Abstract
An inverse relationship between BCL-2 expression and cell cycle transition has been suggested by recent studies in murine models. To investigate the clinical relevance of these laboratory studies, a group of 116 paraffin-embedded non-Hodgkin's lymphoma (NHL) biopsy specimens (Working Formulation Groups D-H, and J) from a cooperative group study of cellular DNA content were analyzed for the 14;18 translocation using polymerase chain reaction (PCR)-based methods and, if sufficient tissue remained, for BCL-2 and BAXexpression by immunohistochemistry. The results of these studies were then compared with the results of the previously performed flow cytometric analysis of ploidy and proliferative activity (S-phase-fraction). BCL-2 expression was inversely associated with proliferative activity (P = .001; n = 41), but there was no association between staining for Bax and %S-phase. Ploidy was not associated with either BCL-2 or BAX expression. The t(14;18) was detected in 21 of the 54 cases in which PCR-amplifiable DNA was recovered; 20 of these occurred at the major breakpoint region and 1 at the minor breakpoint region. High levels of BCL-2 orBAX expression occurred independently of t(14;18). There was no association between t(14;18) and either ploidy or proliferative activity. The inverse relationship between BCL-2 expression and proliferative activity in the intermediate- and high-grade NHLs is consistent with recent studies suggesting that Bcl-2 both retards entry into the cell cycle and inhibits apoptosis.
THE PROTEIN ENCODED by the BCL-2(B-cell lymphoma/leukemia-2) gene is a major regulator of programmed cell death, the process by which many chemotherapeutic agents ultimately effect tumor cell kill.1,2,BCL-2 was first identified because of its association with the t(14;18) chromosomal translocation in which the BCL-2 gene at 18q21 is juxtaposed with the Ig heavy chain locus at 14q32, resulting in deregulation of transcription and overexpression of the Bcl-2 protein.3-5 This balanced translocation is characteristic of the majority of follicular non-Hodgkin's lymphomas (NHLs) and a subset of intermediate- and high-grade lymphomas.6,7 Bcl-2 protein levels may also be elevated in NHLs that do not harbor the t(14;18), suggesting that other mechanisms may result in overexpression.8 Recently, high levels of the Bcl-2 protein detected by immunostaining have been associated with early relapse in the intermediate- and high-grade NHLs. 9-12
Proliferative activity measured by flow cytometric analysis of cellular DNA content or Ki-67 expression correlates inversely with clinical outcome in the NHLs in some series.13-15 To confirm early reports that proliferative activity is a significant independent determinant of clinical behavior in the intermediate- and high-grade NHLs, a large prospective study of cellular DNA content in archival paraffin-embedded biopsy specimens from uniformly staged and treated patients was initiated through the Eastern Cooperative Oncology Group (E6486). Although flow cytometric analysis of ploidy and proliferative activity showed a probable association between increasing proliferative activity (%S-phase) and shortened survival, clinical parameters were more powerful prognostic indicators than cellular DNA content analysis.16 No association with disease-free survival or time to treatment failure was observed. Using residual paraffin-embedded material from 116 of the patients enrolled on E6486, polymerase chain reaction (PCR)-based detection of the 14;18 translocation and immunohistochemical analysis of the Bcl-2 and Bax proteins were performed. Comparison was then made with the results of the previous flow cytometric analysis of ploidy and proliferative activity, showing an association between BCL-2 expression and low proliferative activity. These findings are consistent with recent data suggesting that Bcl-2 retards entry into the cell cycle in addition to inhibiting apoptosis.17-19
MATERIALS AND METHODS
Patient materials.
Cases enrolled on two phase-III intergroup clinical trials (E648320 and E348721) for previously untreated patients with intermediate- and high-grade NHLs were eligible for entry onto an ancillary study of cellular DNA content provided that paraffin blocks from the initial diagnostic specimen were available for submission and that the embedded tissue had a minimal transverse diameter of 0.4 cm and sufficient depth to provide a minimum of three 50-micron sections for cellular DNA content analysis and two routine hematoxylin and eosin sections (5 microns). For technical reasons, material fixed in B5-formalin or Zenker's fixative was not acceptable. Patients with antecedent low-grade NHL were ineligible. Cases in which material remained in the block after sections were cut for DNA content analysis were processed first for molecular analysis for the t(14;18) by the PCR and second for immunohistochemical staining with reagents identifying the Bcl-2 and Bax proteins. Five-micron sections for routine staining with hematoxylin and eosin were obtained from each biopsy specimen to show the presence of lymphoma within the material to be studied.
Flow cytometric analysis.
Three 50-micron sections were obtained from formalin-fixed blocks, deparaffinized, and dissociated using a previously reported modification of the method described by Hedley et al.22,23After dissociation, nuclei were permeabilized with Triton X-100 (0.1%), incubated with RNAase A, and stained with propidium iodide (50 μg/mL) as described in previous reports.23 The presence of lymphoma within the material was shown by examination of adjacent hematoxylin and eosin stained sections.
Cellular DNA content and proliferative activity (S-phase fraction) were quantitated using a Coulter Epics 752 flow cytometer (Coulter, Miami, FL), and analyzed using the Multicycle Software (Phoenix Flow Systems, San Diego, CA) as previously reported.16 Cell cycle analysis for determination of the S-phase fraction was based on the average S-phase; consequently, for DNA-aneuploid cases this measurement was based on the weighted average of S-phase fraction of the DNA diploid and DNA aneuploid cellular subpopulations.
PCR for detection of t(14;18).
DNA was prepared from paraffin-embedded tissue sections by incubating 15-micron sections in 1 mL of xylene followed by three incubations with 100% ethanol. The sample was air dried and suspended in 100 μL of 50 mmol/L Tris-HCL pH 8.5, 1 mmol/L EDTA, 0.5% Triton X-100, 1 mg/mL proteinase K.
After overnight incubation at 37°C, the proteinase K was inactivated by boiling for 10 minutes and samples were stored at 4°C (Phenol/CHCl3 extraction followed by ethanol precipitation of this material offered no improvement in template quality). The suitability of the template DNA was determined by amplification of a 267-bp human β-globin product using 10 μL of diluted and 100-fold diluted paraffin block rescued DNA template.24 If neither template concentration amplified the β-globin product, the case was considered unsuitable for t(14;18) PCR. The same two templated concentrations were used before a β-globin–positive case was considered negative for t(14;18).
Templates that were amplifiable were then assessed for the presence of t(14;18) chromosomes involving the BCL-2 major breakpoint region (mbr) using the protocol of Crescenzi et al25 and the primers MBR5: 5′-TTAGAGAGTTGCTTACGTG and SKJH: 5′-ACCTGAGGAGACGGTGCCAGGGT. The products were then subjected to electrophoresis in 2% agarose, transferred to nylon membranes, and probed with an end-labeled BCL-2 mbr oligonucleotide (5′-GCCTGTTCAACACAGAC).25 DNA from the t(14;18)/mbr cell lines SU-DHL-6 or RL-7 was used as a positive control.
Those specimens that were amplifiable but negative for the t(14;18) using the BCL-2 mbr primers were then evaluated for the presence of a t(14;18) involving the minor breakpoint cluster region (mcr) using a primer MC12 from the mcr 5′-GATGGCTTGCTGAGAGGTAT-3′ and an Ig JH primer (SKJH) by the method of Ngan et al.26 The products were analyzed by Southern blotting with a 32P-end labeledBCL-2 mcr oligonucleotide probe (5′-GACTCCTTTACGTGCTGGTACC).26 DNA from the mcr t(14;18) containing cell line, SU-DHL-16, served as a positive control. In all PCR experiments, DNA from the myeloid leukemia cell line HL60 served as a negative control. All experiments included a no-template DNA control.
Immunohistochemical staining.
Five-micron tissue sections mounted on poly-L-lysine–coated glass slides were deparaffinized, dehydrated, and stained as previously described using polyclonal antisera raised in rabbits against synthetic peptides corresponding to amino acids 41 to 54 of the human Bcl-2 protein and to amino acids 43 to 61 of the human Bax protein.27 28 The slides were scored according to the number of neoplastic cells that stained positively with the antisera. To be consistent with previous reports, cases with high levels ofBCL-2 or BAX expression were distinguished from those considered to have minimal expression. A breakpoint of 20% positive neoplastic cells proved to be a reproducible cutoff.
Statistical methods.
Univariate associations between dichotomous variables were evaluated with Fisher's exact test. Associations involving ordered categorical variables were evaluated with Wilcoxon's rank sum test.29
RESULTS
Clinical characteristics.
Paraffin-embedded material was available for 116 of 257 cases enrolled on the parent study of ploidy and proliferative activity in the intermediate- and high-grade NHLs (E6486) for which blocks were received. These specimens represent the diagnostic material from previously untreated patients with NHLs representing histological groups D through H and J by the Working Formulation30(Table 1). All specimens underwent secondary pathology review to confirm the histological diagnosis.
Pathological Subtype by Working Formulation (n = 116)
| Group . | Number of Patients . |
|---|---|
| Intermediate grade | |
| D. Follicular, large cell | 12 |
| E. Diffuse, small-cleaved cell | 5 |
| F. Diffuse, mixed small and large cell | 11 |
| G. Diffuse, large cell | 53 |
| High grade | |
| H. Diffuse, large cell immunoblastic | 33 |
| J. Diffuse, small noncleaved cell | 2 |
| Group . | Number of Patients . |
|---|---|
| Intermediate grade | |
| D. Follicular, large cell | 12 |
| E. Diffuse, small-cleaved cell | 5 |
| F. Diffuse, mixed small and large cell | 11 |
| G. Diffuse, large cell | 53 |
| High grade | |
| H. Diffuse, large cell immunoblastic | 33 |
| J. Diffuse, small noncleaved cell | 2 |
Molecular studies.
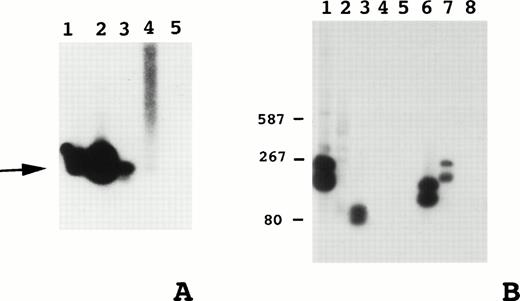
PCR-based assays were used to detect the t(14;18) in paraffin-embedded material.25,26 The sensitivity of these assays was in the range of one t(14;18)-positive cell per 100,000 cells making it highly unlikely that a t(14;18) in non-neoplastic cells would be detected (Fig 1A).31 DNA was prepared from 115 cases but amplification was successfully achieved in fewer than half of these (Table 2). The t(14;18) was detected in 21 of the 54 cases in which amplification occurred (Fig1B). Some positive cases showed two bands most likely resulting from priming from more than one JH gene. Twenty of the translocations occurred at the major breakpoint region, and only one at the minor breakpoint region. No two patients had identical t(14;18) PCR products, and t(14;18) PCR products ranged in size from 80 to 500 base pairs.
(A) Southern blot of t(14;18) major breakpoint region PCR products to assess assay sensitivity. Dilutions of positive control (SU-DHL-6) DNA in negative control (HL60) DNA. 1, 1:103; 2, 1:104; 3, 1:105; 4, 1:106; 5, HL60 DNA; arrow, t(14;18) PCR product. (B) Example of Southern blot of t(14;18) major breakpoint region PCR products from patient biopsy specimens: 1, RL-7 positive control; 2, negative control; 3 to 8, patient PCR products. Size standards at left in base pairs.
(A) Southern blot of t(14;18) major breakpoint region PCR products to assess assay sensitivity. Dilutions of positive control (SU-DHL-6) DNA in negative control (HL60) DNA. 1, 1:103; 2, 1:104; 3, 1:105; 4, 1:106; 5, HL60 DNA; arrow, t(14;18) PCR product. (B) Example of Southern blot of t(14;18) major breakpoint region PCR products from patient biopsy specimens: 1, RL-7 positive control; 2, negative control; 3 to 8, patient PCR products. Size standards at left in base pairs.
Results of Molecular Studies and Immunohistochemical Staining
| . | No. Cases . |
|---|---|
| Total number of cases | 116 |
| DNA available | 115 |
| Amplification achieved | 54 |
| t(14;18) detected by PCR | 21 |
| Major bcr | 20 |
| Minor bcr | 1 |
| Immunohistochemical staining | |
| anti–BCL-2 | 56 |
| >20% cells positive | 35 |
| 1-20% cells positive | 10 |
| Negative | 7 |
| Inevaluable | 4 |
| anti-BAX | 25 |
| >20% cells positive | 19 |
| 1-20% cells positive | 3 |
| Negative | 1 |
| Inevaluable | 2 |
| . | No. Cases . |
|---|---|
| Total number of cases | 116 |
| DNA available | 115 |
| Amplification achieved | 54 |
| t(14;18) detected by PCR | 21 |
| Major bcr | 20 |
| Minor bcr | 1 |
| Immunohistochemical staining | |
| anti–BCL-2 | 56 |
| >20% cells positive | 35 |
| 1-20% cells positive | 10 |
| Negative | 7 |
| Inevaluable | 4 |
| anti-BAX | 25 |
| >20% cells positive | 19 |
| 1-20% cells positive | 3 |
| Negative | 1 |
| Inevaluable | 2 |
Amplification occurred in only 2 of 12 follicular large-cell (Group D) and 2 of 5 diffuse small-cleaved cell (Group E) lymphoma specimens. Both Group D and one of the two Group E biopsy specimens were positive for the t(14;18). Fifty-seven percent of the amplifiable cases from Group F (4 of 7), 31% from Group G (8 of 26), and 38% from Group H (6 of 16) were also positive. The t(14;18) was not detected in the one amplifiable specimen from Group J.
Immunohistochemical studies.
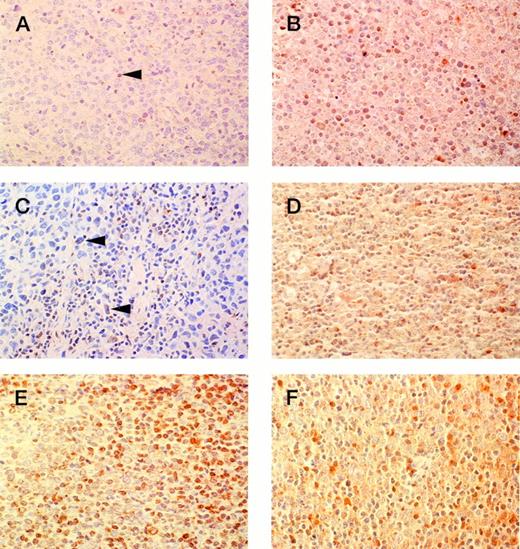
Immunohistochemistry was performed on 56 cases in which material remained after processing for molecular studies. Expression was scored as high (>20% cells positive), intermediate (1% to 20% cells positive), or negative (summarized in Table 2). High levels ofBCL-2 expression were found in 35 of 56 (63%) cases including all three follicular large-cell cases (Group D) and both diffuse, small-cleaved cell cases (Group E) that were immunostained. Intermediate expression was seen in 10 of 56 cases (18%). Seven of 56 (13%) cases were immunonegative. Four cases were inevaluable for technical reasons. The majority of cases analyzed contained high levels of BAX expression (19 of 25; 76%). Twelve of the 19 cases with high levels of BAX expression also had intense Bcl-2 immunostaining. Four cases with >20% of neoplastic cells positive for Bax were negative for Bcl-2, whereas two cases with low or negative staining for Bax were positive (>20%) for Bcl-2. No association was found between Bcl-2 and Bax immunostaining. In all Bcl-2 immunopositive cases, discrete cell associated cytoplasmic staining was present. The anti-Bax staining was typically uniform from cell to cell; however, considerable cell-to-cell variability was the norm for the Bcl-2 immunostaining. All Bcl-2 and Bax immunonegative cases contained positive staining in occasional benign small lymphocytes, thus validating the immunostaining results. Figure 2 provides examples of the Bax and Bcl-2 immunostaining.
Representative immunoperoxidase with anti–Bcl-2 and anti-Bax antisera. (A) anti–Bcl-2 study of lymphoma negative for Bcl-2 (arrowhead indicates positive staining in background small lymphocyte); (B) same case as (A) showing positive staining in greater than 20% of lymphoma cells with anti-Bax antisera; (C) anti–Bcl-2 study of lymphoma showing Bcl-2 positivity in fewer than 20% of the neoplastic cells (arrowheads indicate positive large atypical cells); (D) same as (C) showing positive staining in greater than 20% of lymphoma cells with anti-Bax antisera; (E) anti–Bcl-2 study of lymphoma showing Bcl-2 positivity in greater than 20% of the neoplastic cells; (F) same case as (E) showing lymphoma with greater than 20% of cells positive for Bax.
Representative immunoperoxidase with anti–Bcl-2 and anti-Bax antisera. (A) anti–Bcl-2 study of lymphoma negative for Bcl-2 (arrowhead indicates positive staining in background small lymphocyte); (B) same case as (A) showing positive staining in greater than 20% of lymphoma cells with anti-Bax antisera; (C) anti–Bcl-2 study of lymphoma showing Bcl-2 positivity in fewer than 20% of the neoplastic cells (arrowheads indicate positive large atypical cells); (D) same as (C) showing positive staining in greater than 20% of lymphoma cells with anti-Bax antisera; (E) anti–Bcl-2 study of lymphoma showing Bcl-2 positivity in greater than 20% of the neoplastic cells; (F) same case as (E) showing lymphoma with greater than 20% of cells positive for Bax.
Associations between variables.
BCL-2 expression was inversely associated with proliferative activity (P = .001; Table 3). Cases with >20% neoplastic cells positive for Bcl-2 had the lowest median S-phase, whereas those that were entirely negative for Bcl-2 had the highest %S-phase. This analysis was based on the 41 cases for which both flow cytometric quantitation of %S-phase and immunohistochemical analysis for Bcl-2 were available. This association was maintained if the five cases representing Working Formulation categories D and E were excluded from the analysis. There was no evidence of an association between anti-Bax immunopositivity and proliferative activity (P= .79 by Wilcoxon's test). Consistent with the results of the larger parent study of ploidy and proliferative activity, 57% of cases included in this study were aneuploid by flow cytometry and the median %S-phase was 10.2 (interquartile range: 6.4-17.6). Ploidy was not associated with positive immunostaining for either Bcl-2 or Bax.
Proliferative Activity (% S-Phase) According to Degree of Immunohistochemical Staining (n = 41)
| Histological Categories . | % S-Phase Median (interquartile range) . | ||
|---|---|---|---|
| Bcl-2− (n = 6) . | 1%-20% Bcl-2+ (n = 8) . | >20% Bcl-2+ (n = 27)* . | |
| D-H, J | 19.8 | 11.8 | 7 |
| (12.4-21.2) | (9.8-15.7) | (5.1-12.6) | |
| F, G, H, J† | 19.8 | 11.8 | 8.1 |
| (12.4-21.2) | (9.8-15.7) | (5.4-12.8) | |
| Histological Categories . | % S-Phase Median (interquartile range) . | ||
|---|---|---|---|
| Bcl-2− (n = 6) . | 1%-20% Bcl-2+ (n = 8) . | >20% Bcl-2+ (n = 27)* . | |
| D-H, J | 19.8 | 11.8 | 7 |
| (12.4-21.2) | (9.8-15.7) | (5.1-12.6) | |
| F, G, H, J† | 19.8 | 11.8 | 8.1 |
| (12.4-21.2) | (9.8-15.7) | (5.4-12.8) | |
A nonparametric test for trend assuming an ordering of Bcl-2−, 1%-20% Bcl-2+, and > 20% Bcl-2+ is significant at P = .001 for all cases with and without Groups D, E.
Excluding Groups D and E.
Five of these 27 cases were from Groups D and E.
The presence of the t(14;18) rearrangement at the major breakpoint region was also not associated with either DNA ploidy or proliferative activity. The percent of neoplastic cells positive for Bcl-2 or Bax by immunohistochemistry was independent of the t(14;18); a high percentage of cells stained with anti-Bcl-2 in cases that were negative for the translocation.
DISCUSSION
In this study, immunohistochemically detected BCL-2 expression was associated with low proliferative activity (%S-phase) in biopsy specimens from untreated patients with intermediate- and high-grade NHLs. This result is consistent with recent in vitro data showing that the Bcl-2 protein retards entry into cell cycle.17-19 These findings also confirm trends reported in previous immunohistochemical studies of follicular lymphomas32 33 in which BCL-2 expression was associated with proliferative activity. Bcl-2 has been shown to inhibit programmed cell death under a wide range of circumstances, but a common molecular mechanism has not yet been established. Recent studies linking activators of cell cycle progression to the induction of programmed cell death have led investigators to hypothesize that Bcl-2 may exert at least part of its antiapoptotic effects through regulation of either cell cycle entry (G0 to G1 ) or cell cycle progression at the G1 to S-phase transition. The association of BCL-2 expression with low proliferative activity in the intermediate- and high-grade NHLs seen in this study is consistent with this proposal and provides indirect support for a model in which Bcl-2 suppresses cell proliferation in vivo. The implications of this association for both the process of lymphomagenesis and clinical behavior remain to be elucidated.
Overexpression of wild-type BCL-2 in the B cells of transgenic mice has been shown to inhibit cell death leading to an excess accumulation of lymphocytes without an increase in proliferation.34 Similarly, BCL-2 overexpression has been shown to protect interleukin-3 (IL-3)–dependent lymphoid cells from apoptosis induced by growth factor deprivation without promoting IL-3–independent cell growth. When cells that overexpressBCL-2 are first deprived of IL-3 and then reexposed to the cytokine, they exhibit a prolonged lag phase before S-phase.35 An elegant genetic murine T-cell model providing a gradient of BCL-2 expression has been used to show that the level of BCL-2 expression determines the duration of G0 to S-phase transition, with cells not expressingBCL-2 showing a shortened G0 to S-phase transition and cells that overexpress wild-type BCL-2 showing a delay in progress into cell cycle.17 Studies using other lymphoid systems including one B-cell model have also shown a Bcl-2–induced prolongation of the G0/G1 transition or G1-phase.18,19,36 In one of these studies, Bcl-2–induced accumulation of cells at the G0/G1 stage was reversed by the Bax protein, which heterodimerizes with Bcl-2 promoting apoptosis.19Therefore, the association of BCL-2 expression with a lower proliferative index would appear to be reasonable in light of current molecular theory.
An association between low proliferative activity and apoptotic indices has been previously shown in one small series of gastrointestinal lymphomas of mucosa-associated lymphoid tissue (MALT)37 and in two studies of follicular NHLs,32,33 but not in previous studies of the intermediate- and high-gradeNHLs.38-40 In a compilation of 40 MALT lymphomas (19 low-grade, 21 high-grade), proliferative indices quantitated by immunostaining for the Ki-67 antigen and apoptotic indices calculated by in situ end-labeling were loosely correlated (r = 0.586;P < .001).37 The low proliferative activity characteristic of the low-grade lymphomas and the high frequency of the t(14;18) with its associated BCL-2 expression are likely to favor an association between proliferative activity and apoptotic indices whenever low- and intermediate-grade NHLs are combined. Nevertheless, Barrans et al32 could not show a statistically significant correlation between the number of cells expressing BCL-2 and either p53 or MIB1(anti-Ki-67) in follicular center cell lymphomas, although cases with high levels of expression of BCL-2 did tend to have lower rates of proliferation.32 Among diffuse large-cell lymphomas, proliferative activity was significantly lower in cases in which the t(14;18) was detected and was comparable with that observed in follicular lymphomas.41 In a large series of NHLs with diffuse architectural patterns, an apparent inverse correlation between growth fraction and percentage of Bcl-2 immunopositive cells was noted but this association lost statistical significance when the low- and high-grade lymphomas were examined separately.38 Mitotic and apoptotic indices quantitated by counting mitoses and the number of pyknotic cells per 30 high-powered fields were loosely correlated in a nonlinear fashion in a series of diffuse centrocytic and/or centroblastic NHLs.39 In the intermediate-grade subset of biopsy specimens from patients with relapsed or refractory NHL undergoing salvage therapy, there was a trend towards an inverse correlation between anti–Ki-67 staining and BCL-2expression.40
In contrast to the previously reported series, the NHLs examined here were restricted to the intermediate and high-grade NHLs (groups D-H and J). The parent study from which these specimens were drawn included all intermediate- and high-grade categories, excluding lymphoblastic lymphoma, based on the eligibility for the clinical trials in which these patients participated.16,20,21 Proliferative activity, measured by flow cytometry correlates with the morphologically based low-, intermediate-, and high-grade categories of the Working Formulation.42 Categories D and E have proliferative activity that is intermediate between that of the diffuse mixed (Category F) and diffuse large-cell lymphomas (Category G) and the follicular low-grade NHLs.42 Whereas the frequency of the t(14;18) is greater among Groups D and E than the other intermediate-grade NHLs, a greater proportion of Group D and E NHLs are likely to stain for Bcl-2 using immunohistochemical techniques.43-45 A preponderance of cases of low S-phase with a high likelihood of Bcl-2 positivity might favor an association between these two characteristics. Hence, the analysis was performed with and without these two categories, and the inverse correlation was maintained even when cases from categories D and/or E were excluded. Of note, the one case from category J included in the Bcl-2 versus proliferative activity analysis was Bcl-2 positive, with an exceptionally low %S-phase (8.8) for this histological subtype. None of the cases included in this series were known to have evolved from low-grade NHLs, although a proportion of large-cell lymphomas may derive from previously undetected follicular lymphomas that have retained the t(14;18).46
The Bcl-2 homolog and heterodimerization partner, Bax, is a cell death promoter.47,48 The ratio of Bax to Bcl-2 determines the relative sensitivity or resistance of cells to apoptotic stimuli. In previous in vitro studies, Bax has been shown to override the inhibitory effects of Bcl-2 on the cell cycle.19 The majority of cases in which material was available for immunostaining with anti-Bax antibodies was strongly positive, but too few cases were analyzed overall to evaluate the Bcl-2/Bax ratio and its association with proliferative activity. In recent studies, BAX expression has been associated with increased proliferative activity.49 In the NHLs, the ratio of Bcl-2 to Bax seems likely to play an important role in determining both proliferation and susceptibility to chemotherapy.2 47
PCR amplifiable DNA was isolated from the paraffin-embedded specimens in only 47% of the cases in this series. These specimens were obtained from both academic and community centers belonging to the Eastern Cooperative Oncology Group and the Southwestern Oncology Group in the United States and South Africa; differences in the fixation methodology between institutions may underlie the low success rate. Although all blocks contained formalin-fixed material, the duration of fixation was not specified and may have varied widely from institution to institution. The generation of a PCR product is dependent in part on the duration of formalin fixation.50 In addition, prolonged fixation may interfere with the amplification of larger products such as those generated by the minor breakpoint region primers.51 This may explain the lower than expected percentage of cases positive for the t(14;18) minor breakpoint cluster type translocations.7
False-negative PCR reactions may also occur when the translocation breakpoint is not encompassed by the PCR primer used. Comparative studies of PCR and Southern blot and/or cytogenetic detection of the t(14;18) indicate that, depending on the protocol, PCR can detect 75% to 83% of translocations.52,53 In addition, there will be false negatives resulting from DNA degradation during fixation which precludes the amplification of larger products. A study comparing fresh versus formalin-fixed material from the same cases that used very similar DNA extraction and PCR protocols to those used in this study indicated up to 20% false negatives related to fixation and embedding techniques.54 This may be especially problematic in the detection of translocations producing long PCR products that are beyond the range of confirmed amplification of the DNA isolated in our series. These factors may have also contributed to the detection of only one minor breakpoint cluster type t(14;18). However, one large North American mapping study found only 7 of 91 (8%) of cytogenetically proven t(14;18) lymphomas to have the minor breakpoint cluster type translocations.53 Although these technical considerations suggest that there were false negatives in the molecular analysis, the overall percentage of cases positive for the t(14;18) in this report (39%) is consistent with earlier cytogenetic, Southern, and PCR based studies of diffuse B-cell lymphoma.55-57 The fact that there were cases with high level BCL-2 expression without evidence of the t(14;18) is consistent with the results of other studies and supports the hypothesized existence of mechanisms other than chromosomal translocation for dysregulated BCL-2expression.8 57
Three large clinical trials have now established the prognostic significance of BCL-2 expression in the previously untreated diffuse large-cell lymphomas.9-11 Disease-free survival but not overall survival has been inversely associated with BCL-2expression in these studies. Although drug resistance related to Bcl-2 has been proposed as the underlying basis for this clinical behavior, at least one study of relapsed and refractory patients has shown no relationship between the level of Bcl-2 protein and response to a novel chemotherapeutic regimen.40 In some series, the prognostic significance of p53 mutations were also investigated and showed an association with drug resistance and/or a poor overall survival.40,58 The tumor suppressor gene p53 induces cell cycle arrest and apoptosis and its loss has been associated with drug resistance.59 In the present series, insufficient tissue prevented the investigation of p53 mutations and expression of p21/WAF1, its functional effector. WAF1 has been implicated in growth arrest through interference with transition from G1 into S-phase, and may have prognostic significance either alone or in combination with the identification of p53 mutations.60
There were too few cases in this series to investigate BCL-2expression and proliferative activity as predictors of clinical outcome using a multivariate analysis. Molecular analysis and later immunohistochemical staining for Bcl-2 and Bax were appended to the flow cytometric studies of ploidy and proliferative activity, which were the initial and primary intent of the parent protocol.16 Hence, only a small number of cases had residual material in the submitted block, and in many cases the limited tissue was exhausted during preparation of DNA. Whereas these tissues were submitted by a large number of institutions, there was considerable variation in the quality of fixation resulting in poor preparations for both PCR and flow cytometric analysis in some cases. This further limited the number of cases with both S-phase quantitation and immunohistochemistry.
To be consistent with previous publications,9-12 cases with minimal staining with anti-Bax and anti–Bcl-2 were distinguished from those with greater numbers of positive cells. A cutoff of 20% was chosen, reflecting the fact that a significant number of cases had 10% to 15% positive cells. With this cutoff, 67% of evaluable cases showed staining with anti–Bcl-2 in greater than 20% of the neoplastic cells and were judged to be strong positives, which is in the same range as that reported in the larger series by Hill et al10and by Hermine et al.9
Most studies, including the parent study of DNA content from which these specimens were drawn, have established proliferative activity as a prognostic indicator in the NHLs.13-16 In our previous study,16 a trend associating low proliferative activity with good early survival and very high S-phase with shortened survival was shown leading us to conclude that proliferative activity is probably predictive of survival although clinical parameters represented by the International Index proved to be more powerful prognostic indicators than %S-phase. No variable was predictive of disease-free survival or time to treatment failure. In a much smaller study by Miller15 in which the monoclonal antibody Ki-67 was used to assess proliferative activity, growth fraction was a powerful predictor of survival. In contrast, Bcl-2 has been associated with disease-free survival but not overall survival in most of the larger clinical trials.10-12 The associations between Bcl-2 immunopositvity and low proliferative activity and between proliferative activity and overall survival are not inconsistent with this finding. Bcl-2 expression may identify a subset with low proliferative activity with a predisposition to early relapse, but not necessarily a shortened survival. This subset would have a clinical behavior not unlike the indolent lymphomas. The apparent discrepancies between the reported series will need to be resolved in the context of large studies in which Bcl-2 immunostaining, Southern analysis for the t(14;18), and proliferative activity are measured on fresh tissue.
Although the numbers of specimens studied both for Bcl-2 immunopositivity and S-phase were too few to draw clinical inferences regarding prognosis, the association between BCL-2 expression and low S-phase in clinical specimens complements the results of laboratory investigations into the relationship between apoptosis and cell cycle pathways. The effect of Bcl-2 on cell cycle transition may underlie its apparent effect on chemosensitivity and clinical outcome. In the future, a combination of molecular and immunohistochemical variables may be shown to more accurately predict patient response to therapy and survival than clinical parameters. Classification of NHLs by biological characteristics such as BCL-2 expression may be of greater clinical significance than morphological categories.
Coordinated by the Eastern Cooperative Oncology Group (Robert L. Comis, MD, Chair) and supported in part by the American Cancer Society, Illinois Division, and Public Health Service Grants No. CA17145, CA23318, CA49883, CA 20365, CA2111519, CA60421, CA 32102, and CA66636 from the National Cancer Institute, National Institutes of Health, and the Department of Health and Human Services. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
Address reprint requests to Jane N. Winter, MD, Rm 1456A, Wesley Pavilion, Northwestern Memorial Hospital, 250 E Superior St, Chicago, IL 60611.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal