GM-CSF for 3 minutes; following stimulation, they were lysed on ice in lysis buffer (1% Brij 97 or 1% Triton X-100, 10% glycerol, 150 mmol/L NaF, 1 mmol/L sodium orthovanadate, 100 U/mL aprotinin, 10 mmol/L iodoacetamide, and 25 μg/mLp-nitrophenyl-p′-guanidinobenzoate). For immunoprecipitation, cell lysates (equivalent to 1 × 107 cells) were precleared with Protein G–Sepharose for 2 hours at 4°C and further incubated with various Abs for another 2 hours at 4°C. The immune complexes were precipitated by adding Protein G–Sepharose (Pharmacia Biotech), except in the case of the anti–hIL-5Rα MoAb KM1266, which was covalently bound to Sepharose beads (Pharmacia Biotech) before use. The immune complexes were washed with lysis buffer and subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis ([SDS-PAGE] 7.5% acrylamide gel). The proteins were transferred to Immobilon membranes (Millipore, Bedford, MA), and after blocking with Tris-buffered saline containing 5% bovine serum albumin (Fraction V; Sigma, St Louis, MO), the membranes were probed with anti-JAK2, anti-JAK1, anti–hIL-5Rα, anti-βc, or antiphosphotyrosine Ab (4G10) and visualized by the ECL system (Amersham, Buckinghamshire, UK).
Binding assay for GST fusion proteins with JAK.
The interaction of GST fusion proteins to JAK2 was examined as follows. Equal amounts of GST fusion proteins were incubated with lysates of unstimulated or hIL-5–stimulated TF-h5Rα cells, which were lysed with 0.5% Triton X-100 containing protease inhibitors, for 4 hours at 4°C and washed (0.1% Triton X-100). After adding sample buffer, the precipitates bound to GST fusion proteins were eluted by boiling and examined by immunoblotting with the Ab against JAK2 or JAK1.
Expression of hIL-5R subunits, kinase-negative JAK1, and kinase-negative JAK2.
The expression plasmids for hIL-5Rα (5 μg) and βc (5 μg) with or without kinase-negative JAK1 (15 μg) or kinase-negative JAK2 (15 μg) were transiently transfected into COS7 cells. COS7 cells were suspended in 800 μL cytomix29 (120 mmol/L KCl, 0.15 mmol/L CaCl2, 10 mmol/L K2HPO4/KH2PO4, 25 mmol/L HEPES, 2 mmol/L EGTA, and 5 mmol/L MgCl2, pH 7.6) and mixed with each plasmid. Electroporation was performed using a Gene Pulser (Bio-Rad, Hercules, CA) set at 960 μF and 300 V. After incubation for 36 hours, each group of cells was recovered for analysis.
RESULTS
IL-5–induced interaction between hIL-5Rα and βc.
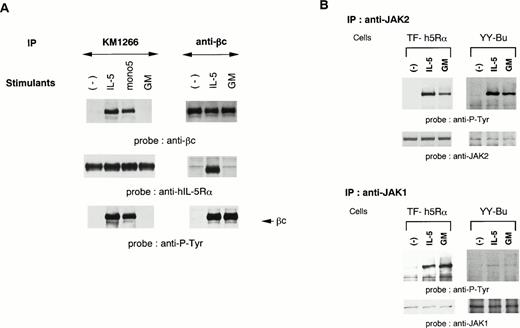
We established two different hIL-5–responsive cell lines, TF-h5Rα and YY-Bu. As we previously reported,22 both TF-h5Rα and YY-Bu respond to hIL-5 and mono5 by proliferation and adhesion to fibronectin, respectively, in a dose-dependent manner to an extent similar to that obtained with GM-CSF stimulation. We also generated an MoAb against hIL-5Rα that could immunoprecipitate hIL-5Rα in the presence of hIL-5. The anti–hIL-5Rα MoAb KM1266 was able to coimmunoprecipitate βc together with hIL-5Rα when lysates were obtained from hIL-5– and mono5-stimulated TF-h5Rα cells, but not with GM-CSF (Fig 1A). Essentially identical results were obtained using anti-βc MoAb. The anti-βc MoAb was able to coimmunoprecipitate hIL-5Rα together with βc only when the cells were stimulated with IL-5 (Fig 1A). Sequential immunoblot analysis showed that the βc coimmunoprecipitated with hIL-5Rα by anti–hIL-5Rα MoAb was tyrosine-phosphorylated. In contrast, the βc immunoprecipitated from unstimulated TF-h5Rα cells by anti-βc MoAb was not tyrosine-phosphorylated. Tyrosine phosphorylation of βc was also observed in extracts of GM-CSF–stimulated TF-h5Rα cells; however, it was not associated with hIL-5Rα (Fig 1A). These observations indicate that a functional hIL-5Rα/βc complex could be formed only in response to IL-5 stimulation.
A functional hIL-5R complex is formed only after IL-5 stimulation, followed by JAK2 and JAK1 activation. (A) Unstimulated, IL-5–stimulated, mono5-stimulated, or GM-CSF–stimulated TF-h5Rα cells were lysed and immunoprecipitated with KM1266 (anti–hIL-5Rα MoAb) or anti-βc MoAb. The immunoprecipitated proteins were analyzed on SDS-PAGE and transferred to Immobilon membranes. The membranes were probed with anti-βc polyclonal Ab or KM1074 (anti–hIL-5Rα MoAb), respectively. The same membranes were reprobed with anti–hIL-5Rα or KM1074. Another immunoprecipitation analysis was made by immunoblotting with antiphosphotyrosine MoAb. (B) Unstimulated, IL-5–stimulated, or GM-CSF–stimulated TF-h5Rα cells were lysed, and immunoprecipitation was performed with anti-JAK1 or -JAK2 Ab. The immunoprecipitates were analyzed on SDS-PAGE and transferred to Immobilon membranes. The membranes were probed with antiphosphotyrosine MoAb 4G10 and reprobed with anti-JAK2 or -JAK1 Ab.
A functional hIL-5R complex is formed only after IL-5 stimulation, followed by JAK2 and JAK1 activation. (A) Unstimulated, IL-5–stimulated, mono5-stimulated, or GM-CSF–stimulated TF-h5Rα cells were lysed and immunoprecipitated with KM1266 (anti–hIL-5Rα MoAb) or anti-βc MoAb. The immunoprecipitated proteins were analyzed on SDS-PAGE and transferred to Immobilon membranes. The membranes were probed with anti-βc polyclonal Ab or KM1074 (anti–hIL-5Rα MoAb), respectively. The same membranes were reprobed with anti–hIL-5Rα or KM1074. Another immunoprecipitation analysis was made by immunoblotting with antiphosphotyrosine MoAb. (B) Unstimulated, IL-5–stimulated, or GM-CSF–stimulated TF-h5Rα cells were lysed, and immunoprecipitation was performed with anti-JAK1 or -JAK2 Ab. The immunoprecipitates were analyzed on SDS-PAGE and transferred to Immobilon membranes. The membranes were probed with antiphosphotyrosine MoAb 4G10 and reprobed with anti-JAK2 or -JAK1 Ab.
IL-5–induced activation of JAKs and STATs.
We have reported that IL-5 induces tyrosine phosphorylation of JAK2, and to a lesser extent JAK1, in a murine B-cell line.24 To examine the involvement of JAK1 and JAK2 in the signal transduction of hIL-5, cell lysates of TF-h5Rα were immunoprecipitated with anti-JAK1 and anti-JAK2 antiserum. Each precipitate was then subjected to immunoblot analysis with antiphosphotyrosine MoAb. JAK2 was tyrosine-phosphorylated upon hIL-5 and GM-CSF stimulation. Moreover, a certain degree of JAK1 tyrosine phosphorylation was also observed. In both cases, tyrosine phosphorylation was observed within 2 minutes of hIL-5 stimulation. The same results were obtained when cell lysates of hIL-5– or GM-CSF–stimulated YY-Bu were used (Fig 1B).
As we have previously demonstrated,22 STAT5 was found to be activated upon hIL-5 stimulation in both TF-h5Rα and YY-Bu. It has also been shown by our group and others20-22 that in human eosinophils STAT1 and STAT5 are activated after hIL-5 stimulation. We infer from these results that not only JAK2 but also JAK1 are tyrosine-phosphorylated in TF-h5Rα and YY-Bu cells in response to hIL-5 followed by STAT5 activation.
Association of JAK2 and JAK1 with hIL-5Rα and βc, respectively.
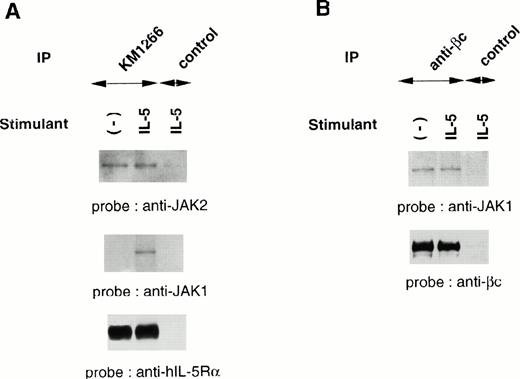
Although IL-5 induces tyrosine phosphorylation of cellular proteins including βc, JAK1, JAK2, and STAT5, both IL-5Rα and βc subunits do not contain an intrinsic kinase domain, suggesting the association of cellular tyrosine kinases. We evaluated the physical association of JAK1 and JAK2 with each component of the hIL-5R complex. Cellular extracts of TF-h5Rα were immunoprecipitated with anti–hIL-5Rα MoAb KM1266 followed by immunoblotting with anti-JAK2 or anti-JAK1 Ab. JAK2 was coimmunoprecipitated with hIL-5Rα regardless of IL-5 stimulation. Human IL-5 stimulation did not induce a significant increase of JAK2 protein coimmunoprecipitation with hIL-5Rα. On the other hand, JAK1 was coimmunoprecipitated with hIL-5Rα only when the cells were stimulated with hIL-5. In the absence of hIL-5 stimulation, no coimmunoprecipitation of JAK1 by anti–hIL-5Rα MoAb was observed (Fig2A).
JAK2 and JAK1 constitutively associate with hIL-5Rα and βc, respectively. (A) Unstimulated or IL-5–stimulated TF-h5Rα cells were lysed and immunoprecipitated with anti–hIL-5Rα, KM1266, or control Ab. The immunoprecipitated proteins were analyzed on SDS-PAGE and transferred to Immobilon membranes. The membranes were probed with anti-JAK2 or anti-JAK1 Ab and reprobed with anti–hIL-5Rα MoAb KM1074. (B) Cell lysates from unstimulated or IL-5–stimulated TF-h5Rα cells were immunoprecipitated with anti-βc MoAb or control Ab and separated on SDS-PAGE. The membrane was probed with anti-JAK1 Ab and reprobed with anti-βc polyclonal Ab.
JAK2 and JAK1 constitutively associate with hIL-5Rα and βc, respectively. (A) Unstimulated or IL-5–stimulated TF-h5Rα cells were lysed and immunoprecipitated with anti–hIL-5Rα, KM1266, or control Ab. The immunoprecipitated proteins were analyzed on SDS-PAGE and transferred to Immobilon membranes. The membranes were probed with anti-JAK2 or anti-JAK1 Ab and reprobed with anti–hIL-5Rα MoAb KM1074. (B) Cell lysates from unstimulated or IL-5–stimulated TF-h5Rα cells were immunoprecipitated with anti-βc MoAb or control Ab and separated on SDS-PAGE. The membrane was probed with anti-JAK1 Ab and reprobed with anti-βc polyclonal Ab.
Next, immunoprecipitation of cellular extracts from TF-h5Rα cells with anti-βc MoAb followed by immunoblotting with anti-JAK1 Ab was performed. It was found that JAK1 coimmunoprecipitated with βc regardless of hIL-5 stimulation (Fig 2B). Taken together, we infer from these results that JAK2 and JAK1 are constitutively associated with hIL-5Rα and βc, respectively.
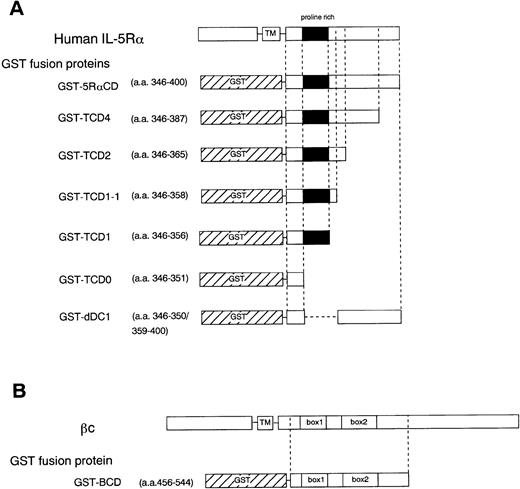
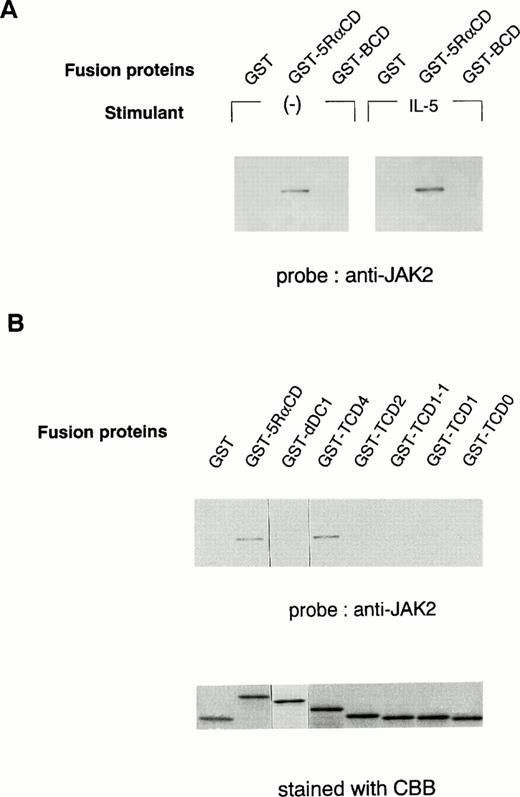
To further determine the role of the cytoplasmic domain of hIL-5Rα in JAK2 association, we generated several GST fusion proteins containing the entire cytoplasmic domain (GST-5RαCD), a deletion mutant of the proline-rich residues of the cytoplasmic domain (GST-dDC1), and several C-terminal truncated cytoplasmic domain mutants of hIL-5Rα (GST-TCD0 to GST-TCD4). Also, a GST fusion protein corresponding to the cytoplasmic region of βc (GST-BCD) was prepared (Fig 3A and B). The molecular weight of each fusion protein was determined by SDS-PAGE analysis, and each was found to have the expected molecular weight (Fig 4B). Using these GST fusion proteins, we performed binding assays for the association of JAK2 to GST fusion proteins. When unstimulated or hIL-5–stimulated cell lysates of TF-h5Rα were incubated with GST alone, no JAK2 protein was detected by immunoblotting. GST fusion proteins containing the entire cytoplasmic domain of hIL-5Rα (GST-5RαCD) bound JAK2 regardless of hIL-5 stimulation (Fig 4A). However, the GST fusion protein with the cytoplasmic region of the βc (GST-BCD), which contained the box1 and box2 motifs, was unable to bind JAK2 (Fig4A), confirming the result that JAK2 does not associate with βc (Fig2A). Furthermore, the GST fusion protein GST-dDC1, which has an eight–amino acid deletion mutation in the proline-rich region (DC1 region)13 of the cytoplasmic domain of hIL-5Rα, was unable to bind JAK2 (Fig 4B). This finding is in agreement with our previous observation, which confirms the role of this region in the activation of JAK2 in response to hIL-5.
Schematic diagram showing various cytoplasmic hIL-5Rα fusion proteins (A) and the cytoplasmic βc fusion protein (B). TM, transmembrane region.
Schematic diagram showing various cytoplasmic hIL-5Rα fusion proteins (A) and the cytoplasmic βc fusion protein (B). TM, transmembrane region.
In vitro binding of the hIL-5Rα cytoplasmic domain to JAK2. (A) GST alone, hIL-5Rα cytoplasmic domain fusion protein (GST-5RαCD), or βc cytoplasmic domain fusion protein (GST-BCD) were incubated with the cell lysates of unstimulated (−) or IL-5–stimulated TF-h5Rα cells, and the precipitated proteins were analyzed on SDS-PAGE and probed with anti-JAK2 Ab. (B) The deletion mutant (dDC1) and C-terminal truncated (TCD4, TCD2, TCD1-1, TCD1, and TCD0) GST fusion proteins were incubated with the lysates of unstimulated TF-h5Rα, separated on SDS-PAGE, and probed with anti-JAK2 Ab. The same amount of fusion proteins were separated on SDS-PAGE and stained with Coomassie brilliant blue (CBB).
In vitro binding of the hIL-5Rα cytoplasmic domain to JAK2. (A) GST alone, hIL-5Rα cytoplasmic domain fusion protein (GST-5RαCD), or βc cytoplasmic domain fusion protein (GST-BCD) were incubated with the cell lysates of unstimulated (−) or IL-5–stimulated TF-h5Rα cells, and the precipitated proteins were analyzed on SDS-PAGE and probed with anti-JAK2 Ab. (B) The deletion mutant (dDC1) and C-terminal truncated (TCD4, TCD2, TCD1-1, TCD1, and TCD0) GST fusion proteins were incubated with the lysates of unstimulated TF-h5Rα, separated on SDS-PAGE, and probed with anti-JAK2 Ab. The same amount of fusion proteins were separated on SDS-PAGE and stained with Coomassie brilliant blue (CBB).
Next, we examined whether the DC1 region containing the proline-rich residues, similar to the box1 region, of the cytoplasmic domain of hIL-5Rα alone was sufficient to associate with JAK2. For this purpose, we used a panel of fusion proteins corresponding to the C-terminal cytoplasmic truncated region of hIL-5Rα. GST-TCD4 was able to bind JAK2 significantly, but neither GST-TCD2, -TCD1-1, -TCD1, nor TCD0 bound to JAK2 (Fig 4B). These results indicate that the downstream region of the proline-rich motif is also indispensable for JAK2 binding to hIL-5α.
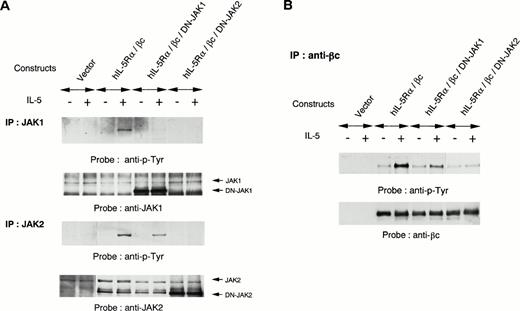
Dominant-negative JAK2 represses tyrosine phosphorylation of endogenous JAK2 and JAK1.
To further evaluate the role of JAK1 and JAK2 in IL-5 signaling, we introduced kinase-negative forms of JAK1 or JAK2 together with expression constructs of hIL-5Rα and βc into COS7 cells. The COS7 transfectants expressing both hIL-5Rα and βc responded to hIL-5 for tyrosine phosphorylation of endogenous JAK2 and JAK1. Overexpression of a kinase-negative form of JAK2 inhibited hIL-5–induced activation of endogenous JAK2, in agreement with a previous report.25Overexpression of a kinase-negative form of JAK1 could also suppress hIL-5–induced activation of endogenous JAK1 (Fig5A). These observations indicate that kinase-negative forms of both JAK1 and JAK2 function as dominant-negative JAK1 (DN-JAK1) and JAK2 (DN-JAK2), respectively, in hIL-5 signaling. Interestingly, overexpression of DN-JAK2 together with hIL-5Rα and βc into COS7 cells also completely inhibited hIL-5–induced JAK1 activation. In contrast, JAK2 activation was conserved regardless of DN-JAK1 introduction into COS7 cells (Fig 5A). The hIL-5–mediated tyrosine phosphorylation of βc was suppressed following transfection with DN-JAK2 (Fig 5B). These results indicate that JAK2 activation alone is sufficient to induce tyrosine phosphorylation of βc in response to hIL-5 and JAK1 activation is not essential.
Effects of DN-JAK1 and in DN-JAK2 on IL-5 signal. (A) hIL-5Rα and βc cDNA were transfected into COS7 cells together with either a vector control, a kinase-negative form of JAK1, or a kinase-negative form of JAK2. After incubation for 36 hours, cells were harvested and lysed after IL-5 stimulation. Immunoprecipitation was performed with anti-JAK1 or -JAK2 Ab. After separation on SDS-PAGE, the transferred membranes were immunoblotted with antiphosphotyrosine MoAb and reprobed with the relevant Ab. (B) Immunoprecipitation with anti-βc MoAb was also performed. After separation on SDS-PAGE, the proteins were probed with antiphosphotyrosine MoAb and reprobed with anti-βc polyclonal Ab.
Effects of DN-JAK1 and in DN-JAK2 on IL-5 signal. (A) hIL-5Rα and βc cDNA were transfected into COS7 cells together with either a vector control, a kinase-negative form of JAK1, or a kinase-negative form of JAK2. After incubation for 36 hours, cells were harvested and lysed after IL-5 stimulation. Immunoprecipitation was performed with anti-JAK1 or -JAK2 Ab. After separation on SDS-PAGE, the transferred membranes were immunoblotted with antiphosphotyrosine MoAb and reprobed with the relevant Ab. (B) Immunoprecipitation with anti-βc MoAb was also performed. After separation on SDS-PAGE, the proteins were probed with antiphosphotyrosine MoAb and reprobed with anti-βc polyclonal Ab.
DISCUSSION
In this study, we report the following three major findings. First, we demonstrated that each component of the hIL-5R complex, hIL-5Rα and βc, exists independently in the absence of IL-5, and βc can be recruited to hIL-5Rα upon hIL-5 stimulation. Second, in unstimulated conditions, JAK2 and JAK1 are constitutively associated with hIL-5Rα and βc, respectively. The cytoplasmic proline-rich region of hIL-5Rα is necessary for JAK2 binding. Third, hIL-5 stimulation induces activation of both JAK1 and JAK2, resulting in tyrosine phosphorylation of cellular proteins including βc and STAT5. Tyrosine phosphorylation of JAK1 depends on the activation of JAK2. These results were obtained by successful generation of MoAbs against hIL-5Rα and by detailed study of the C-terminal truncated cytoplasmic domain of hIL-5Rα using GST fusion proteins.
The hIL-5R complex is composed of two subunits, the ligand-specific hIL-5Rα and βc. Although it has been speculated that a heterodimerization of IL-5R subunits induces the IL-5 signal, the nature of hIL-5Rα and βc heterodimerization has yet to be shown biochemically. It has been suggested that a functional hIL-5R complex is not preformed tightly, based on the finding that GM-CSF and IL-3 can cross-compete with IL-5 for binding and biologic activity.30 We were able to demonstrate that hIL-5Rα and βc were not associated in the absence of IL-5, and that the βc would associate with hIL-5Rα only after IL-5 stimulation (Fig 1A), consistent with a recent report on IL-3R.11,12 The monomeric hIL-5, mono5,26 which is biologically active like the intact dimeric hIL-5, also triggered the interaction of βc with hIL-5Rα, similar to native dimeric hIL-5 (Fig 1A).
hIL-3–induced heterodimerization of an IL-3Rα and βc has been shown to occur by covalent and noncovalent means. It has also been shown that receptor activation such as the tyrosine phosphorylation of βc is associated with the disulfide-linked receptor complex that contains both hIL-3Rα and βc.11,12 We examined the nature of hIL-5–induced heterodimerization of IL-5Rα and βc under nonreducing conditions and found, in addition to a tyrosine-phosphorylated monomeric βc, a 220-kD molecule that was tyrosine-phosphorylated in the presence of hIL-5 to coimmunoprecipitate with anti–hIL-5Rα MoAb (data not shown). However, we were unable to identify the band as a hIL-5Rα and βc heterodimer with our immunoblot analysis. Most of the tyrosine-phosphorylated IL-5R complexes were at least βc-bound to hIL-5Rα noncovalently (data not shown). Moreover, in contrast to the human GM-CSFR where a preformed homodimeric βc forms the functional complex with GM-CSFRα in GM-CSF signaling,10 we have no evidence that a functional hIL-5R complex uses a preformed homodimeric βc. The basis for these differences is not yet known. Further analysis such as crystallization of the IL-5/IL-5Rα/βc, IL-3/IL-3Rα/βc, and GM-CSF/GM-CSFRα/βc complexes will aid in understanding the stoichiometry of these receptor complexes.
The physical interaction between many cytokine receptors and JAKs have been reported in the literature.31,32 In this study, we were able to demonstrate that JAK2 and JAK1 associate with the hIL-5Rα and βc, respectively. Previously, we have been able to detect activation of JAK1, but not JAK2, upon mIL-5 stimulation of FDC-P1 transfectants expressing both the intact βc and the ααβ chimera, which is composed of the extracellular and membrane domains of mIL-5Rα and the cytoplasmic domain of βc.24 We inferred from this observation that homodimerization of the βc cytoplasmic domain by the chimera receptor activates JAK1 but not JAK2, resulting in transduction of the mIL-5 signal. The present finding is consistent with this notion. Furthermore, it was recently reported that JAK2 physically associates with βc only after ligand stimulation in a βc-sharing receptor complex in mammalian cells.33-35Although it was not determined in those reports whether a ligand-specific α subunit is coimmunoprecipitated with the βc, it is possible to speculate that the JAK2 coimmunoprecipitated along with βc may be derived from JAK2 bound to the ligand-specific α subunit.
However, these observations are inconsistent with a previous report that JAK2 constitutively associated with the βc subunit but not the GM-CSFRα subunit when they were expressed in insect cells using the baculovirus expression vector.36 We do not have a good explanation as to why we obtained results different from theirs. The very high concentrations achieved in the insect cells may be involved in changing the affinity of JAK2. What is demonstrated in this study is that JAK2 associates with hIL-5Rα in hIL-5–dependent cell lines (in vivo), and JAK2 associates with the fusion protein containing the cytoplasmic region of hIL-5Rα (in vitro). Furthermore, the region of hIL-5Rα necessary for JAK2 binding was localized to the domain of amino acid residues 346 to 387 of the hIL-5Rα cytoplasmic region. It was also revealed that the region containing particularly the proline-rich residues is indispensable for JAK2 association. We diligently performed point mutation analyses to determine which proline residue(s) would be important for JAK2 binding. Unfortunately, we did not obtain clear-cut and reproducible results, probably because of technical problems. We believe that the point mutation analysis for an in vitro binding assay using fusion protein may be accomplished by a technique with improved protein chemistry. This would be the next issue to be solved.
Finally, we examined the role of JAKs in hIL-5 signaling using the dominant-negative variants DN-JAK1 and DN-JAK2. Interestingly, JAK1 activation was not observed in the absence of JAK2 activation, whereas JAK2 activation was induced upon hIL-5 stimulation irrespective of JAK1 activation (Fig 5A). As in the case of GM-CSFR,25 DN-JAK2 inhibited hIL-5–induced tyrosine phosphorylation of βc. On the other hand, tyrosine phosphorylation of βc could be achieved in the absence of JAK1. Thus, JAK1 activation seems dispensable for hIL-5R activation, similar to a recent report on hIL-2R–mediated signaling37 38 whereby JAK1 but not JAK3 was dispensable for hIL-2R function.
It should be noted that the tyrosine phosphorylation of JAK2 was reduced somewhat by induction of DN-JAK1, which was obtained by three independent experiments. It has also been observed that overexpression of wild-type JAK1 together with βc in COS7 cells induces tyrosine phosphorylation of cotransfected βc, which was also induced by overexpression of wild-type JAK2 (data not shown). These results suggest that JAK1 enhances hIL-5–induced signaling events via further activation of JAK2 and tyrosine phosphorylation of βc.
It has been proposed that the stoichiometry of the hIL-5Rα, βc, and IL-5 complex is 1:1:1.39-41 Based on our present observations, we speculate that JAK2 associated with hIL-5Rα interacts with JAK1 of the βc subunit by heterodimerization and transduces the signal of hIL-5. However, JAK2 activation is observed in the absence of JAK1 activation. These results suggest the possible existence of a non-JAK1 necessary for JAK2 activation. Lyn has been shown to be constitutively associated with βc and activated by hIL-5.42,43 Therefore, it will be interesting to examine the possible involvement of Lyn in the activation of JAKs. We also cannot rule out the possibility that JAK2 binds to βc in a fashion undetected by our experiments, which may result in the cross-phosphorylation of JAK2 itself. Since a recent report has proposed that a βc-sharing receptor complex may be composed of a ligand:α subunit:βc subunit stoichiometry of 2:2:2 configuration,12 it is also possible that the interaction of JAK2 bound to IL-5Rα is activated by cross-phosphorylation.
In conclusion, hIL-5Rα provides not only a ligand-specific binding site but also site(s) for a signaling kinase molecule such as JAK2. Furthermore, JAK2 bound to hIL-5Rα regulates the initiation of IL-5 signaling. Furthermore, JAK1 bound to βc, although dispensable, is involved in IL-5R–mediated signal transduction. These observations provide new clues for understanding the molecular mechanisms of IL-5 signal transduction in human eosinophils.
ACKNOWLEDGMENT
We thank Drs M. Tomonaga and R. Dickason for providing YY-1 cells and mono5, respectively. We also acknowledge Drs Y. Kikuchi, K. Ishizaka, R. Dickason, and E. Barsoumian for suggestions throughout the study and a critical review of the manuscript.
Supported in part by a Special Grant for Advanced Research on Immunology and a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, Sports, and Culture of Japan; a grant from the Japan Research Foundation of Clinical Pharmacology; and research funds from Bayer Pharmaceutical Co and Ono Pharmaceutical Co.
Address reprint requests to Kiyoshi Takatsu, PhD, Department of Immunology, Institute of Medical Science, University of Tokyo, 4-6-1 Shirokanedai, Minato-ku, Tokyo 108, Japan.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal