Abstract
Kaposi's sarcoma (KS) is an angioproliferative disease associated with infection by the human herpesvirus-8 (HHV-8). HHV-8 possesses genes including homologs of interleukin-8 (IL-8) receptor, Bcl-2, and cyclin D, which can potentially transform the host cell. However, the expression of these genes in KS tissues is very low or undetectable and HHV-8 does not seem to transform human cells in vitro. In addition, KS may not be a true cancer at least in the early stage. This indicated that besides its transforming potential, HHV-8 may act in KS pathogenesis also through indirect mechanisms. Evidence suggests that KS may start as an inflammatory-angiogenic lesion mediated by cytokines. However, little is known on the nature of the inflammatory cell infiltration present in KS, on the type of cytokines produced and on their role in KS, and whether this correlates with the presence of HHV-8. Here we show that both acquired immunodeficiency syndrome (AIDS)-KS and classical KS (C-KS) lesions are infiltrated by CD8+ T cells and CD14+/CD68+monocytes-macrophages producing high levels of γ-interferon (γIFN) which, in turn, promotes the formation of KS spindle cells with angiogenic phenotype. γIFN, in fact, induces endothelial cells to acquire the same features of KS cells, including the spindle morphology and the pattern of cell marker expression. In addition, endothelial cells activated by γIFN induce angiogenic lesions in nude mice closely resembling early KS. These KS-like lesions are accompanied by production of basic fibroblast growth factor, an angiogenic factor highly expressed in primary lesions that mediates angiogenesis and spindle cell growth. The formation of KS-like lesions is upregulated by the human immunodeficiency virus Tat protein demonstrating its role as a progression factor in AIDS-KS. Finally, γIFN and HLA-DR expression correlate with the presence of HHV-8 in lesional and uninvolved tissues from the same patients. As HHV-8 infects both mononuclear cells infiltrating KS lesions and KS spindle cells, these results suggest that HHV-8 may elicit or participate in a local immune response characterized by infiltration of CD8+ T cells and intense production of γIFN which, in turn, plays a key role in KS development.
KAPOSI'S SARCOMA (KS) is a proliferative disease of vascular origin particularly frequent and aggressive in human immunodeficiency virus (HIV-1)–infected homosexual men (acquired immunodeficiency syndrome [AIDS]-KS) as compared with classical KS (C-KS) that is rare and indolent.1-3 However, these forms have the same histopathology. In the very early stages, KS is characterized by inflammatory cell infiltration, endothelial cell activation, and angiogenesis. This is followed by the appearance of the typical spindle-shaped cells that represent a heterogeneous population dominated by activated endothelial cells mixed with macrophages and dendritic cells.4-7 In advancing lesions, spindle cells tend to become the predominant cell type, although angiogenesis remains always a prominent feature.
A new herpesvirus, termed KS-associated herpesvirus (KSHV) or human herpesvirus-8 (HHV-8), has been recently identified in KS tissues.8 In contrast with other viruses, HHV-8 has been consistently detected in all forms of KS,9-13 in peripheral blood mononuclear cells (PBMC) from the same patients, and, at a lower frequency, in AIDS patients without KS and in normal individuals, particularly in geographical areas at high risk for KS14-17(and G. Rezza et al, submitted). Other studies showed that HHV-8 infection can be predictive of KS development.18-20Thus, evidence suggests that HHV-8 may have an important role in KS pathogenesis.
HHV-8 possesses several genes acquired from the host, including homologs of macrophage inflammatory protein (MIP) (v-MIP–I and v-MIP–II), interleukin-6 (IL-6) (v-IL–6), IL-8 receptor (v-IL–8R), Bcl-2 (v-Bcl–2), and cyclin D (v-cyclin D).21-29 Most of these genes have been shown to be functional and v-IL–8R has in vitro transforming activity.21,23-26,29 This suggested that they may participate in KS cell transformation or in the induction of basic fibroblast growth factor (bFGF),23 an angiogenic factor that has a key role in KS pathogenesis.30-32 However, to exert a role in KS cell transformation, these viral genes should be expressed in the transformed cells as found in primary effusion lymphomas (PEL) and PEL-derived cell lines.21-23 In contrast, the expression of these viral genes in KS tissues is low or undetectable,21-23,26,28,33 whereas the human bcl-2 and IL-6 are expressed at very high levels in primary lesions.34,35 In addition, the only two tumor cell lines derived from KS do not contain HHV-8,36 and spindle cells derived from the lesions are latently infected, but they lose the virus on culture37-39 (and our unpublished data). Finally, HHV-8 does not seem to transform human endothelial cells, the precursors of KS spindle cells, or B cells in vitro.40
Recent findings show that, in addition to primary B cells,41 HHV-8 is present in circulating monocytes42 (and S. Colombini et al, submitted), in spindle-like macrophagic cell progenitors of the blood of KS patients43,44 and in infiltrating mononuclear cells of KS lesions including monocytes and macrophages where the virus yields a productive infection.42,45 46Altogether these observations suggest that HHV-8 may act through different mechanisms in KS pathogenesis.
Previous studies by us and others showed that KS behaves as a cytokine-mediated disease, and that, at least in early stages, KS is not a true cancer, but a hyperplastic-proliferative disease.47-49 bFGF and vascular endothelial cell growth factor (VEGF) are highly expressed in spindle cells of both AIDS-KS and C-KS, and they mediate the spindle cell growth, angiogenesis, and edema of KS30-32,50,51 (and F. Samaniego et al, submitted). Other data showed that the extracellular HIV-1 Tat protein released by infected cells can increase synergistically the angiogenensis and spindle cell growth mediated by bFGF.33,52-56 As extracellular Tat is present in AIDS-KS lesions and its receptors are highly expressed by vessels and spindle cells,32 57 this suggested that Tat may increase the frequency and aggressiveness of KS in infected individuals acting as a progression factor.
Further data suggested that inflammatory cytokines (IC) may trigger these events. Conditioned media (CM) from activated T cells (TCM) induce the production and release of bFGF and VEGF in cultured KS and/or endothelial cells58,59 (and F. Samaniego et al, submitted). In addition, TCM-treated endothelial cells acquire features similar or identical to KS cells and, as observed with AIDS-KS cells, they become responsive to the growth, invasion, and adhesion effects of Tat.54-57 TCM contain a variety of IC including tumor necrosis factor (TNF), IL-1, IL-6, oncostatin M (OM), and γ-interferon (γIFN) (see Materials and Methods section),53-59 and some of them (TNFα, IL-6, IL-1, OM) have been found in KS,60-62 suggesting a role for the immune system and in particular of host IC in the induction and progression of KS. In particular, the presence of HHV-8 productively infected cells in KS tissues suggests that the virus may trigger an inflammatory response and the expression of cytokines. However, little or nothing is known on the type of inflammatory cell infiltration, specific cytokine production, and role of these cytokines in KS pathogenesis or whether the presence of these cytokines correlates with that of HHV-8. Here we show that (1) KS lesions from both AIDS-KS and C-KS contain a prevalent CD8 T-cell infiltration and infiltration with monocytes-macrophages; (2) these cells produce IC and in particular γIFN; (3) γIFN is required to induce endothelial cells to acquire the phenotypic and functional features of KS spindle cells, both in vitro and in vivo, and to induce angiogenic KS-like lesions in nude mice whose frequency and intensity is increased by the HIV-1 Tat protein; and (4) γIFN and HLA-DR expression in tissues is associated with the presence of HHV-8.
MATERIALS AND METHODS
Immunostaining of tissues and cells.
Frozen sections from KS and uninvolved tissues were fixed in cold acetone and single- or doubly-stained by the alkaline phosphatase antialkaline phosphatase (APAAP) method alone or combined with the peroxidase antiperoxidase (PAP) method. For APAAP single method, monoclonal antibodies (MoAbs) were used directed against γIFN (1:25, Genzyme Diagnostics, Cambridge, MA), TNFα (1:200), IL-1β (1:200), HLA-DR (1:20), CD4 (1:20), CD8 (1:100), CD14 (1:100), CD68 (1:200), CD20 (1:200) (all from DAKO, Golstrup, Denmark). For double-staining experiments, the APAAP and the PAP methods were used by combining the antibodies described above with a rabbit polyclonal antibody directed against γIFN (1:200, Genzyme) as described previously.32All incubations were performed at room temperature. Briefly, for single-staining, the slides were incubated with the MoAb for 30 minutes. After washing with Tris-buffered solution (TBS), the rabbit antimouse IgG (1:25; Dako) was applied for 20 minutes and after additional washing with TBS, the slides were incubated with APAAP (mouse) complex (1:25; Dako) for 20 minutes. The second and the third steps were repeated to amplify the reactions. The reaction was developed with the Fast Red Substrate System (Dako) and slides counterstained with Mayer's hematoxylin solution (Sigma Chemical Co, St Louis, MO). For double-staining (APAAP/PAP)32 the endogenous peroxidase activity was suppressed by using Peroxidase Blocking Reagent (Dako) for 5 minutes. To reduce the background, slides were incubated with normal swine serum (1:5, Dako) for 20 minutes, followed by the addition of MoAbs. After 30 minutes of incubation and washing in TBS, the rabbit polyclonal antibody was added for 30 minutes. After washing in TBS, the slides were incubated with goat antimouse (1:25; Dako) for 30 minutes, rinsed again, and swine antirabbit (1:100; Dako) was applied for an additional 30 minutes. The slides were washed again and APAAP (mouse) complex was applied for 30 minutes, rinsed, and then PAP (rabbit) (1:400; Sigma) was applied for 30 minutes. The APAAP reaction was developed in Fast Red Substrate System (Dako) for 20 minutes and the PAP reaction was developed with 3'3 diaminobenzidin (DAB) solution for 5 minutes. The slides were counterstained as described above. The percentage of red (APAAP) or yellow-brown (PAP) positive cells alone or combined were counted separately in duplicate samples for each experiment and in five high power microscopic fields (HPMF) per slide.
bFGF staining was performed on frozen tissue sections from the sites of inoculation of mice injected with untreated, TCM-treated, or γIFN-treated human umbilical vein endothelial (HUVE) cells by using a rabbit polyclonal anti-bFGF antibody (Santa Cruz Biotechnology Inc, Santa Cruz, CA) diluted 1:20 or 1:40 in 1% phosphate-buffered saline-bovine serum albumine (PBS-BSA) and the PAP method as described above, but by using the peroxidase-antiperoxidase from Dako (1:100 dilution) as described previously.31 59
The staining of HUVE cells for CD34, vascular endothelial (VE)-cadherin, FVIII-RA, EN-4, vascular cell adhesion molecule-1 (VCAM-1), intercellular adhesion molecular-1 (ICAM-1), endothelial leukocyte adhesion molecule (ELAM), and HLA-DR was performed by the APAAP method with cytospin preparations or cells grown on gelatine-coated slides. Slides were fixed in cold acetone for 10 minutes, air dried, and then stained as described for tissue staining. The primary antibodies were applied for 20 minutes. The percentage of positive cells in duplicate samples for each experiment and in five HPMF per slide was then evaluated. The specificity and derivation of the primary antibodies used in these experiments are shown in Table 1.
Cell Marker Expression in HUVE Cells Before or After Treatment With TCM, RTCM Lacking or Containing γIFN, or γIFN Alone
| Antibody . | Specificity . | % Positive Cells . | ||||
|---|---|---|---|---|---|---|
| HUVE . | TCMHUVE . | RTCM-HUVE (−γIFN) . | RTCM-HUVE (+γIFN) . | γIFNHUVE . | ||
| CD34 | Hematopoietic precursors cells, vascular endothelial cells, fibroblasts, smooth muscle cells; monoclonal (Monosan) | 75 ± 5 | 68 ± 12 | 73 ± 8 | 62 ± 2 | 73 ± 10 |
| VE-cadherin | Endothelial cells; monoclonal-150 | 76 ± 9 | 84 ± 8 | 93 ± 1 | 86 ± 5 | 87 ± 3 |
| FVIII-RA | Endothelial cells; monoclonal (Dako) | 61 ± 8 | 13 ± 4 | 28 ± 16 | 27 ± 14 | 18 ± 13 |
| EN-4 | Endothelial cells, platelets, monocytes, granulocytes, B cells; monoclonal (Monosan) | 89 ± 6 | 52 ± 6 | 72 ± 13 | 58 ± 10 | 40 ± 5 |
| VCAM-1 | Activated endothelial cells, monocytes, dendritic cells, myoblasts; monoclonal (Amac) | 11 ± 4 | 42 ± 12 | 55 ± 6 | 42 ± 5 | 45 ± 18 |
| ICAM-1 | Activated endothelial cells, monocytes, T and B cells, dendritic cells, epithelial cells; monoclonal (Amac) | 23 ± 12 | 66 ± 13 | 96 ± 3 | 94 ± 3 | 40 ± 1 |
| ELAM-1 | Activated endothelial cells; monoclonal (Amac) | 2 ± 1 | 54 ± 8 | 65 ± 3 | 53 ± 12 | 30 ± 1 |
| HLA-DR | Activated endothelial cells, B cells, monocytes, macrophages, activated T and NK cells; monoclonal (Dako) | Neg | Neg | Neg | 2 ± 1 | 60 ± 4 |
| Antibody . | Specificity . | % Positive Cells . | ||||
|---|---|---|---|---|---|---|
| HUVE . | TCMHUVE . | RTCM-HUVE (−γIFN) . | RTCM-HUVE (+γIFN) . | γIFNHUVE . | ||
| CD34 | Hematopoietic precursors cells, vascular endothelial cells, fibroblasts, smooth muscle cells; monoclonal (Monosan) | 75 ± 5 | 68 ± 12 | 73 ± 8 | 62 ± 2 | 73 ± 10 |
| VE-cadherin | Endothelial cells; monoclonal-150 | 76 ± 9 | 84 ± 8 | 93 ± 1 | 86 ± 5 | 87 ± 3 |
| FVIII-RA | Endothelial cells; monoclonal (Dako) | 61 ± 8 | 13 ± 4 | 28 ± 16 | 27 ± 14 | 18 ± 13 |
| EN-4 | Endothelial cells, platelets, monocytes, granulocytes, B cells; monoclonal (Monosan) | 89 ± 6 | 52 ± 6 | 72 ± 13 | 58 ± 10 | 40 ± 5 |
| VCAM-1 | Activated endothelial cells, monocytes, dendritic cells, myoblasts; monoclonal (Amac) | 11 ± 4 | 42 ± 12 | 55 ± 6 | 42 ± 5 | 45 ± 18 |
| ICAM-1 | Activated endothelial cells, monocytes, T and B cells, dendritic cells, epithelial cells; monoclonal (Amac) | 23 ± 12 | 66 ± 13 | 96 ± 3 | 94 ± 3 | 40 ± 1 |
| ELAM-1 | Activated endothelial cells; monoclonal (Amac) | 2 ± 1 | 54 ± 8 | 65 ± 3 | 53 ± 12 | 30 ± 1 |
| HLA-DR | Activated endothelial cells, B cells, monocytes, macrophages, activated T and NK cells; monoclonal (Dako) | Neg | Neg | Neg | 2 ± 1 | 60 ± 4 |
Marker expression in untreated HUVE cells or in cells treated with TCM, RTCM in the presence or absence of γIFN (4 U/mL), or γIFN alone (103 U/mL). HUVE cells were cultured for 5 to 6 days and stained by immunohistochemistry as described in Materials and Methods. The results shown are the average of the percentage of positive cells from 4 independent experiments ± standard deviations.
Abbreviations: Neg, negative; NK, natural killer; HUVE, untreated HUVE cells; γIFN-HUVE, γIFN-treated HUVE cells; RTCM-HUVE, HUVE cells treated with RTCM (+γIFN, 4 U/mL) or RTCM (−γIFN); TCM-HUVE, TCM-treated HUVE cells.
Donated by E. Dejana (Institute Mario Negri, Milan, Italy).
PCR and Southern blot analysis or liquid hybridization.
High molecular weight DNA was extracted from frozen tissues using a standard phenol/chloroform procedure. Polymerase chain reaction (PCR) analysis was performed with the following sets of primers. Two sets of primers derived from the published sequence8 were used to amplify HHV-8 sequences. Set 1: (700-810) 5′ TAG CCG AAA GGA TTC CAC CAT 3′, and (1207-1228) 5′ GGA TCC GTG TTG TCT ACG TC 3′; Set 2: (112-130) 5′ TGC GAT CTG TTA GTC CGGA 3′, and (430-453) 5′ ATT CGC CAA GGA CGT ACA GCA 3′. The probe was a 45mer (nucleotides 980-1025 of the published sequences) for primers set 1, and a 51mer (nucleotides 181-232 of the published sequence) for primers set 2, respectively. Primers used for Epstein-Barr virus (EBV) amplification were: 5′ AGG CTG CCC ACC CTG AGG AT 3′, and 5′ GCC ACC TGG CAG CCC TAA AG 3′ and the probe was the internal oligonucleotide 5′ GTT GCC GCC AGG TGG CAGC 3′. Primers used for HHV-6 amplification were: 5′ GCG TTT TCA GTG TGT AGT TCG GCA G 3′ and 5′ TGG CCG CAT TCG TAC AGA TAC GGA GG 3′, and the probe was 5′ GCT AGA ACG TAT TTG CTG CAG AAC G 3′. Primers used for HHV-7 amplification were (1-26): 5′TAT CCC AGC TGT TTT CAT ATA GTA AC 3′ and (186-161) 5′ GCC TTG CGG TAG CAC TAG ATT TTT TG 3′, and the probe was (82-111) 5′ CCT AAT GAA GGC TAC TTT GAA GTA CAA ATG 3′. Primers used for cytomegalovirus (CMV) were: (2038-2057) 5′ GGT GCT CAC GCA CAT TGA TC 3′ and (2300-2281) 5′ AGA CCT TCA TGC AGA TCT CC 3′ and the probe was (2133-2162) 5′ TGA TGA CCA TGT ACG GGG GCA TCT CTC TCT 3′. Primers used for β-globin were (54-73) 5′ CAA CTT CAT CCA CGT TCA CC 3′ and (-195 through -176) 5′ GAA GAG CCA AGG ACA GGT AC 3′. PCR was performed under standard buffer conditions (1 mmol/L MgCl2) using the ampliTAQ PCR amplification kit (Perkin-Elmer-Cetus, Norwalk, CT) according to the manufacturer's instructions. After an initial denaturation of 5 minutes at 94°C, 35 cycles of denaturation (92°C for 1 minute), annealing (55°C for 2 minutes) and extension (72°C for 2 minutes) were performed on a DNA thermal cycler 480 (Perkin-Elmer-Cetus). All PCRs were subjected to a final extension of 7 minutes at 72°C. PCR products were analyzed by agarose gel fractionation and Southern blot hybridization or by liquid hybridization using internal oligonucleotides as32P-end–labeled probes. For liquid hybridization, 10 μL of amplified DNA was mixed with 1 μL of 32P-labeled oligonucleotide and 5 μL of OH1.1 buffer (66.7 mmol/L NaCl and 44 mmol/L EDTA). The sample was then overlaid with mineral oil and subjected to 5 minutes of denaturation at 94°C and 15 minutes annealing at 55°C in a Perkin Elmer Thermal Cycler 480. The product was then loaded in a 10% TB acrylamide minigel (Novex, San Diego, CA) and exposed with Kodak XOMAT film for 1 hour to 12 hours. All cases negative by Southern blot analysis were tested again by liquid hybridization.
Preparation of TCM and reconstituted TCM (RTCM).
TCM were prepared from human T-lymphotropic virus type II-infected/transformed (nonvirus-producing) CD4+ T cells as previously described.53-55 These CM contain the same cytokines produced by mitogen-activated PBL or enriched T cells from normal donors and do not contain viral proteins.53 The average concentration of these cytokines as determined by enzyme-linked immunosorbent assay (ELISA) is: IL-1α (0.5 ng/mL), IL-1β (3.5 ng/mL), IL-2 (0.3 ng/mL), IL-6 (35 ng/mL), TNFα (0.2 ng/mL), TNF-β (50 pg/mL), granulocyte-macrophage colony-stimulating factor (GM-CSF) (0.4 ng/mL), OM (0.5 to 1 ng/mL) and γIFN (150 pg/mL, corresponding to 3 to 4 U/mL of the recombinant γIFN [Boehringer Mannheim, Indianapolis, IN] used in these experiments). No bFGF is present in the CM. RTCM were prepared by combining recombinant cytokines at the concentrations described above. OM was purchased by R & D Systems (Minneapolis, MN) or obtained by B.C. Nair (Advanced BioScience Laboratories, Inc, Kensington, MD). All of the other cytokines were purchased from Boheringer Mannheim.
Cell cultures.
HUVE cells (passage 5 to 10) were cultured as previously described30-32 on gelatinized flasks in complete medium composed of RPMI 1640, 15% fetal bovine serum (FBS), 45 μg/mL of endothelial cell growth supplement (ECGS) (Collaborative Products, Bedford, MA) and 30 μg/mL of heparin (Sigma), 1% nutridoma HU (100 × solution) (Boehringer Mannheim), 1% essential amino acids (50 × solution) (GIBCO, Grand Island, NY), 1% nonessential amino acids (100 × solution) (GIBCO), 1 mmol/L of sodium pyruvate (GIBCO), 100 U/mL penicillin G-sodium, 100 mg/mL streptomycin sulfate, 0.25 mg/mL amphotericin B (GIBCO). Cytokine-treatment was performed by culturing HUVE cells for 5 to 6 days in the presence of TCM, RTCM, or γIFN.
Animal experiments.
γIFN-treated (102 U/mL), TCM-treated (1:4 dilution), or untreated HUVE cells (3 × 106 cells in 200 μL of media), were injected subcutaneously into the lower back (right side) of Balb/c nu/nu athymic mice, in the presence or in the absence of Tat (10 μg) as described previously.32 The negative control (media in which the cells were resuspended) was injected into the left side of the same mice, as previously described.31,32 Cells or media were mixed with an equal volume (200 μL) of Matrigel (Collaborative Biomedical Products) before inoculation.32Mice were killed 6 days later and the sites of injection were evaluated for the presence of macroscopic vascular lesions. Tissue samples were taken from all inoculated sites and fixed in formalin for histologic examination after hematoxylin and eosin (H & E) staining. The histologic changes observed at the site of injection, blood vessel formation, spindle cell proliferation, and edema were evaluated by comparison with the negative controls and graded according to intensity from 1 to 8 with the minimal alteration observed given a value of 1 (intensity value), as described previously.32
RESULTS
CD8+ T cells and monocytes-macrophages are the predominant inflammatory cell types of KS and produce γIFN.
To analyze the type of infiltrating immune cells and the prevalent IC production in KS, immunohistochemical analyses were performed on frozen sections from both AIDS-KS and C-KS lesions and uninvolved tissues from the same patients by using antibodies specific for CD4, CD8, CD14, CD68, CD20, and HLA-DR and for the IC IL-1β, TNFα, and γIFN.
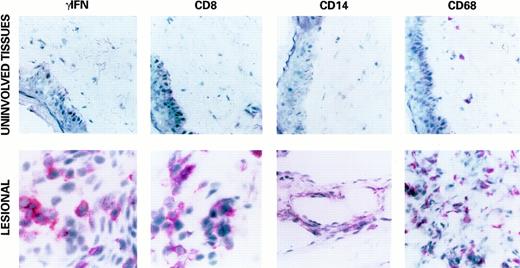
A prevalent CD8+ T-cell infiltration and infiltration with CD14+ and CD68+ monocytes-macrophages were detected in all lesions examined and were more evident in early stage lesions (Fig 1). A variable proportion of CD4+ T cells was also found, whereas B cells (CD20+) were few or absent in all lesions examined (data not shown). This cell infiltration was associated with a detectable expression of IL-1β and TNFα (data not shown), as described previously,60-62 but particularly with a high level of expression of γIFN (Fig 1). γIFN was found expressed in 100% (19/19) AIDS-KS and in 100% (3/3) C-KS lesions examined. Anti-γIFN antibodies stained mostly mononuclear cells, but also spindle-shaped cells (Fig 1). In contrast, in uninvolved tissues, only some mononuclear cells were stained (Fig 1). HLA-DR, which was evaluated to estimate the cell activation and the biologic activity of γIFN, was expressed in all of the γIFN+ KS lesions and tissues tested, and, at a low level, in one uninvolved tissue in which γIFN was not detected. Positivity for HLA-DR was also strong in vessels and endothelial cells of KS lesions.
Expression of γIFN, CD8, CD14, and CD68 in a representative KS lesion (lower panels, original magnification × 400) and uninvolved tissue (upper panels, original magnification × 1,000) by immunohistochemistry (APAAP) as described in Materials and Methods. Positivity for γIFN (red stain) was mostly observed in mononuclear cells, but also in spindle-shaped cells, as compared with uninvolved tissues. A strong increase in CD8+ and CD14+/CD68+ infiltrating cells, often with a subendothelial localization, was also observed in KS lesions, whereas these infiltrating cells are rare in uninvolved tissues. Similar results were obtained in all KS lesions analyzed, but were prevalent in early stage lesions.
Expression of γIFN, CD8, CD14, and CD68 in a representative KS lesion (lower panels, original magnification × 400) and uninvolved tissue (upper panels, original magnification × 1,000) by immunohistochemistry (APAAP) as described in Materials and Methods. Positivity for γIFN (red stain) was mostly observed in mononuclear cells, but also in spindle-shaped cells, as compared with uninvolved tissues. A strong increase in CD8+ and CD14+/CD68+ infiltrating cells, often with a subendothelial localization, was also observed in KS lesions, whereas these infiltrating cells are rare in uninvolved tissues. Similar results were obtained in all KS lesions analyzed, but were prevalent in early stage lesions.
To identify the cell types producing γIFN, double-staining experiments were performed with anti-γIFN and anti-CD8, -CD4, -CD14, -CD68, or anti-FVIII–RA antibodies. The results indicated that the cells producing γIFN (double positive cells) were mostly of the CD8+ phenotype (Fig 2). Spindle-shaped cells producing γIFN had a CD14+ or CD68+ phenotype, whereas endothelial cells (FVIII-RA+) in vessels did not stain for γIFN (Fig 2).
Expression of γIFN by CD8+, CD14+, or CD68+ cells infiltrating KS lesions. Examples of double-staining by immunohistochemistry (APAAP/PAP) of γIFN and CD8, γIFN, and CD14 or CD68, γIFN, and FVIII-RA in a representative KS lesion. Similar results were obtained with other specimens. CD8+ cells (red stain) coexpressing γIFN (brown stain) had a mononuclear morphology (upper right panel), whereas anti-CD14 or anti-CD68 antibodies (red stain), recognizing monocytes-macrophages, costain γIFN+ (brown stain) spindle-shaped cells (lower left and right panels). In contrast, anti-γIFN antibodies (brown stain) do not stain FVIII-RA+ vessels (red stain, upper right panel), but γIFN+ cells are often localized in the proximity of vessels.
Expression of γIFN by CD8+, CD14+, or CD68+ cells infiltrating KS lesions. Examples of double-staining by immunohistochemistry (APAAP/PAP) of γIFN and CD8, γIFN, and CD14 or CD68, γIFN, and FVIII-RA in a representative KS lesion. Similar results were obtained with other specimens. CD8+ cells (red stain) coexpressing γIFN (brown stain) had a mononuclear morphology (upper right panel), whereas anti-CD14 or anti-CD68 antibodies (red stain), recognizing monocytes-macrophages, costain γIFN+ (brown stain) spindle-shaped cells (lower left and right panels). In contrast, anti-γIFN antibodies (brown stain) do not stain FVIII-RA+ vessels (red stain, upper right panel), but γIFN+ cells are often localized in the proximity of vessels.
γIFN is the principal inducer of the KS spindle cell phenotype.
Previous observations indicated that KS spindle cells represent a heterogeneous population dominated by activated endothelial cells.4-7,63-68 IC and in particular γIFN are known to have profound effects on endothelial cells and to induce changes generally described as activation,69-71 which are the same phenotypic changes found in KS lesions. To investigate the role of IC and of γIFN in these changes, specific endothelial cell markers and activation molecules were analyzed after culture of HUVE cells in the presence of TCM that contain the same IC expressed in KS tissue. These experiments were also performed with RTCM that was obtained by adding together recombinant cytokines at the same concentration measured in TCM with or without γIFN, or in the presence of γIFN alone (103 U/mL) (Table 1).
Similar levels of CD34 and VE-cadherin were observed in HUVE cells independently from the treatment. On the contrary, all cytokine combinations (TCM, RTCM) and γIFN alone downregulated FVIII-RA and EN-4 expression (Table 1). This same pattern of marker expression, including FVIII-RA downregulation, is found in KS cells both in vivo and in vitro,55,68 and both IL-1 and γIFN can downregulate FVIII-RA expression.55,71,72 TCM and RTCM also increased the expression of VCAM-1, ICAM-1, and ELAM-1 as found for KS cells.55,67,68 γIFN alone had a similar effect, although less intense than in the presence of combined cytokines (Table 1). In addition, γIFN-treated cells expressed HLA-DR, another marker found to be expressed in spindle cells and vessels of KS; in contrast, cells exposed to TCM or RTCM stained negative due to the counteraction of IL-1.73
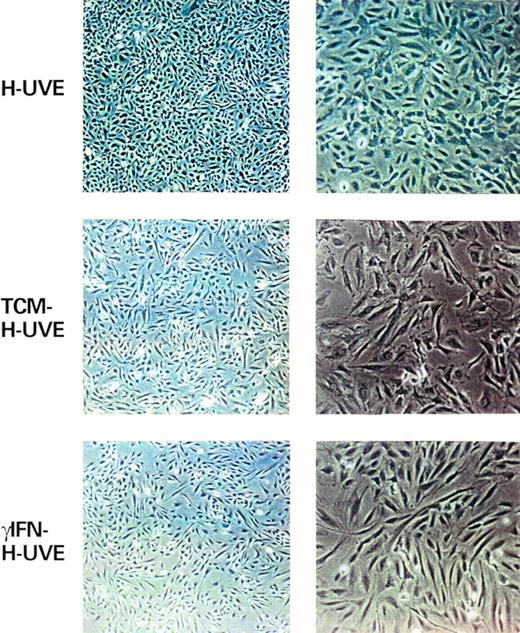
Finally, as previously found with other cell types74,75TCM-treated or γIFN-treated HUVE cells acquired a typical spindle morphology indistinguishable from that of KS cells (Fig 3). Thus, γIFN can induce phenotypic changes similar or identical to those found in spindle cells in vitro and in most spindle cells of the lesions. However, this effect is maximal in the presence of other IC that contribute to these changes directly or by increasing γIFN function.69 71
γIFN and TCM induce HUVE cells to acquire a spindle morphology. Shown are HUVE cells (left panels, original magnification × 40; right panels, original magnification × 100) cultured under standard conditions (HUVE) or after 6 days of culture in the presence of TCM (TCM-HUVE) or 103 U/mL of γIFN (γIFN-HUVE).
γIFN and TCM induce HUVE cells to acquire a spindle morphology. Shown are HUVE cells (left panels, original magnification × 40; right panels, original magnification × 100) cultured under standard conditions (HUVE) or after 6 days of culture in the presence of TCM (TCM-HUVE) or 103 U/mL of γIFN (γIFN-HUVE).
γIFN induces endothelial cells to acquire angiogenic properties and to induce KS-like lesions in nude mice.
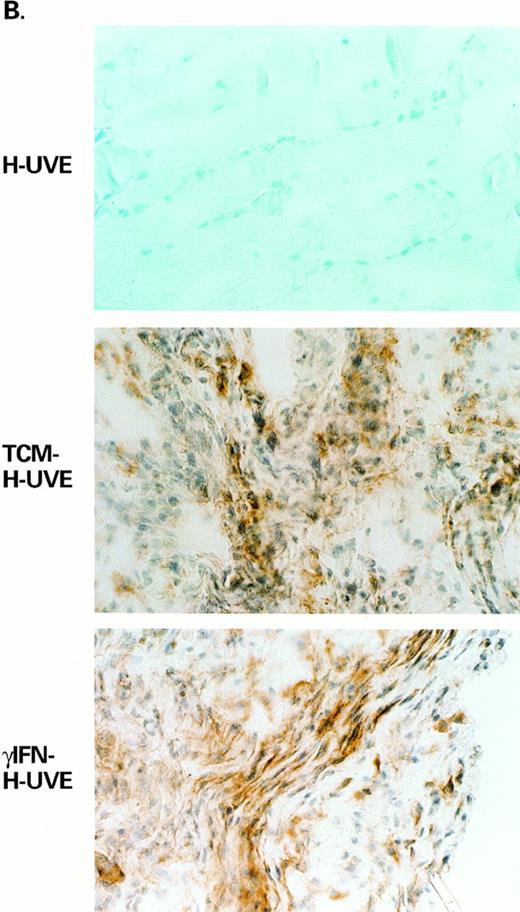
Inoculation of cultured KS spindle cells in nude mice induces vascular lesions of mouse cell origin closely resembling early KS.31,32,76 These KS-like lesions develop in response to the cytokines produced by AIDS-KS spindle cells, such as bFGF, which mediates angiogenesis and spindle cell growth, and VEGF, which synergizes with bFGF in inducing angiogenesis and edema30-32,50 (and F. Samaniego et al, submitted). Angiogenic cytokine production, on the other hand, is induced in vitro in KS cells or endothelial cells by TCM or IC50,58,59 (and F. Samaniego et al, submitted). To determine whether γIFN alone could induce normal endothelial cells to acquire the capability to promote KS-like lesions, untreated, TCM-treated, or γIFN-treated HUVE cells were inoculated in nude mice (Fig 4 [see page 959], Table 2). γIFN-treated or TCM-treated HUVE cells induced macroscopic vascular lesions in 41% and 59% of the inoculated mice, respectively. Histologic alterations typical of KS such as angiogenesis, spindle cell growth, and edema were present in 70% to 82% and 100% of the mice inoculated with γIFN-treated or TCM-treated cells, respectively. In contrast, no lesions were induced by untreated HUVE cells (Fig 4A, Table 2). Tissue samples from the sites inoculated with TCM-treated, γIFN-treated cells, or from untreated cells were then analyzed for bFGF expression by immunohistochemistry (Fig 4B). No bFGF was detected in sites inoculated with untreated HUVE cells, in contrast, high levels of bFGF were found in sites inoculated with both TCM-treated or γIFN-treated HUVE cells. These results indicated that γIFN induces normal endothelial cells to acquire angiogenic, KS-promoting activity due to induction of bFGF whose production is activated by γIFN in cultured endothelial cells.59 In addition, the presence of other IC as in TCM increased the angiogenic effect of γIFN consistent with in vitro data of synergistic effects of combined γIFN, IL-1, and TNF on bFGF production.58 59 As both γIFN and bFGF are expressed in KS lesions (from all forms of KS), these data suggest that these mechanisms are operative in vivo.
γIFN-treated or TCM-treated HUVE cells induce KS-like lesions in nude mice. Lesion formation is associated with expression of bFGF. (A) Shows examples of the histopathology (H & E staining, original magnification × 400) and (B) shows bFGF expression by immunohistochemistry in the same tissues from mice inoculated with untreated HUVE cells (HUVE), TCM-treated HUVE cells (TCM-HUVE), or γIFN-treated HUVE cells (γIFN-HUVE), respectively.
γIFN-treated or TCM-treated HUVE cells induce KS-like lesions in nude mice. Lesion formation is associated with expression of bFGF. (A) Shows examples of the histopathology (H & E staining, original magnification × 400) and (B) shows bFGF expression by immunohistochemistry in the same tissues from mice inoculated with untreated HUVE cells (HUVE), TCM-treated HUVE cells (TCM-HUVE), or γIFN-treated HUVE cells (γIFN-HUVE), respectively.
γIFN-Treated or TCM-Treated HUVE Cells Induce in Nude Mice Vascular Lesions Closely Resembling KS That Are Increased by the HIV-1 Tat Protein
| Cells . | Treatment . | Lesion* . | Angiogenesis† . | Spindle Cells† . | Edema† . |
|---|---|---|---|---|---|
| HUVE | — | 0% (12) | 0% (0) | 0% (0) | 0% (0) |
| HUVE | TCM | 59% (17) | 100% (5) | 100% (6) | 100% (5) |
| HUVE | γIFN | 41% (17) | 70% (2) | 82% (3) | 82% (4) |
| HUVE | TCM + Tat | 100% (10) | 100% (8) | 100% (8) | 100% (5) |
| HUVE | γIFN + Tat | 60% (15) | 73% (3) | 100% (4) | 100% (5) |
| Cells . | Treatment . | Lesion* . | Angiogenesis† . | Spindle Cells† . | Edema† . |
|---|---|---|---|---|---|
| HUVE | — | 0% (12) | 0% (0) | 0% (0) | 0% (0) |
| HUVE | TCM | 59% (17) | 100% (5) | 100% (6) | 100% (5) |
| HUVE | γIFN | 41% (17) | 70% (2) | 82% (3) | 82% (4) |
| HUVE | TCM + Tat | 100% (10) | 100% (8) | 100% (8) | 100% (5) |
| HUVE | γIFN + Tat | 60% (15) | 73% (3) | 100% (4) | 100% (5) |
Reported is the percentage of mice developing macroscopic vascular lesions and the percentage of mice developing histologic alterations. HUVE cells were treated with γIFN (102 U/mL) or TCM (1:4) and 3 × 106 cells were inoculated in nude mice in the presence or in the absence of Tat (10 μg) as described in Materials and Methods. Mice were killed 6 to 7 days after inoculation and tissue slides examined after H & E staining and graded as described previously.32 Inoculation of Tat alone did not induce lesions or histologic alterations, as described previously.32
Percentage of mice developing macroscopic vascular lesions (size ranging from 4 × 5 to 7 × 7 mm). Parenthesis show the number of inoculated mice.
Percentage of mice developing histologic alterations. Parenthesis show the average “intensity value” for each histopathologic feature observed per each experimental condition.
HIV-1 Tat protein increases the KS-like forming activity of endothelial spindle cells induced by γIFN or TCM.
Previous data indicated that the Tat protein of HIV-1 can increase the frequency and aggressiveness of KS in HIV-1–infected individuals32 and may act as a progression factor. In fact, inoculation of mice with Tat alone has little or no effect. However, when Tat is injected in the presence of suboptimal amounts of bFGF, synergistic angiogenic KS-promoting effects are observed and a higher number of mice develop lesions as compared with injection of bFGF alone.32 These are due to the enhancement by Tat of endothelial cell growth, migration, and invasion induced by bFGF and to bFGF-induced expression of the integrins α5β1 and αvβ3 that function as the receptors for Tat.32,57 Thus, bFGF is required for the in vivo effect of Tat. Because TCM or γIFN induce production of bFGF (Fig 4B) and expression of the same integrins57 (and data not shown), this suggested that Tat could exert its effect on KS lesion formation. To investigate this, mice were inoculated with γIFN-treated or TCM-treated cells in the presence of Tat. As shown in Table 2, Tat increased the number of mice developing lesions from 41% to 60% with γIFN-treated cells and from 59% to 100% with TCM-treated cells, respectively. In addition, Tat enhanced each histologic alteration induced by treated cells (Table 2). Thus, Tat can augment the angiogenic activity of TCM- or γIFN-treated endothelial cells and this results in enhancing effects on KS lesion formation. This effect is maximal in the presence of other IC as shown by the more potent effect of Tat on KS-like lesions induced by TCM-treated cells. As Tat is relesased by HIV-1 infected cells52,56,57,77 and is present in AIDS-KS lesions,32 these data support its role as a progression factor for HIV-1–infected individuals and indicate that γIFN alone or, more efficiently, in combination with other IC renders the tissues responsive to the effect of Tat.
HHV-8 infection and expression of γIFN and HLA-DR in AIDS-KS and C-KS.
Herpesviruses are known to activate CD8 T cells and to induce production of γIFN.78-82 Thus, the presence of HHV-8 in all forms of KS suggested that it may induce or contribute to the local immune response and inflammatory cell infiltration that leads to production of cytokines and in particular of γIFN. To verify whether HHV-8 is detectable in the KS lesions and uninvolved tissues examined for γIFN and whether it is the prevalent infectious agent as compared with other herpesviruses, the same tissues were analyzed by regular PCR and Southern blot or liquid hybridization for the presence of HHV-8, EBV, HHV-6, HHV-7, and CMV. β-Globin was used to confirm that the DNA extracted from tissues was amplifiable. As shown in Table 3, HHV-8 was detected in the majority (86%) of both forms of KS analyzed (18/21). Specifically, HHV-8 sequences were amplified in 89% of AIDS-KS (16/18) and 67% (2/3) of C-KS lesions examined. In addition, 40% (2/5) of the uninvolved tissues examined were positive for HHV-8 specific amplification. When the PCR data were compared with the results of the γIFN and HLA-DR staining, we observed that 100% of HHV-8+ KS lesions examined expressed γIFN (18/18) and HLA-DR (14/14), respectively. In contrast, only 14% (3/21) and 12% (2/16) of the γIFN+and HLA-DR+ lesions, respectively, were negative for HHV-8 sequences.
Expression of γIFN and HLA-DR in AIDS-KS, C-KS Lesions, and Uninvolved Tissues and Correlation With the Presence of HHV-8 and EBV
| Specimen . | Average % (range) γIFN . | Positive Cells HLA-DR* . | PCR . | |
|---|---|---|---|---|
| HHV-8 . | EBV . | |||
| AIDS-KS | ||||
| 1 skin | 45 (41-49) | 73 (70-75) | ND | ND |
| 2 skin | 32 (27-36) | 50 (48-52) | + | + |
| 3 skin | 11 (10-11) | 62 (58-65) | + | − |
| 4 skin | 42 (33-50) | 80 (75-84) | + | − |
| 5 skin | 50 (34-50) | 80 (80-81) | + | − |
| 6 skin | 30 (28-32) | 55 (49-61) | + | − |
| 7 skin | 28 (26-30) | 59 (55-62) | + | − |
| 8 skin | 43 (41-44) | 47 (36-65) | + | + |
| 9 skin | 29 (19-39) | 45 (43-64) | + | − |
| 10 skin | 12 (7-17) | 45 (43-64) | + | − |
| 11 skin | 15 (11-21) | ND | − | − |
| 12 skin | 39 (38-40) | 35 (29-41) | − | − |
| 13 skin | 9 (3-15) | ND | + | − |
| 14 skin | 45 (40-50) | 64 (58-70) | + | + |
| 15 skin | 40 (40-40) | ND | + | ND |
| 16 skin | 36 (25-49) | 44 (38-50) | + | ND |
| 17 skin | 35 (29-41) | 47 (38-56) | + | ND |
| 18 skin | 28 (13-49) | ND | + | ND |
| 19 lymphnode | 40 (39-40) | 69 (66-72) | + | + |
| Classical KS | ||||
| 1 skin | 43 (41-60) | 30 (30-31) | − | + |
| 2 skin | 26 (25-27) | 19 (17-21) | + | − |
| 3 skin | 5 (5-7) | ND | + | ND |
| Uninvolved | ||||
| 1 skin | 2 (2-2) | 23 (21-25) | − | ND |
| 2 skin | 5 (5-5) | 19 (19) | − | ND |
| 3 skin | 10 (10-11) | 32 (28-37) | + | ND |
| 4 skin | 12 (7-17) | 41 (32-51) | + | ND |
| 5 skin | 0 | 11 (11-11) | − | ND |
| Specimen . | Average % (range) γIFN . | Positive Cells HLA-DR* . | PCR . | |
|---|---|---|---|---|
| HHV-8 . | EBV . | |||
| AIDS-KS | ||||
| 1 skin | 45 (41-49) | 73 (70-75) | ND | ND |
| 2 skin | 32 (27-36) | 50 (48-52) | + | + |
| 3 skin | 11 (10-11) | 62 (58-65) | + | − |
| 4 skin | 42 (33-50) | 80 (75-84) | + | − |
| 5 skin | 50 (34-50) | 80 (80-81) | + | − |
| 6 skin | 30 (28-32) | 55 (49-61) | + | − |
| 7 skin | 28 (26-30) | 59 (55-62) | + | − |
| 8 skin | 43 (41-44) | 47 (36-65) | + | + |
| 9 skin | 29 (19-39) | 45 (43-64) | + | − |
| 10 skin | 12 (7-17) | 45 (43-64) | + | − |
| 11 skin | 15 (11-21) | ND | − | − |
| 12 skin | 39 (38-40) | 35 (29-41) | − | − |
| 13 skin | 9 (3-15) | ND | + | − |
| 14 skin | 45 (40-50) | 64 (58-70) | + | + |
| 15 skin | 40 (40-40) | ND | + | ND |
| 16 skin | 36 (25-49) | 44 (38-50) | + | ND |
| 17 skin | 35 (29-41) | 47 (38-56) | + | ND |
| 18 skin | 28 (13-49) | ND | + | ND |
| 19 lymphnode | 40 (39-40) | 69 (66-72) | + | + |
| Classical KS | ||||
| 1 skin | 43 (41-60) | 30 (30-31) | − | + |
| 2 skin | 26 (25-27) | 19 (17-21) | + | − |
| 3 skin | 5 (5-7) | ND | + | ND |
| Uninvolved | ||||
| 1 skin | 2 (2-2) | 23 (21-25) | − | ND |
| 2 skin | 5 (5-5) | 19 (19) | − | ND |
| 3 skin | 10 (10-11) | 32 (28-37) | + | ND |
| 4 skin | 12 (7-17) | 41 (32-51) | + | ND |
| 5 skin | 0 | 11 (11-11) | − | ND |
Frozen sections from involved or uninvolved skin and a lymph node of AIDS-KS and C-KS patients were stained by the APAAP method using MoAbs directed against γIFN and HLA-DR. Reported are the average percent of positive cells from 5 HPMF (100× magnification) per slide and in parenthesis is shown the range of positivity from different areas of the lesions. In KS tissues γIFN positive cells were of mononuclear and spindle-shaped morphology. In control tissues γIFN positivity was in mononuclear cells. In KS tissues HLA-DR was strongly expressed in vessels, spindle cells and mononuclear cells. A total of 1 to 10 μg of tissue DNA was analyzed by PCR for HHV-8 and EBV DNA sequences. Each case was tested at least twice. Negative cases resulted negative also by using 10 μg DNA and different tissue sections. HHV-8 negative cases were consistently negative independently from the amount of DNA used and were early KS lesions. All the PCR negative samples were positive for β-globin amplification. PCR analysis was also performed for HHV-6, HHV-7, and CMV. These herpesviruses resulted absent or present at a low frequency (HHV-6 [5%] or CMV [8%]) in association with HHV-8.
Abbreviation: ND, not done.
Positivity for HLA-DR was also present in vessels.
EBV was detected in 4/14 AIDS-KS (28%) and in 1/3 C-KS (33%) lesions examined (Table 3). For AIDS-KS, all cases positive for EBV were also positive for HHV-8 and expressed both γIFN and HLA-DR. Surprisingly, for C-KS, the case positive for EBV was consistently negative for HHV-8 by unnested PCR, but it was still positive for γIFN or HLA-DR expression. No other herpesviruses except for a small percentage of HHV-6 (5%) and CMV (8%) were detected in the lesions examined and they were associated with the presence of HHV-8 (data not shown).
Finally, γIFN and HLA-DR were both found to be expressed in 2/2 (100%) HHV-8+ uninvolved tissues. In addition, γIFN was expressed in 2/3 (66%) and HLA-DR in 3/3 (100%) HHV-8- uninvolved tissues, respectively. However, the levels of expression of both markers and particularly of γIFN were lower than in HHV-8+ tissues (Table 3). Thus, γIFN is highly expressed by CD8+ and CD14+/CD68+ cells infiltrating the lesions from both AIDS-KS and C-KS that contain HHV-8 and/or EBV specific sequences and these events are associated with endothelial cell activation, as shown by HLA-DR staining of spindle cells and vessels, which are also positive for other activation markers such as ELAM-1, ICAM-1, and VCAM-1.68
DISCUSSION
The consistent presence of HHV-8 in tissues from patients with all forms of KS8-12 and the high rate of infection in individuals at risk of KS indicate that this virus plays an important role in KS development.18,83-85 The specific role of HHV-8 in KS pathogenesis, however, has not yet been delineated. HHV-8 possesses genes encoding for potential transforming proteins, in particular cyclin D, IL-8R, and bcl-2. Other viral genes encode for proteins, like v-IL–6, that can act with paracrine mechanisms on neighbor cells.21-23,86 These viral genes have been shown to be functional and some display a transforming activity in vitro. In addition, their homologs are present in herpesviruses with known or suggested transforming activities, like EBV, herpesvirus Sahiri (HVS) and CMV.87-89 These observations suggested that these viral products may play a key role in the pathogenesis of KS. However, HHV-8 cyclin D, IL-8R, and IL-6 are expressed at very low levels or are undetectable in KS tissues.21-23,26,28,33 In addition, it is unclear whether these genes contribute to the transforming activity of EBV and HVS whose transforming functions are encoded by other genes.90-92 Furthermore, EBV-bcl–2 is expressed in cells undergoing lytic infection after reactivation from latency.93 Consistent with this, HHV-8 viral homologs are induced at high levels in PEL cell lines after reactivation of lytic infection by TPA.21,33 Because only a small percentage of HHV-8–infected cells of monocytic-macrophagic origin undergo viral lytic infection in KS tissues,33,42,45 the low levels of expression of v-IL–8R, v-cyclin D, and v-IL–6 are consistent with these functions being expressed in this small percentage of cells. In contrast, the human IL-6 and bcl-2 are expressed at high levels in KS.34,35 In addition, HHV-8 is absent in the only two KS tumor cell lines obtained to date,36 and although endothelial spindle cells present in KS lesions are latently infected with HHV-8, they lose the virus on culture33,37-39,94-96(and our unpublished data). Finally, HHV-8 does not seem to transform cells in vitro.40
It appears therefore conceivable that HHV-8 may act in KS pathogenesis also through indirect mechanisms, for example by activating the expression of host molecules able to induce the KS spindle cell phenotype and the cascade of events leading to KS lesion formation. This is also in agreement with experimental and clinical data indicating that, at least in early stage, KS is not a true cancer, but an hyperplastic angiogenic proliferation that may regress.47-49 These considerations prompted us to identify host factors triggering KS formation and to investigate their correlation with HHV-8 infection.
Our results show that γIFN may play a pivotal role in KS development. γIFN is strongly expressed in both AIDS-KS and C-KS lesions and in HHV-8+ uninvolved tissues from the same patients. γIFN expression is associated with endothelial cell activation, as indicated by the expression of HLA-DR and adhesion molecules in vessels and spindle cells of the lesions. Although it is mostly produced by infiltrating CD8+ T cells, γIFN is also expressed by spindle cells of macrophage origin with a subendothelial localization, in agreement with previous studies with macrophages.97,98Data from the report by Sirianni et al99further support these findings by showing that a prevalent CD8+ T-cell infiltration is present in both AIDS-KS and C-KS lesions, and that PBMC, tumor infiltrating lymphocytes, and spindle cells of macrophage origin cultured from KS lesions produce prevalently γIFN.
γIFN induces the formation of spindle cells with angiogenic activity. Specifically, γIFN promotes phenotypical and functional changes and activities in endothelial cells closely resembling KS spindle cells including the angiogenic activity and the angiogenic synergy with Tat. In fact, γIFN alone is sufficient to induce a modulation of marker expression that is similar or identical to the pattern of markers expressed by KS spindle cells and it is accompanied by the acquisition of the typical spindle morphology. In addition, when normal endothelial cells are treated with TCM or γIFN alone, they acquire the capability of inducing KS-like lesions and histologic alterations of mouse cell origin, which are indistinguishable from those induced by KS cells. As for KS cells, this is associated with the upregulation of bFGF production in the inoculated mice, as shown in vitro58,59and it is consistent with the presence of high levels of expression of bFGF in all forms of KS.32 Finally, these effects are increased synergistically by Tat demonstrating its role as a progression factor in AIDS-KS, in fact, bFGF and Tat are both present in AIDS-KS lesions and Tat increases the KS-forming activity of bFGF.32 Further, Tat can amplify HHV-8 viral load100 and can activate a further increase of IC (reviewed in Chang et al101). All of the effects of γIFN are potentiated by the presence of additional IC that increase γIFN function or synergize with γIFN. For example, IL-1 and TNF synergize with γIFN in activating bFGF expression and release.58,59Similar enhancing effects are observed for adhesion molecule expression and spindle morphology.69-72,74 75 Thus, although γIFN alone is sufficient, other IC present in KS, such as IL-1 and TNFα, cooperate with γIFN to induce the histological and phenotypical features of the lesion.
γIFN and HLA-DR expression correlate with the presence of HHV-8 in involved or uninvolved tissues. Data from the accompanying paper by Sirianni et al99 show that the infiltration of CD8+ T cells and the presence of macrophages producing γIFN is associated with that of HHV-8 in PBMC, KS tissues, and macrophagic spindle cells cultured from KS lesions of the same patients. The blood of these patients contains spindle-like macrophagic cells that are infected by HHV-8.43,44 This is consistent with recent data indicating HHV-8 lytic infection in mononuclear cells infiltrating KS lesions.42,45 These data therefore suggest that an immune response to HHV-8–infected cells present in tissues triggers or amplifies KS development through induction of γIFN. It is remarkable that one early C-KS lesion expressing both γIFN and HLA-DR was negative for HHV-8 (or contained viral DNA in such a low amount to prevent its detection by unnested PCR), but it was infected by EBV. In fact, EBV, as well as other herpesviruses, are known to activate CD8+ T cells and to induce γIFN production.78-82 Indeed, an oligoclonal expansion of CD8 T+ cells has been described following EBV infection in patients with HIV and in macaques infected with the simian immunodeficiency virus.82,102,103 Although γIFN and HLA-DR expression are increased in HHV-8+ versus HHV-8- tissues, a low production of γIFN is also found in HHV-8- uninvolved tissues from KS patients, suggesting that γIFN expression may precede a detectable HHV-8 infection. As γIFN has also been shown to act as a major mediator in recruiting cells into the skin,104 this may represent a process to increase the tissue localization of HHV-8–infected cells, and it may explain why γIFN detection can precede HHV-8 detection. This would be in agreement with recent evidence that HHV-8 prevalence and viral load can increase with lesion progression.37 105
In conclusion, although other IC may cooperate in the pathogenesis of KS, γIFN seems to play a major role in KS development and this may be in response to HHV-8 infection. In support of this concept are studies that have shown that unstimulated106 or activated107 purified blood mononuclear cell cultures from HIV-1–infected homosexual men with documented early phase infection produce more γIFN than the seronegative controls. Furthermore, CD8 activation, generally accompanied by γIFN production, is higher in HIV-1 seronegative homosexual men than in healthy donors, as documented by the increased serum levels of CD8 and soluble ICAM in these individuals.108,109 These patients are also infected by HHV-8 and are at high risk of KS development. Finally, the administration of γIFN to KS patients has resulted in disease progression.110 111 Although it remains to be determined whether HHV-8 itself can induce a CD8+ T-cell activation and consequent γIFN production, this may represent a pathway by which this virus can trigger or amplify the development of KS.
ACKNOWLEDGMENT
We thank E. Dejana (Istituto di Ricerche Farmacologiche “Mario Negri,” Milano, Italy) for the anti–VE-cadherin MoAbs; B.C. Nair (Advanced BioScience Laboratories, Inc, Kensington, MD) for OM; P. Secchiero (Institute of Human Virology, Baltimore, MD) for the HHV-6 and HHV-7 PCR primers; V. Kao (Laboratory of Tumor Cell Biology [LTCB], NCI) for technical help; G. Barillari (Department of General Pathology, II University of Rome), P. Verani (Laboratory of Virology, Istituto Superiore di Sanità, Rome) for helpful discussions, and Angela Lippa for editorial assistance.
Supported in part by a grant from Associazione Italiana Ricerca sul Cancro (AIRC; Milan) and from the IX AIDS Project from the Ministry of Health, Rome, Italy. V.F. was partially supported by Associazione Nazionale per la Lotta contro l'AIDS, Ministry of Health in Rome, Italy.
Address reprint requests to Barbara Ensoli, MD, PhD, Laboratory of Virology, Istituto Superiore di Sanità, Viale Regina Elena 299, 00161 Rome, Italy.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal