Abstract
Leukocyte adhesion deficiency or LAD is a congenital immunodeficiency disease characterized by recurrent bacterial infections in which the leukocytes from affected children fail to adhere to endothelial cells and migrate to the site of infection due to heterogeneous defects in the leukocyte integrin CD18 subunit. To assess the feasibility of human gene therapy of LAD, we transduced granulocyte colony-stimulating factor (G-CSF)-mobilized, CD34+peripheral blood stem cells derived from a patient with the severe form of LAD using supernatant from the retroviral vector PG13/LgCD18. The highest transduction frequencies (31%) were found after exposure of the cells to retroviral vector on a substrate of recombinant fibronectin fragment CH-296 in the presence of growth factors interleukin-3 (IL-3), IL-6, and stem cell factor. When the phenotype of the transduced cells was monitored by fluorescence-activated cell sorting following in vitro differentiation with growth factors G-CSF and granulocyte-macrophage CSF (GM-CSF), CD11a surface expression was detected immediately after transduction. CD11b and CD11c were expressed at low levels immediately following transduction, but increased over 3 weeks in culture. Adhesion of the transduced cells was nearly double that of nontransduced cells in a cell adhesion assay using human umbilical vein endothelial cells. Transduced cells also demonstrated the ability to undergo a respiratory burst in response to opsonized zymosan, a CD11/CD18-dependent ligand. These experiments show that retrovirus-mediated gene transfer of the CD18 subunit complements the defect in LAD CD34+ cells resulting in CD11/CD18 surface expression, and that the differentiated myelomonocytic cells derived from the transduced LAD CD34+ cells display CD11/CD18-mediated adhesion function. These results indicate that ex vivo gene transfer of CD18 into LAD CD34+ cells, followed by re-infusion of the transduced cells, may represent a therapeutic approach to LAD.
LEUKOCYTE ADHESION deficiency type 1 (LAD), a congenital immunodeficiency disorder characterized by defects in neutrophil adhesion, results in recurrent, life-threatening bacterial infections in affected individuals.1 Considerable experimental evidence indicates that heterogeneous molecular defects in the leukocyte integrin CD18 are responsible for the inability to express the CD11/CD18 heterodimeric complex in LAD.2
LAD is an attractive disease for human gene therapy from a clinical standpoint in that conventional therapy with antibiotics and granulocyte transfusions improves the symptoms but does not correct the phenotype. Although transplantation of hematopoietic stem cells from normal donors has been shown to be curative in LAD, indicating that LAD results from a defect in a hematopoietic stem cell,3 4 the majority of patients do not have a suitably matched donor. Despite a matched donor, allogeneic stem cell transplantation still carries the risk of life-threatening regimen-related toxicity, as well as acute and chronic graft-versus-host disease.
In previous studies, our lab and others have shown that transfer of the CD18 subunit into Epstein-Barr virus (EBV)-transformed B-lymphoblastoid cells derived from children with LAD reconstitutes a CD11a/CD18 heterodimer on the cell surface, and that this dimer mediates homotypic aggregation.5-7 These results indicate that a dominant negative CD18 protein is not present in the four LAD patients whose EBV B cells were transduced.
In the current experiments we extend our retroviral-mediated gene transfer studies to granulocyte colony-stimulating factor (G-CSF)-mobilized peripheral blood CD34+ cells from a patient with LAD. Functional studies on the transduced LAD CD34+ cells indicate that adhesion-related and CD18-dependent respiratory burst activities are reconstituted.
MATERIALS AND METHODS
Isolation of peripheral blood CD34+ cells.
A patient with the severe form of LAD8,9 (22-year-old man, 53 kg) and a normal donor received recombinant human G-CSF (6 μg/kg/d) (Amgen, Inc, Thousand Oaks, CA) subcutaneously for 5 days. The concentration of CD34+ cells in the peripheral blood of the LAD patient just before apheresis was measured at 17 CD34+ cells/μL. Apheresis of peripheral blood leukocytes was performed on days 5 and 6 according to approved protocols and after having obtained informed consent (University of Washington School of Medicine). Apheresis samples were pooled, and CD34+ cells were isolated on Cellpro CD34+ columns (Cellpro Inc, Bothell, WA) according to the manufacturer's directions. Cell yields from the LAD patient were 418 million total nucleated cells, with 187.42 million CD34+ cells (47% CD34+ purity).
Retroviral vector containing human CD18.
Construction of the retroviral vector PG13/LgCD18, containing a human CD18 cDNA, has been described previously.10 The vector contains a novel glutamine tRNA PBS sequence {TGGAGGTTCCACCGAGAT} at the beginning of the extended packaging signal sequence.
Transduction of LAD CD34+ cells.
CD34+ cells from a patient with LAD were transduced for 3 days using vector-containing medium from producer line PG13/LgCD18. Approximately 4 × 105 cells were exposed to 2 mL of vector-containing medium with 50 ng/mL each of growth factors interleukin-3 (IL-3), IL-6, and stem cell factor (SCF) (Amgen) under several conditions including: (1) 8 μg/mL protamine sulfate (Sigma Chemical Co, St Louis, MO); (2) recombinant fibronectin fragment CH-29611 (RetroNectin; Takara Shuzo Co, Ltd, Shiga, Japan) or full-length fibronectin (Collaborative Biomedical Products, Bedford, MA); or (3) CH-296–coated 35-mm petri dishes (Falcon 1008; Becton Dickinson Labware, Franklin Lakes, NJ) (day 0). After overnight exposure to virus-containing medium, the medium was replaced by centrifugation of the cells at 300g for 10 minutes followed by resuspension of the cell pellet in new vector-containing medium plus growth factors (day 1). After 6 to 8 hours exposure to retrovirus-containing medium, the medium was replaced with 2 mL of IMDM/30% media (1× Iscove's Modified Dulbecco's Medium [Life Technologies, Gaithersburg, MD], 30% defined fetal bovine serum [FBS; HyClone Laboratories, Inc, Logan, UT], 1% wt/vol bovine serum albumin [BSA; Sigma, Fraction V], and 3 mmol/L L-glutamine [Life Technologies]). This cycle was repeated on day 2. Cells were harvested on day 3. For cocultivation experiments, 0.7 × 106 PG13/LgCD18 producer cells and 4 × 105CD34+ cells were plated in 6-well tissue culture plates in the presence of IMDM/30% media, 8 μg/mL protamine sulfate, and growth factors.
For surface phenotyping, integrin expression, adherence, and chemiluminescence experiments, LAD CD34+ cells were transduced as described above by exposure to PG13/LgCD18-containing supernatant for 3 days on CH-296 coated tissue culture flasks (at 10 μg/cm2) without protamine sulfate. Untransduced LAD CD34+ cells and normal CD34+ cells were grown in IMDM/30% media plus growth factors. After 3 days of transduction, a portion of the transduced LAD cells was sorted for CD18 expression (see below) and all populations (untransduced, transduced, transduced and sorted LAD CD34+ cells, and normal CD34+ cells) were expanded in culture for a total of 21 days by incubation with IL-3, IL-6, and SCF plus 50 ng/mL each of G-CSF and granulocyte-macrophage (GM-CSF) (Amgen). Cells were split 1/5 and fresh media added approximately every 3 to 4 days.
Flow cytometric analysis of CD34+ cells.
After the transductions, cells were harvested using cell disassociation buffer (Life Technologies) according the manufacturer's directions, washed with phosphate-buffered saline (PBS), and immunostained with one of several fluoroscein isothiocyanate (FITC)-conjugated antibodies, including anti-human CD18 (Dako Corp, Carpenteria, CA), anti-human CD11a (Dako), anti-human CD11b (Sigma), anti-human CD11c (Sigma), and phycoerythrin (PE)-conjugated anti-human CD34 (Becton Dickinson Immunocytometry Systems, San Jose, CA). All antibodies were of the IgG1 isotype. Isotype controls included an FITC-conjugated mouse IgG1 (Sigma) and a PE-conjugated mouse IgG1 (Sigma). Cells were incubated with 1 μg/mL 7-Amino-Actinomycin D (7AAD; Calbiochem-NovaBiochem Intl, La Jolla, CA) in PBS + 1% bovine serum albumin (BSA) for a minimum of 30 minutes at 4°C to allow gating of dead cells.12 Live cells were analyzed by gating out cell debris and dead and apoptotic cells using forward scatter (FSC), side scatter (SSC), and 7AAD staining (FL3). Cells were analyzed on a Becton Dickinson FACScan (San Jose, CA) using FACScan Research Software. Additional software analysis of the data was performed using the program WinMDI version 2.5 (Joseph Trotter, The Scripps Research Institute, La Jolla, CA).
For integrin expression and adherence experiments, transduced cells were immunostained with filter-sterilized anti-human CD18 FITC (Dako) plus 1 μg/mL 7AAD, sorted on a B-D FACStar Plus Cell Sorter at day 3, and the sorted cells expanded for a total of 21 days.
Preparation of purified neutrophils.
Venous blood was collected from the LAD patient and a normal volunteer using 0.2% dipotassium EDTA as anticoagulant. Neutrophils were isolated by sequential sedimentation in 3% Dextran (Sigma) in 0.9% sodium chloride, centrifugation over Histopaque-1077 (Sigma), and hypotonic lysis of erythrocytes as previously described.13The preparations contained >97% polymorphonuclear cells, of which 95% were neutrophils. Cell viability was greater than 98% as determined by trypan blue exclusion.
Cell adherence studies.
Human umbilical vein endothelial cells (HUVEC) were isolated and cultured as previously described14 on surfaces coated with 2% gelatin (Sigma). The cells were grown in RPMI 1640 with the addition of 2 mmol/L glutamine, sodium pyruvate, nonessential amino acids, 1 mmol/L HEPES, 100 U/mL penicillin, 100 U/mL streptomycin, 250 ng/mL amphotericin B (BioWhittaker, Walkersville, MD), 90 μg/mL heparin (Sigma), bovine hypothalamic extract (gift of R. Ross, University of Washington), 10% bovine calf serum (BCS), and 10% BCS supplemented with iron (Hyclone, Logan, UT).
Differentiated normal and LAD CD34+ cells were labeled with 2.5 μmol/L calcein-AM (Molecular Probes, Eugene, OR)15at room temperature for 20 to 40 minutes, washed, and resuspended in medium without phenol red. Preincubation of calcein-labeled cells with antibodies was performed in the same medium. Cells were allowed to adhere to HUVEC with and without the addition of 100 ng/mL phorbol 12-myristate 13-acetate (PMA) (Sigma) for 15 to 20 minutes at 37°C. Adherence was assessed in a Cytofluor Series 4000 fluorescence plate reader (PerSeptive Biosystems, Farmingham, MA). Plates were scanned before and after washing for total and adherent cells, respectively, and calculations of percent adherence were performed using Excel (Microsoft, Redmond, WA).
Assay of luminol-enhanced chemiluminescence.
Luminol-enhanced chemiluminescence was used as a sensitive measure of the respiratory burst of human phagocytes as previously described.16,17 Either CD34+ cells or mature neutrophils (5 × 105) were preincubated for 15 minutes in a 0.5-mL vol of RPMI 1640 (BioWhittaker), with 10 mmol/L HEPES (BioWhittaker) and 15 μg/mL human serum albumin (HSA; Sigma), in polystyrene chemiluminescence cuvettes (Analytical Luminescence Laboratory, San Diego, CA) at room temperature. At the start of the assay, 10 μmol/L luminol (Sigma) and 1 mg/mL opsonized zymosan were added to the reaction mixture. Luminol-enhanced chemiluminescence was read for 10-second intervals at the designated time points with a Monolight 1500 luminometer (Analytical Luminescence Laboratory, San Diego, CA) set to integration mode. The assay was performed at room temperature. Chemiluminescence is reported as relative light units (RLU)/106 cells/10 s. Selected cells were also preincubated with monoclonal antibody (MoAb) 60.3 (1 μg/mL), an antagonistic MoAb directed against CD18,18before the start of the reaction. Zymosan (ICN Pharmaceutical, Cleveland, OH) was opsonized with pooled human serum (OZ) as previously described.19
RESULTS
Conditions for transduction of LAD CD34+ cells.
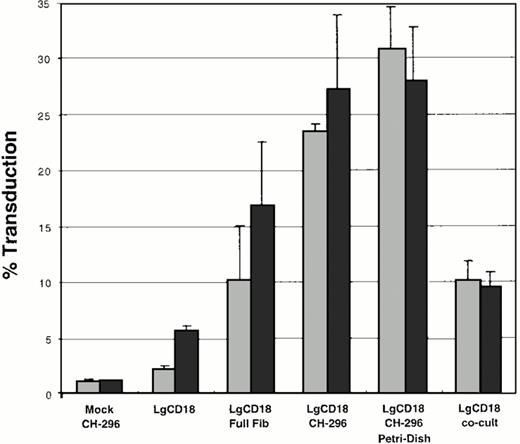
To optimize the conditions for transduction of LAD CD34+cells by retroviral vector PG13/LgCD18, several conditions were examined (Fig 1). First, the presence or absence of protamine sulfate at 8 μg/mL was examined in all experiments. Results in the presence of protamine sulfate are shown as darkly shaded bars (Fig 1). Second, the presence or absence of fibronectin was examined. Without the fibronectin substrate and without protamine sulfate, a transduction frequency of approximately 2.1% was achieved, and this transduction frequency increased to 6% in the presence of protamine sulfate (Fig 1, bars second from left). Full-length fibronectin (at 20 μg/cm2) resulted in a transduction rate of 10%, with an increase to 17% with protamine sulfate (Fig 1, bars third from left). In contrast, recombinant fibronectin fragment CH-296 plated in tissue culture–treated 6-well plates yielded a 23% transduction rate, with a 4% increase upon the addition of protamine sulfate (Fig 1, bars fourth from left). Despite an overall increase in transduction frequency, an average decrease of 3% (from 31% to 28%) was seen with transductions on recombinant fibronectin fragment-coated petri dishes when protamine sulfate was added (Fig 1, bars second from right). Cocultivation of the target CD34+ cells with the PG13/LgCD18 producer cells resulted in fewer transduced cells (approximately 10%) than transductions on recombinant fibronectin (31%) (Fig 1, bars at far right). Thus, in the presence of recombinant fibronectin fragment CH-296,11 the addition of protamine sulfate resulted in only a small to no increase in transduction.
Bar graph of transduction conditions of LAD CD34+ cells by retroviral vector PG13/LgCD18. Cells were transduced under the conditions shown and analyzed by FACS using FITC conjugated anti-human CD18. LgCD18, transduction using vector PG13/LgCD18. Mock, mock transduction using supernatant from PG13 cells only. Full Fib, full-length fibronectin; CH-296, recombinant fibronectin fragment CH-296; co-cult, cocultivation on producer line PG13/LgCD18. Data represent the average of two separate experiments. Error bars indicate the standard deviation. (▧), No protamine sulfate; (▪), 8 μg/mL protamine sulfate.
Bar graph of transduction conditions of LAD CD34+ cells by retroviral vector PG13/LgCD18. Cells were transduced under the conditions shown and analyzed by FACS using FITC conjugated anti-human CD18. LgCD18, transduction using vector PG13/LgCD18. Mock, mock transduction using supernatant from PG13 cells only. Full Fib, full-length fibronectin; CH-296, recombinant fibronectin fragment CH-296; co-cult, cocultivation on producer line PG13/LgCD18. Data represent the average of two separate experiments. Error bars indicate the standard deviation. (▧), No protamine sulfate; (▪), 8 μg/mL protamine sulfate.
Surface phenotype of transduced LAD CD34+.
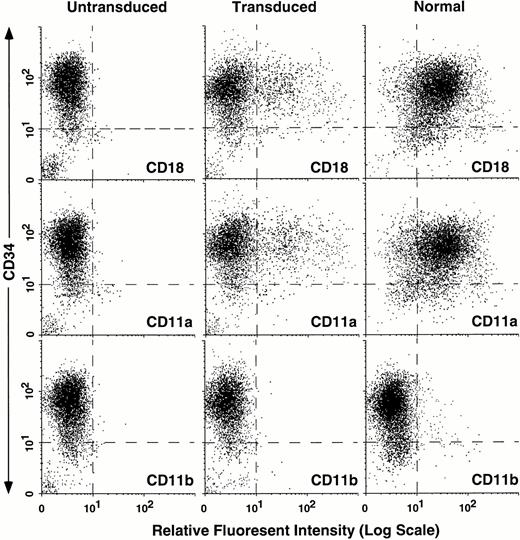
To compare the surface phenotype of untransduced and transduced LAD CD34+ cells to normal CD34+ cells, the three cell populations were analyzed by FACS for expression of surface CD11a, CD11b, CD18, and CD34 (Fig 2).Approximately 20% of the LAD CD34+ cells were transduced as detected by CD18 expression (Fig 2, top middle panel). At 3 days the majority of the cells still retained the CD34+ phenotype. Both transduced and normal CD34+ cell populations displayed primarily CD11a with only low numbers of CD11b+ cells (Fig2, bottom panels) and CD11c+ cells (data not shown), consistent with the relative immaturity of these cells. Importantly, the level of expression of the leukocyte integrins when expressed on the cell surface of the transduced CD34+ cells from the LAD patient was comparable to the level seen on normal CD34+cells (Fig 2, compare top right panel to top middle panel), demonstrating the high efficiency expression of CD18 from the retroviral vector.
Flow cytometric analysis of untransduced LAD CD34+ cells, transduced LAD CD34+ cells, and normal CD34+ cells. LAD CD34+ cells were transduced using the retroviral vector PG13/LgCD18 in a 3-day supernatant transduction on CH-296. Untransduced LAD CD34+ cells and normal CD34+ cells were grown for 3 days in IMDM/30% plus growth factors. After 3 days, all three cell populations were immunostained with either anti-human CD18, CD11a, or CD11b (X-axis where noted) and CD34 (Y-axis). 7AAD was used to gate out dead cells (not shown).
Flow cytometric analysis of untransduced LAD CD34+ cells, transduced LAD CD34+ cells, and normal CD34+ cells. LAD CD34+ cells were transduced using the retroviral vector PG13/LgCD18 in a 3-day supernatant transduction on CH-296. Untransduced LAD CD34+ cells and normal CD34+ cells were grown for 3 days in IMDM/30% plus growth factors. After 3 days, all three cell populations were immunostained with either anti-human CD18, CD11a, or CD11b (X-axis where noted) and CD34 (Y-axis). 7AAD was used to gate out dead cells (not shown).
Time course of leukocyte integrin expression on transduced LAD CD34+ cells.
Transduced LAD CD34+ cells were examined for expression of CD18, CD11a, CD11b, and CD11c on the cell surface at days 3, 7, 14, and 21 after in vitro expansion and differentiation with IL-3, IL-6, SCF, G-CSF, and GM-CSF (Fig 3A). Additionally, transduced cells were sorted for CD18 expression by flow cytometry at day 3 and examined after 3 weeks of expansion with the same growth factors (Fig 3B). Transduced CD34+ cells expressed primarily CD11a, with expression of CD11b and CD11c occurring by days 7-21 in response to incubation in the presence of growth factors. The CD34+ cells displayed a progressive decrease in CD34 positivity over the first 2 weeks in culture. The transduced CD34+ cells sorted for CD18 expression at day 3 expressed high levels of CD11a and CD18 at day 21, and lower, but detectable, levels of CD11b and CD11c surface expression (Fig 3B, right-hand column).
Flow cytometric analysis of transduced and differentiated LAD CD34+ cells. In these studies, transduced LAD CD34+ cells (from Fig 2) were selected by FACS at day 3. The unsorted (A) and sorted (B) cells were differentiated in vitro for a total of 21 days and analyzed at the designated time points (days 3, 7, 14, and 21 for [A], day 21 for [B]) for surface expression of CD18, CD11a, CD11b, CD11c (X-axis where noted; percent positive cells noted in upper right corner of each dotplot), and CD34 (Y-axis).
Flow cytometric analysis of transduced and differentiated LAD CD34+ cells. In these studies, transduced LAD CD34+ cells (from Fig 2) were selected by FACS at day 3. The unsorted (A) and sorted (B) cells were differentiated in vitro for a total of 21 days and analyzed at the designated time points (days 3, 7, 14, and 21 for [A], day 21 for [B]) for surface expression of CD18, CD11a, CD11b, CD11c (X-axis where noted; percent positive cells noted in upper right corner of each dotplot), and CD34 (Y-axis).
Adherence of transduced LAD cells.
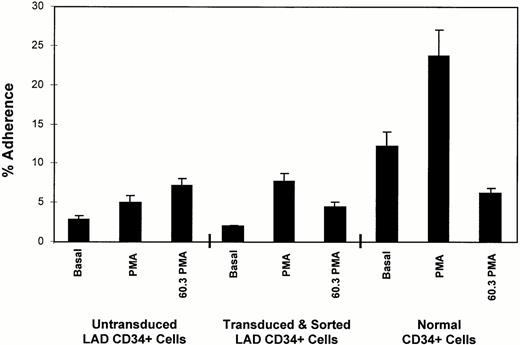
To demonstrate reconstitution of CD18-mediated functional activity after retroviral-mediated transfer of the CD18 into LAD CD34+ cells, transduced LAD CD34+ cells were sorted for CD18 expression. These cells were then expanded and differentiated for 21 days, and used in a neutrophil adherence assay with HUVEC. A representative experiment out of three separate experiments is shown in Fig 4. PMA-induced cell binding to HUVEC is mediated primarily through CD11/CD18 binding to ICAMs.20 Although binding of the untransduced LAD CD34+ cells did increase somewhat in response to PMA, this increase was not present in two other experiments, and the binding was not blocked by the anti-CD11/CD18 MoAb 60.3 (Fig 4, left three bars). The transduced and sorted LAD cells expanded in the presence of differentiation factors displayed increased binding to HUVEC from 2% to 8% in response to PMA. The anti-CD18 MoAb 60.318blocked the PMA-induced binding to HUVEC of the transduced cells by 43% (Fig 4). This blocking of adhesion was present in three separate experiments, with an average of 56%. Similar blocking was present for the expanded normal CD34+ cells (74% inhibition of binding).
Adherence of transduced LAD CD34+ cells to HUVEC. Untransduced LAD CD34+ cells, transduced and sorted LAD CD34+ cells, and normal CD34+cells were expanded for 21 days, labeled with calcein, and allowed to bind to HUVEC. Basal represents the basal or background level of cell adherence. PMA indicates cells stimulated with 100 ng/mL PMA. 60.3 PMA indicates cells incubated with anti-CD18 MoAb 60.3 and stimulated with PMA.
Adherence of transduced LAD CD34+ cells to HUVEC. Untransduced LAD CD34+ cells, transduced and sorted LAD CD34+ cells, and normal CD34+cells were expanded for 21 days, labeled with calcein, and allowed to bind to HUVEC. Basal represents the basal or background level of cell adherence. PMA indicates cells stimulated with 100 ng/mL PMA. 60.3 PMA indicates cells incubated with anti-CD18 MoAb 60.3 and stimulated with PMA.
Chemiluminescence assay of neutrophil respiratory burst.
In a second assay of CD11/CD18-mediated function, normal and LAD neutrophils, along with in vitro differentiated normal CD34+ cells, untransduced LAD CD34+ cells, and transduced LAD CD34+ cells were compared in a chemiluminescence assay of neutrophil respiratory burst activity in response to complement-opsonized zymosan. Activation of the respiratory burst in response to C3bi-opsonized zymosan has been shown to be a CD11/CD18-mediated function activity.21 22 Neutrophil respiratory burst was measured by chemiluminescence over time (Fig 5). Normal polymorphonuclear leukocytes (PMN) displayed a prominent respiratory burst (Fig 5, filled squares), whereas PMN from a LAD patient displayed no detectable respiratory burst (Fig 5, open squares). Normal CD34+cells differentiated in vitro displayed a respiratory burst in response to opsonized zymosan (Fig 5, filled diamonds), whereas the untransduced, expanded LAD CD34+ cells did not (Fig 5, open diamonds). Transduced and expanded LAD CD34+cells showed a low but detectable level of chemiluminescence (Fig 5, dashed X), indicating partial restoration of PMN function in response to opsonized zymosan, a stimulant that requires CD11b/CD18 expression for uptake and triggering of the respiratory burst. Opsonized zymosan-induced luminol-enhanced chemiluminescence was inhibited greater than 80% by MoAb 60.3, an antagonist CD18 MoAb, in normal PMN, and in both normal and LAD CD34+ cells differentiated in vitro (data not shown).
Enhanced chemiluminescence of transduced LAD CD34+ cells. Cells from a normal individual (normal PMN [▪] and normal CD34 [⧫]) and an LAD patient (LAD 1 PMN [□], LAD 1 CD34 [◊], LAD 1 CD34 Trans [X]) were used in a luminol/zymosan chemiluminescence assay with opsonized zymosan as a stimulus to assess neutrophil respiratory burst. In this experiment Trans indicates transduced (unsorted) cells.
Enhanced chemiluminescence of transduced LAD CD34+ cells. Cells from a normal individual (normal PMN [▪] and normal CD34 [⧫]) and an LAD patient (LAD 1 PMN [□], LAD 1 CD34 [◊], LAD 1 CD34 Trans [X]) were used in a luminol/zymosan chemiluminescence assay with opsonized zymosan as a stimulus to assess neutrophil respiratory burst. In this experiment Trans indicates transduced (unsorted) cells.
DISCUSSION
In this report we examined the structural and functional reconstitution of the molecular defect in primary CD34+ cells from a patient with leukocyte adhesion deficiency type 1 (LAD). A transduction efficiency of up to 31% with these cells was achieved using recombinant fibronectin fragment CH-296,11 growth factors IL-3, IL-6, and SCF, and CD18-retrovirus–containing medium. When the phenotype of the transduced cells was analyzed, these cells were shown to possess structural reconstitution of surface CD11a, CD11b, CD11c, and CD18 expression in the form of CD11/CD18 heterodimers. Functional reconstitution of the transduced LAD CD34+ cells was demonstrated using assays for CD11/CD18-mediated cell adhesion and respiratory burst activity.
The severe form of LAD is characterized by delayed umbilical cord detachment, recurrent, life-threatening bacterial infections, lack of pus formation, severe periodontitis, and persistent leukocytosis. Bowen et al8 studied in vitro neutrophil function using cells from the same patient with the severe form of LAD reported here. The investigators found that the phagocytes from these children displayed defective adherence to surfaces, depressed chemotaxis, and a reduced response of chemiluminescence to stimulation with opsonized zymosan (an indirect measure of phagocytosis). In vivo PMN and monocyte chemotaxis as assessed by skin window and skin chamber methods were dramatically impaired.
Since the original description of LAD in 1982, considerable experimental evidence has accumulated indicating that a variety of molecular defects in the leukocyte integrin CD18 are responsible for the clinical phenotype and the defects in in vitro adhesion-related activities in LAD. These defects result in the inability to express the CD11/CD18 heterodimers on the leukocyte surface. Furthermore, MoAbs directed against the CD11/CD18 complex block normal neutrophil adhesion, mimicking the defects observed in LAD neutrophils.18
LAD represents a disease for which human gene therapy may be therapeutic. The reasons for this are several. First, previous studies of patients with the moderate deficiency form of LAD indicate that only 5% to 10% surface expression of CD18 on cells is sufficient to prevent most severe bacterial infections.1 Second, studies in neutropenic patients indicate that as few as 2% of normal neutrophil numbers (100 cells per microliter of blood) appear to be sufficient to provide normal host defense.23 Lastly, gene mutations in CD18 are responsible for LAD, and gene replacement could substitute a normal CD18 gene for the defective CD18 gene.
Several laboratories have explored the use of CD18-containing retroviral vectors for gene transfer into EBV-transformed B cells derived from LAD patients.5-7 Those results have been reviewed elsewhere.24 Recently, we have explored the use of modified retroviral vectors and packaging cell lines and reported increased transduction efficiency when vectors packaged in the PG13 cell line were used.10 Improved transduction was correlated with increased transcription of the retrovirus receptor, glvr-1, which binds the PG13 gibbon ape leukemia virus envelope protein.25
Primary LAD bone marrow cells have been the target of CD18 gene transfer in one previous study.26 In that report LAD bone marrow cells were transduced using the retroviral vector am-hCD18 (GP +envAm12/pΔN2-hCD18). Viral transcripts were detected in floating cells from long-term cultures of transduced LAD bone marrow cells by reverse transcriptase-polymerase chain reaction (RT-PCR). CD18 expression was detected on the surface of the transduced cells by immunofluorescence with an anti-human CD18 antibody; however, the transduced cells were not further characterized.
To identify transduction conditions for future ex vivo gene transfer into peripheral blood stem cells from an individual with LAD, CD34+ were obtained from a patient with the severe form of LAD by G-CSF mobilization. Cell numbers were in the range of those obtained using normal donors (approximately 200 × 106CD34+ cells; about 4 × 106CD34+ cells/kg). This indicates that mobilization of peripheral blood stem cells from LAD patients is not impaired.
We explored a variety of transduction conditions, including the use of recombinant fibronectin fragment CH-296, to improve transduction efficiency of the LAD CD34+ cells by the retroviral vector PG13/LgCD18. CH-296 has been reported by Hanenberg et al27to improve retroviral-mediated transduction efficiency by colocalization of the retrovirus and target cells. Maximal transduction (average of 31%) of LAD CD34+ cells occurred using petri dishes coated with recombinant fibronectin fragment CH-296. Transductions without fibronectin, or with full-length fibronectin, resulted in lower transduction efficiencies than with the recombinant fibronectin fragment. Although transduction efficiencies using cocultivation were higher than transductions without fibronectin, they were much lower than those observed with the recombinant fibronectin fragment. These studies demonstrate a clear advantage for conducting the transductions on recombinant fibronectin fragment, and indicate that supernatant infection on fibronectin is superior to cocultivation using these transduction conditions.
Surface phenotyping of the LAD CD34+ cells by FACS was performed to determine the success of in vitro differentiation of the transduced cells into neutrophil-like cells. CD11a expression on transduced cells was detectable immediately after transduction, whereas CD11b and CD11c expression increased with time. These results indicate that the transduced LAD CD34+ cells retain expression of CD18 with differentiation, that the expression of CD18 is equivalent to levels found in normal cells, and that endogenous CD11b and CD11c expression is inducible with growth factors.
Improvement of the clinical manifestations of LAD is expected following the infusion of transduced LAD CD34+ cells which have reconstituted function. For this reason, we tested the transduced cells in functional assays. Neutrophil migration to the site of inflammation or infection is primarily mediated by interaction of CD11/CD18 on the neutrophil's surface with the intercellular adhesion molecules located on the endothelial surface.20 This interaction can be measured in vitro by the binding of neutrophils to HUVEC. CD34+ cells from an LAD patient were transduced for 3 days, flow cytometry sorted into a CD18 enriched population, and expanded and differentiated to allow CD11b and CD11c expression. When used in an HUVEC adhesion assay, increased binding to HUVEC in response to PMA was demonstrated for the transduced LAD CD34+ cells and normal CD34+ cells. The enhanced binding of the transduced cells and normal CD34+ cells was inhibited by use of an anti-human CD18-specific antibody (60.3), demonstrating the specificity of the CD18 binding. This inhibition was not seen with untransduced LAD CD34+ cells. These data argue that the transduced cells have acquired the ability to function in in vitro assays.
The respiratory burst in neutrophils plays an important role in host defense against pathogens and has been shown to be a CD11/CD18-mediated function when C3bi-opsonized zymosan is used as the stimulus. Neutrophils from individuals with LAD are able to respond to soluble stimuli by reducing nitro-blue-tetrazolinium (NBT) as part of the respiratory burst; however, neutrophils from LAD patients do not respond to C3bi-opsonized zymosan particles with NBT reduction due to the inability of these cells to adhere to C3bi-complement opsonized zymosan. Neutrophils and untransduced and differentiated CD34+ cells from a LAD patient displayed no chemiluminescence in this assay. In contrast, neutrophils and differentiated CD34+ cells from normal individuals displayed activity, and transduced and differentiated LAD CD34+ cells showed a clearly detectable respiratory burst, indicating enhancement of neutrophil function by retroviral-mediated gene transfer of the CD18 molecule.
These studies, demonstrating structural and functional correction of the genetic defect in primary CD34+ cells from a child with LAD using retroviral-mediated CD18 gene transfer, support the feasibility of ex vivo transduction of LAD CD34+ cells, followed by reinfusion of the transduced cells, as a therapeutic approach to this disease.
ACKNOWLEDGMENT
We thank Dr John Harlan for his helpful advice.
Supported by National Institutes of Health NIH Grants and Contracts No. DK48456 (D.D.H.) and HL54881 (D.D.H.), HL53515 (W.C.L.), Pfizer Postdoctoral Fellowship (W.C.L.), the March of Dimes Birth Defects Foundation (D.D.H.), and the Department of Veterans Affairs Merit Review Program (D.D.H.). A portion of this work was conducted through the Clinical Research Center facility at the University of Washington, supported by the National Institute of Health, Grant No. M01-RR-00037.
Address reprint requests to Thomas R. Bauer, Jr, PhD, VA Puget Sound Health Care System, GMR 151, 1660 S Columbian Way, Seattle, WA 98108.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

![Fig. 3. Flow cytometric analysis of transduced and differentiated LAD CD34+ cells. In these studies, transduced LAD CD34+ cells (from Fig 2) were selected by FACS at day 3. The unsorted (A) and sorted (B) cells were differentiated in vitro for a total of 21 days and analyzed at the designated time points (days 3, 7, 14, and 21 for [A], day 21 for [B]) for surface expression of CD18, CD11a, CD11b, CD11c (X-axis where noted; percent positive cells noted in upper right corner of each dotplot), and CD34 (Y-axis).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/5/10.1182_blood.v91.5.1520/3/m_blod4054003.jpeg?Expires=1769378871&Signature=X~lQP2u2tti8xovcprE7MYU4uDlGgd~2sf8j3Njx6DeBMMpj0MuVNaDgptp8NZlpCLt8-oTuxabExv-xb1LLuwnOChAYJhpsIp-b25gOpuRmOlTpVE4Hzdb7WHGFKLskzI51AzJSsYVZ1SrOxwbILbMwzJ1TnCe7iTPB~yoH6JhVyqXM6BbASoNWWkaXDcAYRqGnwOuYmVr4gDetB41b8Q0kkct91h2HZ-qjU~kTftWb6WaGTt1n4NtNwB0vWgPHJcBfKyKPZcM9MMqzeMhNbOAp2idjkTGLfj4vSbQvalC9oXZ93zjV43ETGpaWm22PwlzXyDqFuNHM0wAhOG6n-w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Enhanced chemiluminescence of transduced LAD CD34+ cells. Cells from a normal individual (normal PMN [▪] and normal CD34 [⧫]) and an LAD patient (LAD 1 PMN [□], LAD 1 CD34 [◊], LAD 1 CD34 Trans [X]) were used in a luminol/zymosan chemiluminescence assay with opsonized zymosan as a stimulus to assess neutrophil respiratory burst. In this experiment Trans indicates transduced (unsorted) cells.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/5/10.1182_blood.v91.5.1520/3/m_blod4054005.jpeg?Expires=1769378871&Signature=ng7T8Lmpe-tSLY4cW53KVpFc5r13J8VCDXVh4nA84Z0GEAynUdkLh4eZNUVzPG0ptl5TiEsm5hV4~fpTgxK1PYnFph7WSD5vDarXtQW1tbhk81x7JMDfPgF4sZ1uXePUCTfertj3K7kxku4HoZxLHMTo0JaawFL0XGbgq7-u3MGBlk9we7PoUKqrh-IMH2r0u~sFi0H~Om5PdpzdQuWRiBePyeYL-LSi4Wl8mu4HYIpARPG726aHsaCDhugmpBIsiA0QOQjx9WRq1s1VPBh6~h1v1nnGesWh3Z0mOpW261SZFkBhWYCUHMTsBua3cyNtZnWZU42NV05uFXVLB23jTg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal