Abstract
Ultrasound reversibly alters the structure of polymerized fibrin, an effect that could influence tissue-plasminogen activator (t-PA) binding. We have, therefore, characterized the effects of ultrasound on binding of t-PA to fibrin using a novel system in which radiolabeled, active-site blocked, single chain tissue-plasminogen activator flowed through a fibrin gel at constant rate, and specific binding was determined by monitoring incorporation of radiolabel. Results using polymerized fibrin were compared with those using a surface of fibrin immobilized on Sepharose beads in a similar system. Interaction of t-PA with surface-immobilized fibrin involved two classes of binding sites (Kd = 31 nmol/L and 244 nmol/L) and a maximum binding ratio of 3.8 mol t-PA/mol fibrin. Ultrasound increased Kd for the high affinity site to 46 nmol/L (P < .0001), but it had no significant effects on the Kd 244 nmol/L site nor on Bmax. Tissue-plasminogen activator binding to noncrosslinked fibrin involved two sites with Kds of 267 nmol/L and 952 nmol/L, while a single Kd 405 nmol/L site was identified for crosslinked fibrin. Ultrasound had no significant effect on the binding affinity for noncrosslinked fibrin, but Bmaxwas increased in the presence of ultrasound, from 31 μmol/L to 43 μmol/L (P < .0001). Ultrasound decreased the Kd for crosslinked fibrin to 343 nmol/L (P = .026) and also increased Bmax from 22 μmol/L to 25 μmol/L (P = .015). Ultrasound also affected the kinetics of t-PA binding to fibrin, significantly accelerating the rate of dissociation by 77% ± 5% for noncrosslinked fibrin and by 69% ± 3% for crosslinked fibrin (P < .001 for each). These results indicate that ultrasound exposure accelerates t-PA binding, alters binding affinity, and increases maximum binding to polymerized fibrin, effects that may result from ultrasound-induced changes in fibrin structure.
FIBRINOLYSIS IS CAREFULLY regulated by efficiently generating the serine protease plasmin at sites of fibrin formation, and limiting its systemic activation. Several mechanisms contribute to the localized activation of fibrinolysis including the specific binding of reactants to fibrin, including t-PA, the enzyme which converts plasminogen to plasmin.1-6 In addition to binding t-PA, fibrin also facilitates the t-PA–mediated conversion of plasminogen to plasmin.1,7,8 Specificity for binding to fibrin rather than to its circulating precursor, fibrinogen, depends on unique structural features that arise from the conversion of fibrinogen to fibrin. The structure of fibrin may, however, also limit binding because it is polymeric, composed of two-stranded protofibrils aggregated laterally to form larger fibers varying in width.9,10 Previous studies examining binding of t-PA to fibrin have used several approaches to overcome the problem of binding to such a polymeric solid. These include binding of t-PA to fibrin immobilized on a plastic surface,5,6 and also mixing t-PA and fibrinogen before clotting.1-3 In another approach, access of t-PA to binding sites on polymerized fibrin was facilitated by sonicating the fibrin to form a suspension.4 The latter most closely approximates important pathologic conditions in which t-PA is administered in pharmacologic doses into the blood outside of a clot as treatment for obstructive thrombosis. The particles in the sonicate were, however, still large and could restrict access of enzyme, possibly explaining the failure to achieve saturation binding in that study.4
Fibrinolysis is accelerated in the presence of high frequency, low intensity ultrasound (US) both in vitro11-17 and in vivo.17-21 The enhanced fibrinolysis results from accelerated enzymatic degradation rather than from mechanical disruption of fibrin. The effects are non-thermal and may be mediated in part by increasing transport of reactants into the fibrin matrix. Thus, US increases clot uptake of t-PA in the absence of flow,22 and it also increases pressure-mediated flow through fibrin.23 The latter finding suggests that US alters fibrin structure, which is the primary determinant of flow resistance, and scanning electron microscopy has documented reversible alteration in fiber density and diameter caused by US.24
Because changes in fibrin structure could affect t-PA binding, we have investigated the effect of US on interactions of t-PA with fibrin, using a permeation system to maximize access of t-PA to binding sites with the gel matrix. Since permeation plays an important role in transport of fibrinolytic reactants into thrombi in vivo, this system approximates t-PA delivery during pharmacologic administration. Binding of t-PA to unpolymerized fibrin used a similar system with fibrin immobilized on Sepharose beads. Using this approach, we have characterized the binding of t-PA to non-crosslinked fibrin, crosslinked fibrin and non-polymerized fibrin, and examined the effects of US on binding parameters.
MATERIALS AND METHODS
Tissue plasminogen activator.
Recombinant t-PA (Activase; Genentech, South San Francisco, CA) was inactivated by incubation with a five-fold molar excess of D-Phe-Pro-Arg-chloromethylkeytone (PPACK) (Bachem, Torrence, CA), and excess PPACK was removed by gel filtration chromatography on Sephadex G-10 (Bio-Rad Laboratories, Hercules, CA). The inactivated t-PA showed no enzymatic activity when incubated with the chromogenic substrate H-D-Ile-L-Pro-L-Arg-P-nitroanalid (S-2288) (Kabi Vitrum, Stockholm, Sweden). Radio-iodination of inactivated t-PA was performed using the iodogen method,25 and unbound 125I was removed by Sephadex G-10 chromatography. The radiolabeled, inactivated t-PA was affinity purified using a column of fibrin celite as described previously4 and modified with one additional step of washing the fibrin-bound celite column with 0.5 mol/L arginine hydrochloride before t-PA application to remove unbound proteins. The fibrin-binding form of inactive t-PA that eluted with 0.5 mol/L arginine hydrochloride comprised approximately 90% of the total inactivated, labeled t-PA, and this was used for binding experiments. The t-PA had a specific activity of 4.1 × 107 cpm/mg, and its mobility on sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) was identical to active, unlabeled t-PA.
Preparation of polymerized fibrin.
Fibrin gels were prepared in segments of plastic transfer pipettes with a 4 mm internal diameter (Samco, Marsh Biomedical Products, Rochester, NY) using a modification of previously described methods.26 27 To increase adhesion of fibrin to the plastic, a 10 mm segment of the tube was scratched symmetrically using a 20 gauge needle and then lined with a thin layer of fibrin prepared by addition of human thrombin (Cal-Biochem, LaJolla, CA) at a concentration of 1 U/mL in 0.5 mL fibrinogen (Kabi Vitrum) at a concentration of 6 mg/mL in 0.018 mol/L sodium citrate, 0.132 mol/L sodium chloride, pH 7.4. Following clotting, the fibrin was expelled from the tube under high pressure leaving a thin coating of fibrin over the scratched segment.
Non-crosslinked fibrin clots for perfusion were formed within the 10 mm scratched and fibrin-lined segment. Fibrinogen (6.0 mg/mL) in 0.018 sodium citrate, pH 7.4 containing 0.057 mol/L sodium chloride and 100 kallikrein inhibitory U/mL aprotinin (Trasylol® FBA Pharmaceuticals, Westhaven, CT) was clotted by addition of human thrombin to a concentration of 0.5 U/mL, and 0.1 mL of the mixture was rapidly added to the scratched segment and incubated for one hour at 37°C before perfusion. Crosslinked fibrin clots were prepared in the same way with the addition of 20 mmol/L calcium chloride and 5 U/mL of factor XIII (Behring, Marburg, Germany). The polypeptide chain composition of non-crosslinked and crosslinked fibrin was characterized by SDS-PAGE, which confirmed cleavage of fibrinopeptides and demonstrated the absence of γ or α chain crosslinking in non-crosslinked fibrin and extensive crosslinking for crosslinked fibrin.
Surface-immobilized fibrin preparation.
Fibrin immobilized on Sepharose beads was also used for perfusion experiments. Purified IgG of monoclonal antibody J88B, reactive with an epitope within the sequence arg63-met78 of the γ chain of human fibrinogen28 was kindly provided by Dr P.J. Simpson-Haidaris, Rochester, NY and immobilized by incubating 1 mL of hydrated, activated CNBr Sepharose (Pharmacia Biotech, Piscataway, NJ) with 1 mL of IgG in 0.2 mol/L sodium bicarbonate buffer, pH 8.3 and gently mixing for 18 hours at 4°C. Residual unbound sites were blocked by incubation in 1 mol/L glycine, and the suspension was then washed twice with 50 mL each of 0.2 mol/L sodium bicarbonate buffer pH 8.3 containing 1 mol/L sodium chloride and then with 50 mL of 0.1 mol/L sodium acetate buffer pH 4.0 containing 0.5 mol/L sodium chloride. The suspension was then packed in a column and equilibrated with 0.05 mol/L tris buffer, pH 7.4 containing 0.13 mol/L sodium chloride and 1% bovine serum albumin. A 1 mL volume of fibrinogen (20 mg/mL) was perfused through the column, and it was then washed with 4 mL of the equilibrating buffer to remove any unbound fibrinogen. The eluate was collected and the protein concentration determined, indicating that 2.5 mg of fibrinogen was bound per 1 mL of beads. To convert fibrinogen to fibrin, 0.24 mL of suspension was incubated with 1 mL of 0.5 U/mL of human thrombin.
Binding measurements.
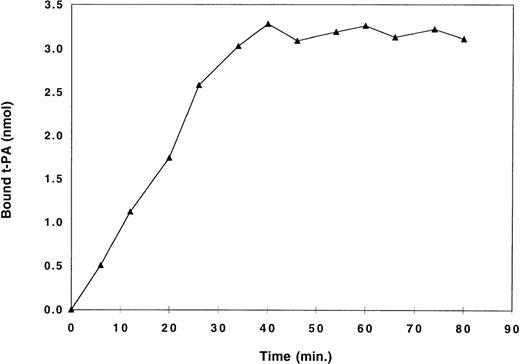
Radiolabeled, active-site blocked t-PA at selected concentrations from 1.8 to 1,000 μg/mL in 0.05 mol/L tris buffer, pH 7.4 containing 0.13 mol/L sodium chloride and 1% bovine serum albumin was perfused through fibrin gels or fibrin-bound Sepharose at a constant rate of 15 μL/min using a pump, and aliquots of 50 μL or 100 μL were collected to calculate binding. For each perfusion, equilibrium binding was considered to occur when there was no additional binding of t-PA to the fibrin column, and each column was used to derive a single equilibrium binding point (Fig 1). Perfusions at each t-PA concentration were done in triplicate, and nonspecific binding was determined using the same system with a 1,000-fold molar excess of unlabeled t-PA. Linear regression using Scatchard analysis was performed using the Ligand program.29 Non-linear regression analysis was also used, fitting data to an equation of the form B = B1F/K1F + B2F/K2 + F, where B the bound concentration and F free.
Binding of t-PA to non-crosslinked fibrin in a single perfusion. Radiolabeled, active-site blocked t-PA was perfused at a constant rate of 15 μL/min through a column of fibrin. The amount of bound t-PA was calculated by measuring radioactivity in aliquots of effluent from the column. Equilibrium was considered to occur when there was no additional binding of t-PA to the fibrin column as indicated by the flat portion of the curve. A single point representing mean equilibrium binding taken from that part of the curve was used for binding isotherms (Fig 3) and Scatchard analysis (Fig 4).
Binding of t-PA to non-crosslinked fibrin in a single perfusion. Radiolabeled, active-site blocked t-PA was perfused at a constant rate of 15 μL/min through a column of fibrin. The amount of bound t-PA was calculated by measuring radioactivity in aliquots of effluent from the column. Equilibrium was considered to occur when there was no additional binding of t-PA to the fibrin column as indicated by the flat portion of the curve. A single point representing mean equilibrium binding taken from that part of the curve was used for binding isotherms (Fig 3) and Scatchard analysis (Fig 4).
This provided a good estimate of Bmax from B1 + B2. It also determined Kd when a single binding site was identified and the higher affinity site if two binding sites were found. It was not accurate in calculating the Kd of the lower affinity site when two were identified because the relative affinities were not sufficiently different for resolution, and results are presented using both types of analysis. Rates of dissociation were determined for each perfusion by fitting the dissociation of radiolabeled t-PA after addition of a four-fold excess of unlabeled t-PA to f(x) = ae−bx using b as the apparent rate constant.
Ultrasound exposure.
All perfusions were performed in temperature-controlled water at 25°C in a tank fitted with a piezoelectric transducer as described.11 The US transducer had an area of 4 cm2 and operated at a frequency of 1 MHz. The US field at the site of exposure was adjusted to 2 W/cm2 for each experiment, and US was generated in cycles of 5 ms on and 5 ms off. For experiments with US exposure, a 3 cm rubber block was placed behind the perfusion apparatus as an acoustic absorber to minimize standing waves and a US blocking material was placed after the rubber to block insonification of control samples.
Statistics.
Equilibrium binding data in the presence and absence of US was compared. Linearlized data of bound versus bound/free from the Scatchard analysis was analyzed for each site by linear regression to derive the best fit line. The resulting lines for binding in the presence and absence of US were compared using analysis of covariance to determine if the slopes were significantly different. This provided a statistical comparison of Kd values for each site. Each regression analysis included an examination of residuals as a check on the required assumptions of normally distributed errors with constant variance. The significance of differences in Bmax in the presence and absence of US was determined from the nonlinear regression by comparing the estimated values using an approximate t test.
RESULTS
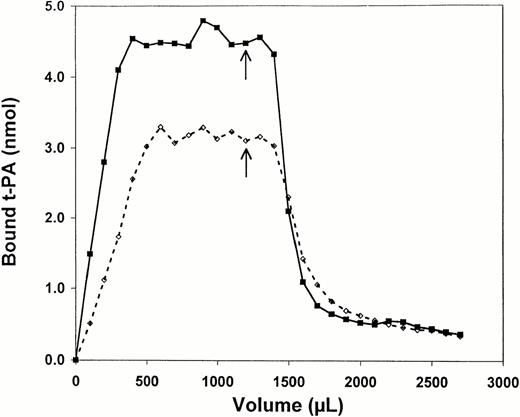
Perfusion of t-PA through fibrin led to a progressive increase in binding until a stable level was achieved and addition of a fourfold molar excess of unlabeled t-PA then resulted in displacement of bound, radiolabeled t-PA after a lag period representing time for perfusion through the fibrin gel as shown in Fig 2using non-crosslinked fibrin. The overall pattern was similar when the experiment was conducted in the presence of 1 MHz US at 2 W/cm2, but there were differences in both the amount and rate of binding. Binding increased more rapidly in the presence of US, and equilibrium was achieved faster. For example, in the experiment shown in Fig 2 total bound t-PA was approximately 4.5 x 10−9 mol in the presence of US, but only 3.0 × 10−9 mol in its absence. Dissociation of bound t-PA after addition of excess unlabeled ligand was also more rapid as indicated by the slope of the dissociation curve, and the baseline level was reached sooner in the presence of US despite an initial higher level of equilibrium binding. The level of baseline binding observed in the presence of excess unlabeled t-PA was the same with or without US.
Binding of t-PA to non-crosslinked fibrin during perfusion. Radiolabeled, active-site blocked t-PA was perfused at a constant rate of 15 μL/min through a column of non-crosslinked fibrin. Aliquots of 100 μL were collected to calculate binding. At the arrow, a 1,000-fold molar excess of unlabeled t-PA was added to the perfusate. Results are shown for binding without ultrasound (dotted line) and in the presence of 1 MHz ultrasound at 2 W/cm2and 50% duty cycle.
Binding of t-PA to non-crosslinked fibrin during perfusion. Radiolabeled, active-site blocked t-PA was perfused at a constant rate of 15 μL/min through a column of non-crosslinked fibrin. Aliquots of 100 μL were collected to calculate binding. At the arrow, a 1,000-fold molar excess of unlabeled t-PA was added to the perfusate. Results are shown for binding without ultrasound (dotted line) and in the presence of 1 MHz ultrasound at 2 W/cm2and 50% duty cycle.
These data were analyzed to compare rates of association and dissociation of t-PA and fibrin in the presence and absence of US. A total of 33 surface-immobilized fibrin, 32 non-crosslinked fibrin, and 28 crosslinked fibrin perfusion experiments with US and an equal number of perfusions without US were compared over a range of t-PA concentrations from 1.8 μg/mL to 1,000 μg/mL. These were used for quantitative analysis of both equilibrium binding and dissociation rates. The dissociation rate constant was calculated using time as a variable; however, the rates of association and dissociation are dependent on the flow rate in the system. The association of t-PA with fibrin was, therefore, limited in all experiments by the flow rates. Therefore, the rate of binding and dissociation was dependent on the flow rate and does not represent the true rate constant. Since flow was constant in all experiments, however, these apparent rate constants can be used for comparison of relative rates with and without US. Each dissociation was fit to a single exponential decay model of f(x) = ae−bx with b as the apparent rate constant. For all perfusions, the apparent rate constants without US were normalized to 100%, and the change in the rate of US-dependent dissociation was then determined. Surface-immobilized fibrin showed no significant change in binding kinetics in the presence of ultrasound. However, for both non-crosslinked and crosslinked polymerized fibrin US exposure resulted in significant increases in the rates of dissociation of 77% ± 5% and 69% ± 3%, respectively (P < .001). The increases in dissociation rates with US were independent of t-PA concentration over the range tested.
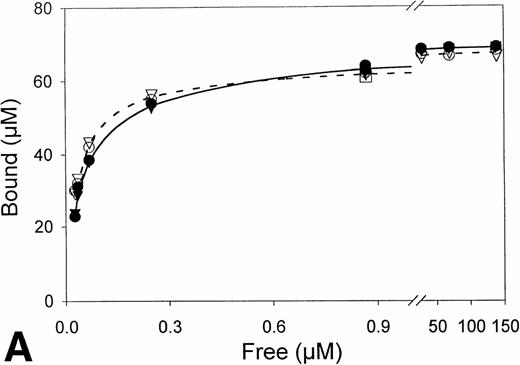
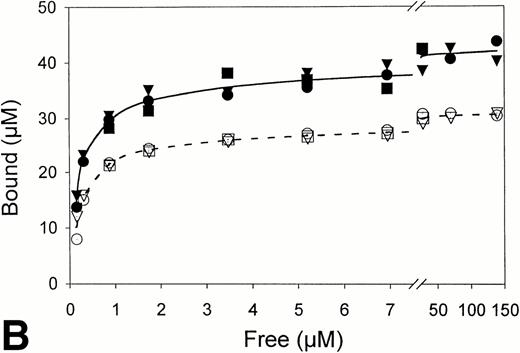
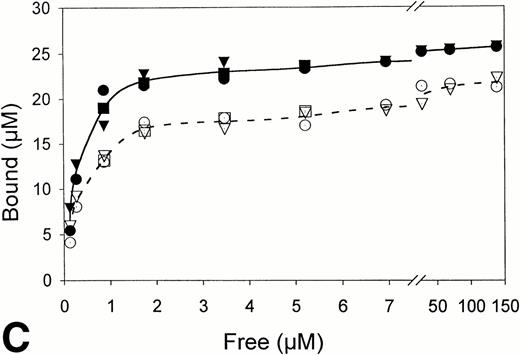
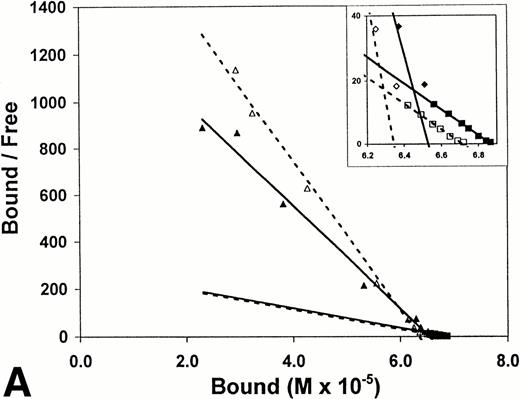
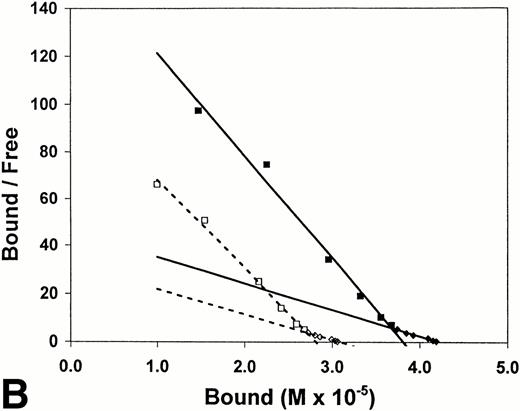
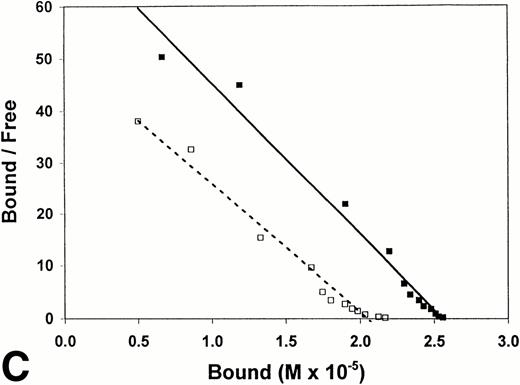
Equilibrium binding constants were also determined from analysis of the perfusion experiments, considering that the mean of the measured points on the flat portion of the binding curve represented equilibrium binding at a single concentration of t-PA (Fig 1). Experiments over a range of t-PA concentrations from 1.8 μg/mL to 1,000 μg/mL were then used to determine binding isotherms (Fig3). For fibrin immobilized on Sepharose beads, saturation was achieved at approximately 1 μmol/L t-PA with little difference in binding in the presence or absence of US (Fig 3A). Compared with fibrin monomer, saturation occurred at higher t-PA concentrations for both non-crosslinked fibrin (Fig 3B) and crosslinked fibrin (Fig 3C), and there was an increase in maximum binding for both in the presence of US. Binding of t-PA was modeled quantitatively using Scatchard analysis and nonlinear regression, comparing binding in the presence and absence of US. Binding to fibrin monomer (Fig4A) was described best with a two-site model with Kds of 31 nmol/L and 244 nmol/L (Table1). Ultrasound had a small effect on the binding of t-PA to fibrin monomer, increasing Kd from 31 nmol/L to 46 nmol/L for the high affinity site (P < .0001), which was also reflected in the change of slope in the reciprocal binding plot (Fig 4A), but US had no effect on the Kd 244 nmol/L site. Bmax was 67 μmol/L in the absence and 69 μmol/L in the presence of US (P = .09). Similar results were obtained when the data was analyzed using nonlinear regression with a high affinity Kd 31 nmol/L site identified, Bmax of 67 μmol/L, and a maximum molar binding ratio of 3.8 ± .03 mol t-PA/mol fibrin.
Fibrin binding isotherms in the presence and absence of ultrasound. Equilibrium binding to fibrin of t-PA at a single concentration was determined from individual perfusion experiments as in Fig 1. One perfusion experiment generated a single point for binding in either the absence (▵, ○, □; -----) or presence (▴, •, ▪; _____) of ultrasound. (A) Surface-immobilized fibrin. (B) Non-crosslinked fibrin. (C) Crosslinked fibrin.
Fibrin binding isotherms in the presence and absence of ultrasound. Equilibrium binding to fibrin of t-PA at a single concentration was determined from individual perfusion experiments as in Fig 1. One perfusion experiment generated a single point for binding in either the absence (▵, ○, □; -----) or presence (▴, •, ▪; _____) of ultrasound. (A) Surface-immobilized fibrin. (B) Non-crosslinked fibrin. (C) Crosslinked fibrin.
Scatchard analysis of t-PA binding to fibrin. Scatchard plots derived using data from binding isotherms (Fig 2). Binding in the absence (▵, ◊, □; ------) and presence (▴, ⧫, ▪; ______) of ultrasound is shown. (A) Surface-immobilized fibrin. (B) Non-crosslinked fibrin. (C) Crosslinked fibrin.
Scatchard analysis of t-PA binding to fibrin. Scatchard plots derived using data from binding isotherms (Fig 2). Binding in the absence (▵, ◊, □; ------) and presence (▴, ⧫, ▪; ______) of ultrasound is shown. (A) Surface-immobilized fibrin. (B) Non-crosslinked fibrin. (C) Crosslinked fibrin.
Binding Constants for the Association of t-PA With Fibrin in the Absence and Presence of Ultrasound
| . | Ultrasound . | Kd1-150 . | Kd2-150 . | Bmaxmax-151 . | Molar Binding Ratio-152 . |
|---|---|---|---|---|---|
| Fibrin monomer | No | 31 nmol/L (1) | 244 nmol/L (8) | 67 μmol/L (0.5) | 3.8 ± .03 |
| Yes | 46 nmol/L (2) | 243 nmol/L (8) | 69 μmol/L (0.6) | 3.8 ± .03 | |
| P < .0001 | P = NS | P = NS | |||
| Non-crosslinked fibrin | No | 267 nmol/L (17) | 952 nmol/L (67) | 31 μmol/L (0.6) | 1.77 ± .04 |
| Yes | 232 nmol/L (10) | 917 nmol/L (47) | 43 μmol/L (1) | 2.42 ± .06 | |
| P = NS | P = NS | P < .0001 | |||
| Crosslinked fibrin | No | 405 nmol/L (23) | — | 22 μmol/L (1) | 1.27 ± .06 |
| Yes | 343 nmol/L (14) | — | 25 μmol/L (0.4) | 1.44 ± .02 | |
| P = .026 | P < .015 |
| . | Ultrasound . | Kd1-150 . | Kd2-150 . | Bmaxmax-151 . | Molar Binding Ratio-152 . |
|---|---|---|---|---|---|
| Fibrin monomer | No | 31 nmol/L (1) | 244 nmol/L (8) | 67 μmol/L (0.5) | 3.8 ± .03 |
| Yes | 46 nmol/L (2) | 243 nmol/L (8) | 69 μmol/L (0.6) | 3.8 ± .03 | |
| P < .0001 | P = NS | P = NS | |||
| Non-crosslinked fibrin | No | 267 nmol/L (17) | 952 nmol/L (67) | 31 μmol/L (0.6) | 1.77 ± .04 |
| Yes | 232 nmol/L (10) | 917 nmol/L (47) | 43 μmol/L (1) | 2.42 ± .06 | |
| P = NS | P = NS | P < .0001 | |||
| Crosslinked fibrin | No | 405 nmol/L (23) | — | 22 μmol/L (1) | 1.27 ± .06 |
| Yes | 343 nmol/L (14) | — | 25 μmol/L (0.4) | 1.44 ± .02 | |
| P = .026 | P < .015 |
Numbers in parentheses are 1 SD.
Determined by Scatchard analysis.
Determined from nonlinear regression.
Expressed as moles of t-PA per mole of fibrin. Determined at maximum t-PA binding.
US had greater effects on t-PA binding to polymerized fibrin. Scatchard analysis of binding to noncrosslinked fibrin (Fig 4B) identified two sites with Kds of 267 nmol/L and 952 nmol/L. Using nonlinear regression, the Kd of the higher affinity site was similar, 239 nmol/L. Exposure to US caused no significant changes in affinity as reflected by the apparently parallel lines in the Scatchard plot (Fig 4B); and analysis of covariance confirmed the visual impression that there was no statistically significant difference in the slopes of lines characterizing the two binding sites in the presence and absence of US. There was, however, a significant change in maximum binding with US as reflected by both an increase in x-intercept for the lines describing both binding sites and by the apparent increase in concentration of bound t-PA at saturation in the binding isotherms (Fig 3B). With US exposure Bmax increased from 31 μmol/L to 43 μmol/L (P < .0001) corresponding to a 36% increase in the molar binding ratio from 1.77 ± .04 to 2.42 ± .06. t-PA bound to a single Kd 405 nmol/L site on crosslinked fibrin, as determined by Scatchard analysis or 350 nmol/L by nonlinear regression. There was no significant difference in affinity in the presence and absence of US as indicated by parallel Scatchard plots (Fig 4C) and by analysis of covariance. There was, however, a significant increase in maximum binding evident from binding isotherms (Fig 3C) and an increase in Bmax from 22 μmol/L to 25 μmol/L (P = .015) estimated from nonlinear regression and increase in maximum molar binding ratio from 1.27 ± .06 to 1.44 ± .02 mol t-PA/mol fibrin.
DISCUSSION
The insoluble, polymeric structure of fibrin complicates characterization of ligand binding because it may limit access to potential binding sites. Therefore, we chose to examine t-PA binding to fibrin using a constant flow permeation system that results in saturation (Figs 1 and 2) and also prevents depletion of free ligand. A further advantage is that it more closely approximates the interaction of t-PA with fibrin during pharmacologic administration when a large performed intravascular thrombus is exposed to circulating t-PA in the blood. Binding of t-PA to fibrin is important in regulating the rate of fibrinolysis, increasing the catalytic efficiency kcat/Km, for conversion of plasminogen to plasmin several hundred-fold in the presence of fibrin as compared with fibrinogen.1 8
Our findings indicate that binding of t-PA to surface-immobilized fibrin is best described by two binding sites with Kds of 31 nmol/L and 244 nmol/L. The monoclonal antibody used to immobilize fibrinogen reacts with a site not known to be involved in t-PA binding, and treatment with thrombin most likely resulted in conversion of fibrinogen to surface-immobilized fibrin monomer, but the absence of polymerization was not confirmed. Binding to polymerized noncrosslinked fibrin also involved two sites, but of lower affinity (267 nmol/L and 952 nmol/L), while a single, Kd 405 nmol/L site was identified for crosslinked fibrin. These results are in general agreement with previous studies, but there are important differences that may be related to the experimental approach. Higgins and Vehar2clotted a solution of fibrinogen and t-PA and identified a single Kd 380 nmol/L binding site with greater affinity for single chain than for two chain t-PA. Such a system eliminates the problem of transport of soluble ligand into an insoluble fibrin matrix, although the equilibrium may be affected by the concurrent processes of polymerization and t-PA binding. Different results were obtained by Larsen et al3 who used a similar method but varied the concentration of fibrin instead of t-PA and observed a single Kd of approximately 15 μmol/L site. Hussain et al4characterized the binding of t-PA to preformed polymerized fibrin, the same problem approached in this report. Recognizing the transport problem, they mixed t-PA with a finely sonicated suspension of fibrin. Similar to our findings, they found two distinct sites for non-crosslinked fibrin with Kds of 320 nmol/L and 1,500 nmol/L and a single, 580 nmol/L site for crosslinked fibrin. They concluded that crosslinking prevented binding to a lower affinity binding site only available on noncrosslinked fibrin, but a particular problem was the inability to achieve saturation of binding, and this limited the analysis. Both Fleury et al5 and Grailhe et al6examined t-PA binding to a nonpolymerized monolayer on plastic and identified a single high affinity site with Kds of 3 nmol/L and 1 nmol/L, respectively.
Previous reports have identified binding sites for t-PA on fibrin and localizing them in the regions of α148-16030-32 and γ311-379.33,34 Since fibrin is dimeric, a total of four potential binding sites per molecule are, therefore, available. This is consistent with our identification of two distinct sites and a maximum molar binding ratio of 3.8 for fibrin monomer. Polymerized fibrin presents a more complex substrate than fibrin monomer for binding. Following thrombin cleavage of four fibrinopeptides from fibrinogen, the resulting monomers aggregate in a half-staggered overlap pattern to form two-stranded protofibrils which then aggregate laterally to form a branching network of fibers each composed of 14 to 22 protofibrils.9,10 Consequently, all potential binding sites on fibrin are not equally available, and t-PA may have greater accessibility to binding sites exposed on the surface of fibers than to similar sites within the interior. As with fibrin monomer, two sites of differing affinities were identified for non-crosslinked fibrin, consistent with binding to the sites on α and γ chains. The lower affinities, however, suggest that polymerization may alter the conformation of binding sites, lowering affinity or reducing the accessibility of t-PA. Our results with crosslinked fibrin are consistent with those of Hussain et al4 who found a single binding site suggesting that structural changes accompanying crosslinking eliminate one binding site.
Because of the effect of US on fibrin structure24 and on fibrinolysis,11-17 we sought to determine whether t-PA binding to fibrin was altered by US. It was found that US had little effect on binding to surface-immobilized fibrin with no change in Bmax, a small increase in Kd from 31 nmol/L to 46 nmol/L, and no effect on the kinetics of binding. US also had little effect on binding affinity for non-crosslinked fibrin and only a small effect on crosslinked fibrin affinity. The reason for the small but significant changes in Kd are unclear but could reflect modest changes in conformation of polymerization sites induced by US. Effects were more apparent, however, on Bmax and also on the apparent rate constant for t-PA binding, which increased by 78% and 69% for non-crosslinked and crosslinked fibrin, respectively. These data are consistent with an effect of US on increasing accessibility of t-PA to binding to sites unavailable in polymerized fibrin that are fully accessible on fibrin monomer.
The overall rate of fibrinolysis is determined by several factors including fibrin structure, plasminogen, and t-PA concentration and the presence of enzyme inhibitors. The transport of t-PA into the clot and binding to fibrin is an important determinant of the rate as indicated by both experimental results35-40 and mathematical modeling.41,42 Diamond and Anand42 have presented a model of fibrinolysis using multicomponent convection-diffusion equations. The model predicts that transport of reactants into the fibrin matrix is a rate-limiting step in fibrinolysis. The model also analyzes the effects of steric hindrance of t-PA binding to fibrin within fibers as compared with that on the surface of fibers. Increasing binding of t-PA by improved penetration into fibrin fibers accelerates fibrinolysis up to four-fold in the model. Therefore, both the increased permeation through fibrin in the presence of US, as shown previously23 and the increased binding of t-PA to fibrin reported herein may contribute to the acceleration of fibrinolysis with US.
Other data also indicates that US causes a reversible alteration in fibrin structure. The flow of fluid through fibrin gels under constant pressure increases reversibly with US exposure.23 Because flow at constant pressure is a function of resistance, which is determined by fiber structure,43 this finding indicates that US causes a reversible alteration in fibrin structure. This was confirmed by electron microscopic observations demonstrating that noncrosslinked fibrin that is fixed during US exposure shows an increased fiber density and a decrease in mean fiber diameter.24 This effect is reversible, with return of fiber structure to baseline when the acoustic field is removed. Fully crosslinked fibrin showed less change in fiber density and size, consistent with the smaller effect on Bmax demonstrated. Therefore, experiments with flow resistance, electron microscopic observations, and t-PA binding data are all consistent with a reversible alteration in the polymerization state of fibrin fibers caused by US. Together, these findings support the novel concept that US can exert bioeffects through a nonthermal mechanism by altering the structure of a biopolymer and influencing its interaction with physiologically important ligands.
ACKNOWLEDGMENT
The authors thank Carol Weed for her help in the preparation of the manuscript.
Supported in part by Grants No. HL-30616 and HL-50497 from the National Heart, Lung and Blood Institute, National Institutes of Health, Bethesda, MD.
Address reprint requests to Charles W. Francis, MD, Vascular Medicine Unit, PO Box 610, 601 Elmwood Ave, Rochester, NY 14642.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal