Abstract
Stem cell factor (SCF) binding to the c-kit receptor triggers homodimerization and intermolecular tyrosine phosphorylation of the c-kit receptor, thus initiating signal transduction. Receptor dimerization is a critical early step in this process. Prior biochemical studies of c-kit receptor dimerization have mainly used affinity cross-linking techniques, which are beset with problems including low efficiency of cross-linking and the usual requirement for radiolabeled SCF to detect the cross-linked complex. We used the fluorescence resonance energy transfer (FRET) technique to examine the effects of SCF and other hematopoietic cytokines on c-kitreceptor dimerization. The nonneutralizing anti–c-kit receptor monoclonal antibody 104D2 was directly conjugated to fluorescein isothiocyanate (FITC) or to the carbocyanine dye Cy3 and used to label cytokine-responsive human hematopoietic cell lines. The ability of SCF to induce c-kit receptor dimerization was assessed by flow cytometric analysis of FRET between the donor fluorochrome FITC and the acceptor fluorochrome Cy3. SCF induced a dose-dependent increase inc-kit receptor dimerization that correlated well with the concentrations of SCF required to stimulate cell proliferation. Receptor dimerization was detectable within 3 minutes after the addition of SCF and was maximal 30 minutes after the addition of SCF. Confocal microscopy showed redistribution of the c-kit receptor (from a diffuse distribution on the cell surface to “caps” at one end of the cell) within 3 minutes after SCF addition, followed by receptor internalization. Reappearance of the c-kit receptor on the cell surface required new protein synthesis, suggesting that thec-kit receptor is not recycled to the cell surface after internalization. Finally, erythropoietin (Epo), but not the structurally and functionally related cytokine thrombopoietin (Tpo), stimulated c-kit receptor dimerization detectable by FRET, and tyrosine phosphorylation of the c-kit receptor. These results suggest that exposure to Epo can activate the c-kit receptor and provide further evidence for cross-talk between the Epo andc-kit receptors in human hematopoietic cell lines. Studies with progeny of burst-forming unit-erythroid (BFU-E) suggest that the FRET technique is sufficiently sensitive to detectc-kit receptor dimerization on normal human hematopoietic cells.
STEM CELL FACTOR (SCF) is a growth factor that stimulates the survival, proliferation, and differentiation of hematopoietic cells.1 SCF also promotes mast cell production and function and plays a role in the development of melanocytes, germ cells, and intestinal pacemaker cells. Endothelial cells, fibroblasts, and certain epithelial cells constitutively produce soluble and transmembrane forms of SCF, and the soluble form of SCF circulates in the blood as a noncovalently associated dimer.2
SCF initiates its biologic effects by binding to the c-kitreceptor,3,4 a member of the type III receptor tyrosine kinase family.5 The c-kit receptor is encoded at the murine W locus. The dominant negative effect of mutations at the W locus that ablate or diminish c-kit receptor tyrosine kinase activity yet permit cell surface expression of the impuissant protein first suggested that the c-kit receptor might be activated by homodimerization and autophosphorylation.6-8 Enforced expression of kinase-deficient W42c-kit receptor in transgenic mice recapitulated some of the phenotypic abnormalities found in mice with naturally occurring mutations at the W locus and provided support for this model of c-kit receptor activation.9
Dimerization of the c-kit receptor in the presence of SCF has been directly demonstrated by affinity cross-linking techniques10-12 and by biophysical methods.13,14 The current concept is that binding of dimeric SCF triggers c-kit receptor homodimerization and intermolecular tyrosine phosphorylation of the receptor, creating a molecular scaffolding that contains docking sites for SH2-containing signal transduction molecules.15 16 Thus, c-kitreceptor dimerization is a key initial step in the SCF signal transduction process.
The biochemical10-12 and biophysical13,14studies of c-kit receptor dimerization do not provide an optimal way to examine a dynamic process such as assembly of receptor subunits on an intact cell membrane. Fluorescence resonance energy transfer (FRET) between a donor fluorochrome and an acceptor fluorochrome is exquisitely dependent on the distance separating the two fluorochromes, and thus can serve as a “molecular ruler”17,18 within the 10 to 75 angstrom range. The FRET technique has been used to investigate assembly of the T-cell antigen receptor complex,19,20 to demonstrate interleukin-1 (IL-1) receptor dimerization after IL-1 binding,21 and to study epidermal growth factor (EGF) receptor oligomerization,22,23 among other uses.24
We wished to define in greater detail the kinetics of c-kitreceptor dimerization in intact cells. Because of the importance of both SCF and erythropoietin (Epo) for normal erythropoiesis and the accumulating evidence for cross-talk between the c-kit and Epo receptors,25-27 we also wished to determine whether exposure to Epo could induce c-kit receptor dimerization. To achieve these goals, we used the FRET technique to permit assessment ofc-kit receptor dimerization without the use of affinity cross-linking. We initially showed that SCF-induced c-kitreceptor dimerization can be detected by FRET between monoclonal anti–c-kit receptor antibodies labeled with the donor fluorochrome fluorescein isothiocyanate (FITC) and the acceptor fluorochrome Cy3. Dimerization of the c-kit receptor was detectable within 3 minutes of SCF addition. We then applied this technique to determine whether other cytokines could inducec-kit receptor dimerization. The results suggest that Epo, but not the structurally and functionally similar cytokine thrombopoietin (Tpo), or the myeloid cytokine granulocyte-macrophage colony-stimulating factor (GM-CSF), can activate the c-kitreceptor in cytokine-responsive cell lines. Minutes after the addition of SCF, redistribution of the c-kit receptor into “caps” on the cell surface occurs. Moreover, the c-kit receptor remains dimerized after internalization of the ligand-receptor complex and does not recycle to the cell surface.
MATERIALS AND METHODS
Cells.
The SCF-responsive human hematopoietic cell line MO7e28,29was maintained in Iscove's modified Dulbecco's medium (IMDM; GIBCO, Grand Island, NY) supplemented with 10% fetal calf serum (FCS, Hyclone, Logan, UT) and recombinant human GM-CSF (3 ng/mL; a gift from Dr Kenneth Kaushansky, University of Washington, Seattle). The UT-7/Epo cell line30 was maintained in IMDM supplemented with 10% FCS and 1 U/mL recombinant human Epo.31 The UT-7/Tpo cell line32 was maintained in IMDM supplemented with 10% FCS and 10 ng/mL recombinant human Tpo (obtained from Zymogenetics, Inc, Seattle, WA). Normal human burst-forming unit-erythroid (BFU-E) progeny were obtained as previously described.33,34Nonhemoglobinized BFU-E progeny were plucked on day 10 of culture and were incubated in IMDM supplemented with 5% FCS plus 1 ng/mL recombinant human IL-3 (obtained from Dr Kenneth Kaushansky) for 3 hours at 37°C to permit internalization and degradation of surface-bound SCF acquired during culture,33 before use for FRET experiments.
FRET technique.
The 104D2 anti–c-kit receptor monoclonal antibody (IgG1) was generated by immunizing a Balb/C mouse with the MOLM-1 cell line and screening the hybridomas for ability to specifically bind to NIH/3T3 cells engineered to express human c-kit receptor, but not to control NIH/3T3 cells.35,36 This antibody does not block binding of 125I-SCF to the c-kit receptor (Table 1). Purified 104D2 (1 mg) was directly conjugated to FITC (Molecular Probes, Inc, Eugene, OR) as recommended by the manufacturer. A separate aliquot of purified 104D2 (1 mg) was directly conjugated to Cy3 using a FluoroLink Cy3 Reactive Dye 5-Pack (Amersham Life Science, Arlington Heights, IL). Both conjugations were performed at 3 different molar ratios of dye to protein. The final coupling ratio was determined as previously described.21 A dye to protein molar ratio of approximately 2.5 to 1 resulted in retention of the ability of 104D2 to recognize thec-kit receptor on MO7e cells and in sufficient fluorescence intensity for use of the FRET technique.
Monoclonal Antibody 104D2 Does not Block Binding of SCF to the c-kit Receptor
| Addition . | cpm Bound . |
|---|---|
| 125I-SCF | 1,050 ± 39 |
| 125I-SCF + unlabeled SCF | 177 ± 22 |
| 125I-SCF + 104D2 | 1,042 ± 22 |
| Addition . | cpm Bound . |
|---|---|
| 125I-SCF | 1,050 ± 39 |
| 125I-SCF + unlabeled SCF | 177 ± 22 |
| 125I-SCF + 104D2 | 1,042 ± 22 |
OCIM1 cells were incubated with 125I-SCF (800 pmol/L) without or with unlabeled SCF (80 nmol/L) or with 104D2 monoclonal antibody (200 μg/mL) for 1 hour at 37°C as previously described.38 The data are presented as the mean (±SEM) of triplicate values.
To assess c-kit receptor dimerization, the SCF responsive cell lines MO7e, UT-7/Epo, or UT-7/Tpo were labeled with a mixture of 104D2-FITC and 104D2-Cy3 (0.1 to 0.3 μg/mL of each conjugate) for 1 hour at 4°C, then incubated with or without various concentrations of recombinant human SCF (expressed in E. coli, provided by Amgen, Inc, Thousand Oaks, CA) for 3 minutes to 1 hour at 37°C. The cells were then fixed in 1% paraformaldehyde and analyzed in a Coulter Epics Elite flow cytometer (Coulter, Miami, FL) with 488 nm excitation. FITC fluorescence emission was detected at 505 to 545 nm, and Cy3 fluorescence emission was detected above 590 nm. Because some FITC fluorescence can be detected at the wavelength used to detect Cy3 fluorescence,21 the fraction of FITC fluorescence that crosses into the Cy3 window was electronically compensated. Fluorescence was measured with linear signal amplification. To determine whether other hematopoietic cytokines could inducec-kit receptor dimerization, the cells labeled with 104D2-FITC plus 104D2-Cy3 were incubated with Epo (4 U/mL), IL-3 (2.5 ng/mL), GM-CSF (2.5 ng/mL), or Tpo (50 ng/mL) for 1 hour at 37°C, andc-kit receptor dimerization was assessed by FRET as described above. To examine the effect of reduced temperature on c-kitreceptor dimerization, MO7e cells labeled with 104D2-FITC plus 104D2-Cy3 at 4°C were incubated without or with SCF at either 37°C or 4°C before analysis.
The FRET data were analyzed as previously described.21 In brief, the mean fluorescence intensity of the 104D2-FITC and of the 104D2-Cy3 bound to the cells was determined by flow cytometric analysis. Each experiment included a sample of cells labeled with 104D2-FITC plus 104D2-Cy3 in the presence of a 20-fold molar excess of unconjugated 104D2. The equation used to calculate the acceptor (Cy3) to donor (FITC) ratio is: Acceptor/Donor Ratio=mean fluorescence intensity of 104D2-Cy3 minus mean fluorescence intensity of (104D2-Cy3 plus unconjugated 104D2)/mean fluorescence intensity of 104D2-FITC minus mean fluorescence intensity of (104D2-FITC plus unconjugated 104D2).
Analysis of c-kit receptor surface display.
The kinetics of SCF-induced c-kit receptor internalization and of c-kit receptor reappearance at the cell surface were analyzed by flow cytometry. MO7e cells were cultured overnight without GM-CSF in IMDM supplemented with 5% FCS and 0.5% bovine serum albumin (BSA; Intergen, Purchase, NY), then incubated with SCF (100 ng/mL) for 1 hour at 37°C. After the incubation with SCF, the cells were washed three times to remove the SCF and resuspended in IMDM supplemented with 5% FCS and 0.5% BSA. At various time points (0 to 4 hours), an aliquot of cells was removed, and cell surface c-kitreceptor display was detected by labeling the cells with 104D2 (2 μg/mL) followed by goat antimouse IgG-PE (Jackson ImmunoResearch, West Grove, PA) and flow cytometric analysis. In parallel, to determine whether reappearance of the c-kit receptor at the cell surface required new protein synthesis, MO7e cells were incubated with SCF (100 ng/mL) plus cycloheximide (10 μg/mL; Sigma Chemical Co, St Louis, MO) for 1 hour at 37°C. The cells were washed three times as described above and resuspended in IMDM supplemented with 5% FCS, 0.5% BSA, and cycloheximide (10 μg/mL). Aliquots of cells were removed at various time points (0 to 4 hours), and cell surface c-kit receptor display was analyzed as described above.
Analysis of c-kit receptor phosphorylation.
UT-7/Epo or UT-7/Tpo cells were washed twice and incubated overnight at 37°C in IMDM supplemented with 5% FCS in the absence of exogenous growth factors. The cells were then exposed to individual growth factors in IMDM (SCF 150 ng/mL, Epo 2.5 U/mL, GM-CSF 5 ng/mL, or Tpo 25 ng/mL) for 10 minutes at 37°C, then lysed by rocking for 20 minutes at 4°C in a solution consisting of 20 mmol/L Tris, 150 mmol/L NaCl, 10 mmol/L EDTA, 10% glycerol, 1% Triton X-100, 1.5 mmol/L MgCl2, 1 mmol/L phenylmethylsulfonyl fluoride (PMSF), 200 μg/mL aprotinin, 10 μg/mL leupeptin, 10 μmol/L pepstatin, 100 μmol/L NaF, and 2 mmol/L Na orthovanadate, as previously described.37 Triton X-100, leupeptin, and aprotinin were obtained from Boehringer Mannheim (Indianapolis, IN). The other chemicals were obtained from Sigma. The cell lysates were centrifuged for 5 minutes at 10,000g to remove insoluble debris, and solubilized cellular proteins were precleared by precipitation with protein A-sepharose beads (Pharmacia LKB, Piscataway, NJ). Thec-kit receptor was immunoprecipitated by incubating with purified SR-1 monoclonal antibody (4 μg/mL)38 for 3 hours at 4°C, followed by the addition of protein A-sepharose beads (1 hour at 4°C). The immunoprecipitates were washed once with 20 mmol/L HEPES (pH 7.5), 150 mmol/L NaCl, 0.1% Triton X-100, 10% glycerol, then resuspended in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer containing 50 mmol/L dithiothreitol,12 boiled for 2 minutes, and analyzed on a 7% SDS polyacrylamide gel. The proteins were transferred to nitrocellulose membranes (Bio-Rad, Richmond, CA), and tyrosine phosphorylated proteins were detected by Western blotting with the 4G10 antiphosphotyrosine monoclonal antibody (Upstate Biotechnology, Inc, Lake Placid, NY) and the enhanced chemiluminescence system (Amersham).
Confocal microscopy.
Confocal microscopy was used to determine whether SCF would alter the localization of the c-kit receptor on the cell surface, and to assess the time course of c-kit receptor internalization. MO7e cells growing in GM-CSF were washed free of GM-CSF and resuspended in IMDM supplemented with 0.5% BSA and SCF (200 ng/mL). Aliquots of the cells were removed after 3 minutes, 10 minutes, or 30 minutes at 37°C. The cells were fixed in 0.5% paraformaldehyde and one half of each aliquot of cells was permeabilized with 0.05% Triton X-100 (to permit detection of intracellular c-kit receptor). All of the samples were labeled with 104D2 (5 μg/mL) for 45 minutes at 4°C, washed, and then incubated with goat antimouse IgG-FITC for 45 minutes at 4°C. Just before confocal microscopy, the cells were incubated with propidium iodide (7.5 μg/mL; Molecular Probes, Inc) in 0.1% citrate plus 0.1% Triton X-100 to stain the nuclei. Surface fluorescence and intracellular fluorescence was analyzed with an ACAS Ultima Laser Cytometer (Meridian Instruments, Okemos, MI) as previously described.39 Fluorescence excitation was at 488 nm and FITC emission was detected with a 530/20 band pass filter. Propidium iodide emission was detected with a 605 long pass filter. A 60× oil immersion objective (numerical aperture 1.3) was used for scanning cells with a step (pixel) size of 0.2 μm in the xy plane and a step size of 0.4 μm for serial optical sections in the z axis. The pinhole setting was 225 μm, which yielded a theoretical optical thickness (full width at half maximum) of approximately 1 μm.
RESULTS
The FRET technique can be used to detect c-kit receptor dimerization on a human hematopoietic cell line (Table 2). MO7e cells were labeled with the nonneutralizing anti–c-kit receptor monoclonal antibody 104D2 that had been directly conjugated to either FITC or to Cy3. After incubation with SCF at 37°C, the mean fluorescence intensity of the donor fluorochrome (FITC) decreased, while the mean fluorescence intensity of the acceptor fluorochrome (Cy3) increased, in comparison to incubation without SCF, indicating that FRET occurred between the two labeled antibodies (Table 2). Because the distance at which 50% of the energy is transferred (Ro) is 55 angstroms for the FITC-Cy3 pair of fluorochromes,21 we interpret these results to mean that a portion of the c-kit receptor monomers must have dimerized in the presence of SCF. When the 104D2-FITC plus 104D2-Cy3–labeled MO7e cells were incubated in the presence of SCF at 4°C, energy transfer between the two fluorochromes was markedly diminished, compared with results obtained at 37°C, suggesting that the c-kit receptor does not dimerize as readily at 4°C as at 37°C (Table 2).
SCF-Induced Dimerization of the c-kit Receptor Is Detectable by FRET
| Temperature . | Growth Factor . | FITC Mean Fluorescence Intensity . | Cy3 Mean Fluorescence Intensity . | Acceptor/ Donor Ratio . |
|---|---|---|---|---|
| 37°C | 0 | 180 | 247 | 1.37 |
| SCF | 85 | 314 | 3.69 | |
| GM-CSF | 174 | 251 | 1.44 | |
| IL-3 | 185 | 244 | 1.32 | |
| Epo | 172 | 236 | 1.37 | |
| 4°C | 0 | 203 | 193 | 0.95 |
| SCF | 196 | 213 | 1.10 |
| Temperature . | Growth Factor . | FITC Mean Fluorescence Intensity . | Cy3 Mean Fluorescence Intensity . | Acceptor/ Donor Ratio . |
|---|---|---|---|---|
| 37°C | 0 | 180 | 247 | 1.37 |
| SCF | 85 | 314 | 3.69 | |
| GM-CSF | 174 | 251 | 1.44 | |
| IL-3 | 185 | 244 | 1.32 | |
| Epo | 172 | 236 | 1.37 | |
| 4°C | 0 | 203 | 193 | 0.95 |
| SCF | 196 | 213 | 1.10 |
MO7e cells were labeled with a mixture of 104D2-FITC plus 104D2-Cy3 for 1 hour at 4°C, then incubated with no added growth factor, SCF (100 ng/mL), GM-CSF (2.5 ng/mL), IL-3 (2.5 ng/mL), or Epo (4 U/mL) for 1 hour at 37°C, or with no added growth factor or SCF (100 ng/mL) for 1 hour at 4°C. The cells were fixed in 1% paraformaldehyde, and analyzed in a Coulter Epics Elite Flow Cytometer to detect FITC fluorescence at 505 to 545 nm, and Cy3 fluorescence above 590 nm.
To define the relationship between SCF concentration and c-kitreceptor dimerization, MO7e cells labeled with 104D2-FITC and with 104D2-Cy3 were exposed to a range of SCF concentrations, and dimerization was assessed by FRET (Table3). In two experiments, FRET was detected at a concentration of SCF less than or equal to 10 ng/mL. Peak FRET was found at an SCF concentration of 100 to 300 ng/mL. These results correlate well with the concentrations of SCF that stimulate proliferation of MO7e cells (Fig 1). Because the extent of FRET is a function of the distance separating the donor-acceptor pairs of fluorochromes and of the number of donor-acceptor pairs of fluorochromes within a threshold distance of each other, these results provide an index of c-kit receptor dimerization.
SCF Induces c-kit Receptor Dimerization in a Dose-Dependent Manner
| . | Concentration of SCF (ng/mL) . | FITC Mean Fluorescence Intensity . | Cy3 Mean Fluorescence Intensity . | Acceptor/ Donor Ratio . |
|---|---|---|---|---|
| Experiment 1 | 0 | 89.7 | 99.6 | 1.11 |
| 1 | 88.2 | 100.1 | 1.13 | |
| 3 | 87.6 | 101.0 | 1.15 | |
| 10 | 85.6 | 109.3 | 1.28 | |
| 30 | 88.9 | 131.2 | 1.48 | |
| 100 | 77.4 | 132.6 | 1.71 | |
| 300 | 80.7 | 137.2 | 1.70 | |
| 1000 | 76.4 | 127.1 | 1.66 | |
| Experiment 2 | 0 | 219 | 320 | 1.46 |
| 2 | 227 | 341 | 1.50 | |
| 10 | 210 | 346 | 1.65 | |
| 50 | 179 | 398 | 2.22 | |
| 250 | 170 | 403 | 2.37 |
| . | Concentration of SCF (ng/mL) . | FITC Mean Fluorescence Intensity . | Cy3 Mean Fluorescence Intensity . | Acceptor/ Donor Ratio . |
|---|---|---|---|---|
| Experiment 1 | 0 | 89.7 | 99.6 | 1.11 |
| 1 | 88.2 | 100.1 | 1.13 | |
| 3 | 87.6 | 101.0 | 1.15 | |
| 10 | 85.6 | 109.3 | 1.28 | |
| 30 | 88.9 | 131.2 | 1.48 | |
| 100 | 77.4 | 132.6 | 1.71 | |
| 300 | 80.7 | 137.2 | 1.70 | |
| 1000 | 76.4 | 127.1 | 1.66 | |
| Experiment 2 | 0 | 219 | 320 | 1.46 |
| 2 | 227 | 341 | 1.50 | |
| 10 | 210 | 346 | 1.65 | |
| 50 | 179 | 398 | 2.22 | |
| 250 | 170 | 403 | 2.37 |
MO7e cells were labeled with 104D2-FITC plus 104D2-Cy3 as described in Table 2, then incubated with a range of concentrations of SCF for 20 minutes at 37°C, fixed, and analyzed by flow cytometry.
Proliferation of MO7e cells in response to SCF correlates with c-kit receptor dimerization detected by FRET. MO7e cells were washed to remove GM-CSF and resuspended at a concentration of 1 × 105 cells/mL in IMDM suppplemented with 10% FCS and various concentrations of SCF (0 to 1,000 ng/mL; •), or 104D2 (0.5 μg/mL). After a 3-day incubation at 37°C, the number of viable cells was counted. The data represent the mean (± standard error of mean [SEM]) of triplicate values from one experiment. The wells supplemented with 104D2 contained 1.1 ± 0.2 × 105 MO7e cells/mL on day 3.
Proliferation of MO7e cells in response to SCF correlates with c-kit receptor dimerization detected by FRET. MO7e cells were washed to remove GM-CSF and resuspended at a concentration of 1 × 105 cells/mL in IMDM suppplemented with 10% FCS and various concentrations of SCF (0 to 1,000 ng/mL; •), or 104D2 (0.5 μg/mL). After a 3-day incubation at 37°C, the number of viable cells was counted. The data represent the mean (± standard error of mean [SEM]) of triplicate values from one experiment. The wells supplemented with 104D2 contained 1.1 ± 0.2 × 105 MO7e cells/mL on day 3.
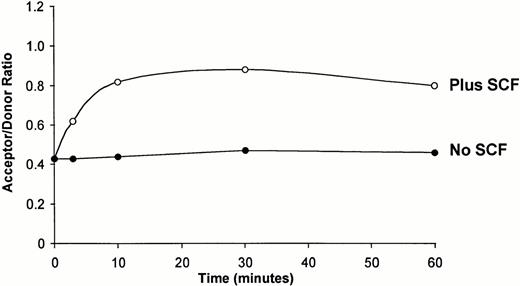
The onset of c-kit receptor dimerization detectable by FRET was assessed by incubating MO7e cells without or with SCF for varying periods of time (Fig 2). In the absence of SCF, no detectable change in energy transfer occurred between the fluorochromes during the 60-minute period. However, c-kitreceptor dimerization was detectable within 3 minutes of SCF addition, peaked at 30 minutes, and was still detectable at 60 minutes after SCF addition (Fig 2).
Dimerization of the c-kit receptor is detectable within 3 minutes after the addition of SCF. MO7e cells were labeled with 104D2-FITC plus 104D2-Cy3 for 45 minutes at 4°C, then incubated in the absence (•) or presence (○) of SCF (200 ng/mL) for 3 minutes, 10 minutes, 30 minutes, or 60 minutes at 37°C, fixed, and analyzed as described in Table 2. Two additional experiments gave similar results. Analysis of variance with a post hoc Student'st-test was used to determine if the acceptor/donor ratios at the various time points in the three experiments were different than the values at time 0. In the presence of SCF, the acceptor/donor ratios at 3 minutes, 10 minutes, 30 minutes, and 60 minutes were different than the acceptor/donor ratio at time 0 (P < .05).
Dimerization of the c-kit receptor is detectable within 3 minutes after the addition of SCF. MO7e cells were labeled with 104D2-FITC plus 104D2-Cy3 for 45 minutes at 4°C, then incubated in the absence (•) or presence (○) of SCF (200 ng/mL) for 3 minutes, 10 minutes, 30 minutes, or 60 minutes at 37°C, fixed, and analyzed as described in Table 2. Two additional experiments gave similar results. Analysis of variance with a post hoc Student'st-test was used to determine if the acceptor/donor ratios at the various time points in the three experiments were different than the values at time 0. In the presence of SCF, the acceptor/donor ratios at 3 minutes, 10 minutes, 30 minutes, and 60 minutes were different than the acceptor/donor ratio at time 0 (P < .05).
The specificity of cytokine-induced c-kit receptor dimerization was assessed by incubating MO7e cells, UT-7/Epo cells, or UT-7/Tpo cells with a number of cytokines (Tables 2 and4). Although MO7e cells can proliferate in response to SCF, GM-CSF, or IL-3, only SCF induced c-kitreceptor dimerization is detectable by FRET (Table 2). MO7e cells do not respond to Epo, and Epo did not induce c-kitreceptor dimerization in this cell line (Table 2). Despite the megakaryocytic features of the MO7e cell line and the ample display of Epo receptors on normal megakaryocytes,40 subsequent experiments showed that MO7e cells fail to bind 125I-Epo (data not shown). The UT-7/Epo and the UT-7/Tpo cell lines, derived from the parental UT-7 cell line by culture in Epo or in Tpo, respectively,29,31 offer an opportunity to explore the concept of receptor cross-talk in cells that are responsive to multiple cytokines. SCF induced c-kit receptor dimerization detectable by FRET in UT-7/Epo cells (Table 4). In each of five experiments with UT-7/Epo cells, Epo appeared to modestly promote FRET between the 104D2-FITC and the 104D2-Cy3 antibodies, in comparison to the results obtained with no added growth factor or GM-CSF (Table 4). In the UT-7/Tpo cell line, only SCF was able to induce c-kit receptor dimerization detectable by FRET. Although Tpo and GM-CSF can support proliferation of UT-7/Tpo cells,31 neither of these cytokines stimulated c-kit receptor dimerization (Table 4).
Effect of Hematopoietic Growth Factors on c-kitReceptor Dimerization
| Cell Line . | Growth Factor . | FITC Mean Fluorescence Intensity . | Cy3 Mean Fluorescence Intensity . | Acceptor/ Donor Ratio . |
|---|---|---|---|---|
| UT-7/Epo | 0 | 86 | 42 | 0.49 |
| SCF | 37 | 59 | 1.59 | |
| Epo | 80 | 47 | 0.59 | |
| GM-CSF | 85 | 41 | 0.48 | |
| UT-7/Tpo | 0 | 46 | 156 | 3.39 |
| SCF | 36 | 175 | 4.86 | |
| Tpo | 54 | 170 | 3.15 | |
| GM-CSF | 46 | 155 | 3.37 |
| Cell Line . | Growth Factor . | FITC Mean Fluorescence Intensity . | Cy3 Mean Fluorescence Intensity . | Acceptor/ Donor Ratio . |
|---|---|---|---|---|
| UT-7/Epo | 0 | 86 | 42 | 0.49 |
| SCF | 37 | 59 | 1.59 | |
| Epo | 80 | 47 | 0.59 | |
| GM-CSF | 85 | 41 | 0.48 | |
| UT-7/Tpo | 0 | 46 | 156 | 3.39 |
| SCF | 36 | 175 | 4.86 | |
| Tpo | 54 | 170 | 3.15 | |
| GM-CSF | 46 | 155 | 3.37 |
UT-7/Epo cells or UT-7/Tpo cells were labeled with 104D2-FITC and 104D2-Cy3, then incubated with no added growth factor, SCF (100 ng/mL), GM-CSF (2.5 ng/mL), IL-3 (2.5 ng/mL), Epo (4 U/mL), or Tpo (50 ng/mL) for 1 hour at 37°C, fixed, and analyzed as described in Table 2. The results of 1 of 5 similar experiments with UT-7/Epo cells, and 1 of 3 similar experiments with UT-7/Tpo cells, are shown. Analysis of variance with a post hoc student's t-test was used to determine the significance of the differences in the acceptor/donor ratio in the absence or presence of growth factors in the 5 experiments with UT-7/Epo cells. The acceptor/donor ratio for the UT-7/Epo cells incubated with either SCF or with Epo was different from the acceptor/donor ratio of the cells incubated with no added growth factor (P < .01, SCF v no added growth factor;P < .01, Epo v no added growth factor).
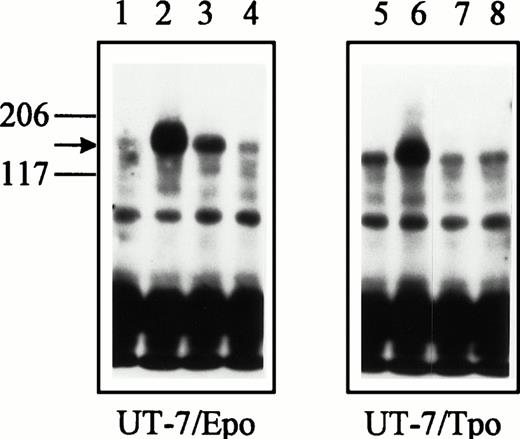
Binding of SCF induces c-kit receptor dimerization and intermolecular tyrosine phosphorylation by the kinase domain of the receptor.15 Because of the suggestion that Epo could stimulate dimerization of the c-kit receptor, the ability of Epo to stimulate c-kit receptor tyrosine phosphorylation was investigated (Fig 3). Exposure of UT-7/Epo cells to SCF or to Epo for 10 minutes resulted in readily detectable tyrosine phosphorylation of the c-kit receptor (Fig 3, upper panel, lanes 2 and 3). The magnitude of c-kit receptor tyrosine phosphorylation in the presence of SCF was greater than that found in the presence of Epo. Exposure of UT-7/Epo cells to GM-CSF did not induce c-kit receptor tyrosine phosphorylation (Fig 3, upper panel, lane 4). In the UT-7/Tpo cell line, only SCF was found to stimulate c-kit receptor tyrosine phosphorylation (Fig 3, upper panel, lane 6). In accord with the results of the c-kitreceptor dimerization experiments, neither Tpo nor GM-CSF inducedc-kit receptor tyrosine phosphorylation in UT-7/Tpo cells (Fig3, upper panel, lanes 7 and 8). Tyrosine phosphorylation of thec-kit receptor in UT-7/Epo cells was detectable within 5 minutes of the addition of either SCF or Epo (Fig 3, lower panel). These experiments demonstrating that Epo can induce tyrosine phosphorylation of the c-kit receptor support the concept that Epo can activate the c-kit receptor in the UT-7/Epo cell line.
Upper panel: Epo induces tyrosine phosphorylation of thec-kit receptor in UT-7/Epo cells. UT-7/Epo cells were exposed to no added growth factor (lane 1), SCF (150 ng/mL, lane 2), Epo (2.5 U/mL, lane 3), or GM-CSF (5 ng/mL, lane 4) for 10 minutes at 37°C. UT-7/Tpo cells were exposed to no added growth factor (lane 5), SCF (lane 6), Tpo (25 ng/mL, lane 7), or GM-CSF (lane 8). Cell lysates were immunoprecipitated with SR-1 anti–c-kit receptor monoclonal antibody, subjected to SDS-PAGE, and blotted with the 4G10 antiphosphotyrosine monoclonal antibody. Molecular weight markers are indicated. The arrow identifies the c-kit receptor. Two additional experiments gave similar results. Lower panel: tyrosine phosphorylation of the c-kit receptor in UT-7/Epo cells is detectable within 5 minutes of Epo stimulation. UT-7/Epo cells were exposed to no added growth factor (lane 1), SCF (150 ng/mL, lane 2) for 5 minutes, SCF (lane 3) for 10 minutes, Epo (2.5 U/mL, lane 4) for 5 minutes, Epo (lane 5) for 10 minutes, or GM-CSF (2.5 ng/mL) for 5 minutes at 37°C.
Upper panel: Epo induces tyrosine phosphorylation of thec-kit receptor in UT-7/Epo cells. UT-7/Epo cells were exposed to no added growth factor (lane 1), SCF (150 ng/mL, lane 2), Epo (2.5 U/mL, lane 3), or GM-CSF (5 ng/mL, lane 4) for 10 minutes at 37°C. UT-7/Tpo cells were exposed to no added growth factor (lane 5), SCF (lane 6), Tpo (25 ng/mL, lane 7), or GM-CSF (lane 8). Cell lysates were immunoprecipitated with SR-1 anti–c-kit receptor monoclonal antibody, subjected to SDS-PAGE, and blotted with the 4G10 antiphosphotyrosine monoclonal antibody. Molecular weight markers are indicated. The arrow identifies the c-kit receptor. Two additional experiments gave similar results. Lower panel: tyrosine phosphorylation of the c-kit receptor in UT-7/Epo cells is detectable within 5 minutes of Epo stimulation. UT-7/Epo cells were exposed to no added growth factor (lane 1), SCF (150 ng/mL, lane 2) for 5 minutes, SCF (lane 3) for 10 minutes, Epo (2.5 U/mL, lane 4) for 5 minutes, Epo (lane 5) for 10 minutes, or GM-CSF (2.5 ng/mL) for 5 minutes at 37°C.
After SCF binds, the SCF-c-kit receptor complex is internalized.41 42 To assess the kinetics of c-kitreceptor reappearance on the cell surface, MO7e cells were exposed to SCF for 1 hour, and c-kit receptor display on the cell surface was examined by flow cytometry (Fig 4). Exposure to SCF for 1 hour decreased c-kit receptor display on the cell surface. When SCF was removed from the medium, c-kitreceptor display on the cell surface increased gradually over several hours, but had not returned to its initial density by 4 hours after removal of SCF (Fig 4). Blocking new protein synthesis by the addition of cycloheximide prevented the increase in c-kit receptor display on the cell surface (Fig 4), arguing that internalizedc-kit receptor does not recycle to the cell surface.
Reappearance of the c-kit receptor on the cell surface requires new protein synthesis. Display of the c-kitreceptor on MO7e cells proliferating in GM-CSF, before exposure to SCF, is shown in the left portion of the figure. MO7e cells were incubated with SCF (100 ng/mL) for 1 hour at 37°C to induce internalization of the c-kit receptor. The cells were washed to remove SCF, and reappearance of the c-kit receptor on the cell surface was assessed by flow cytometry at various time points (0 to 4 hours) after removal of SCF. MO7e cells were incubated in parallel in the presence of cycloheximide (10 μg/mL) to inhibit new protein synthesis. The data are presented as the mean (± SEM) of triplicate values. The cells incubated in the absence of cycloheximide displayed more cell surface c-kit receptor than the cells incubated in the presence of cycloheximide (*P < .01, Student's t-test).
Reappearance of the c-kit receptor on the cell surface requires new protein synthesis. Display of the c-kitreceptor on MO7e cells proliferating in GM-CSF, before exposure to SCF, is shown in the left portion of the figure. MO7e cells were incubated with SCF (100 ng/mL) for 1 hour at 37°C to induce internalization of the c-kit receptor. The cells were washed to remove SCF, and reappearance of the c-kit receptor on the cell surface was assessed by flow cytometry at various time points (0 to 4 hours) after removal of SCF. MO7e cells were incubated in parallel in the presence of cycloheximide (10 μg/mL) to inhibit new protein synthesis. The data are presented as the mean (± SEM) of triplicate values. The cells incubated in the absence of cycloheximide displayed more cell surface c-kit receptor than the cells incubated in the presence of cycloheximide (*P < .01, Student's t-test).
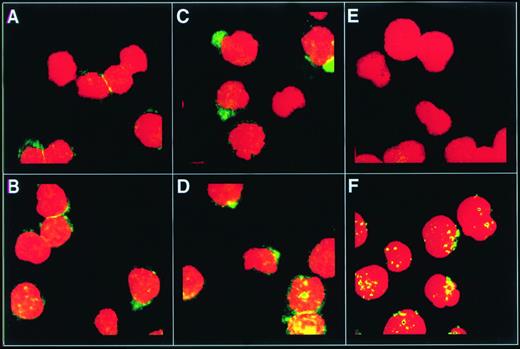
Confocal microscopy was used to determine whether exposure to SCF would alter the localization of the c-kit receptor on the cell surface (Fig 5). The images in Fig 5represent optical slices of approximately 1 μm taken half-way through the cell, to facilitate the distinction between surface and intracellular c-kit receptor. Before exposure to SCF, thec-kit receptor protein was widely distributed on the surface of most of the MO7e cells (Fig 5A and B), and little cytoplasmicc-kit receptor was detected (Fig 5B). By 3 minutes after exposure to SCF, punctate fluorescent foci were detected in one region of many of the cells, suggesting aggregation of c-kit receptors at this site (Fig 5C and D). Thirty minutes after exposure to SCF, little cell surface c-kit receptor was detected (Fig 5E and F), and the majority of the c-kit receptor protein was found inside the cell (Fig 5F).
The c-kit receptor clusters on the cell surface within 3 minutes of exposure to SCF. MO7e cells were exposed to SCF (200 ng/mL) for 0 minutes (A and B), 3 minutes (C and D), or 30 minutes (E and F) at 37°C. One portion of each aliquot of cells was stained with 104D2 followed by goat antimouse IgG-FITC to detect cell surfacec-kit receptor (A, C, and E). The other portion of each aliquot of cells was permeabilized with Triton X-100 to permit detection of intracellular c-kit receptor in addition to cell surfacec-kit receptor (B, D, and F), then stained as described above. The cells were analyzed by confocal microscopy.
The c-kit receptor clusters on the cell surface within 3 minutes of exposure to SCF. MO7e cells were exposed to SCF (200 ng/mL) for 0 minutes (A and B), 3 minutes (C and D), or 30 minutes (E and F) at 37°C. One portion of each aliquot of cells was stained with 104D2 followed by goat antimouse IgG-FITC to detect cell surfacec-kit receptor (A, C, and E). The other portion of each aliquot of cells was permeabilized with Triton X-100 to permit detection of intracellular c-kit receptor in addition to cell surfacec-kit receptor (B, D, and F), then stained as described above. The cells were analyzed by confocal microscopy.
To determine whether the FRET technique could detect c-kitreceptor dimerization in normal human hematopoietic cells, progeny of peripheral blood BFU-E plucked on day 10 of culture were labeled with 104D2-FITC plus 104D2-Cy3 and incubated without or with SCF (Table 5). Although the mean fluorescence intensity of 104D2 binding to the BFU-E progeny was substantially less than the mean fluorescence intensity of 104D2 binding to the MO7e cell line, SCF-induced dimerization of the c-kit receptor in the BFU-E progeny was readily detectable (Table 5).
FRET Technique Can Detect c-kit Receptor Dimerization in Progeny of Normal Human BFU-E
| . | Growth Factor . | FITC Mean Fluorescence Intensity . | Cy3 Mean Fluorescence Intensity . | Acceptor/ Donor Ratio . |
|---|---|---|---|---|
| Experiment 1A | 0 | 16.7 | 17.4 | 1.04 |
| SCF | 7.8 | 26.7 | 3.42 | |
| Experiment 1B | 0 | 16.8 | 18.2 | 1.08 |
| SCF | 8.4 | 25.6 | 3.04 |
| . | Growth Factor . | FITC Mean Fluorescence Intensity . | Cy3 Mean Fluorescence Intensity . | Acceptor/ Donor Ratio . |
|---|---|---|---|---|
| Experiment 1A | 0 | 16.7 | 17.4 | 1.04 |
| SCF | 7.8 | 26.7 | 3.42 | |
| Experiment 1B | 0 | 16.8 | 18.2 | 1.08 |
| SCF | 8.4 | 25.6 | 3.04 |
Normal human peripheral blood BFU-E progeny were plucked on day 10 of culture, pooled, and incubated for 3 hours at 37°C in the absence of SCF to permit internalization and degradation of SCF acquired during culture.33 34 The BFU-E progeny were then aliquoted into individual test tubes for experiment 1A or experiment 1B, labeled with 104D2-FITC plus 104D2-Cy3 at 4°C, incubated without or with SCF (200 ng/mL) for 1 hour at 37°C, fixed, and analyzed by flow cytometry as described in Table 2.
DISCUSSION
The c-kit receptor is a member of the tyrosine kinase type III family of receptors, which includes the M-CSF receptor (c-fms), the platelet-derived growth factor (PDGF) receptors, and the flt3/flk-2 receptor.15 These receptors are characterized by an extracellular region that contains five immunoglobulin-like domains, a single membrane-spanning region, and a cytoplasmic region that encodes a tyrosine kinase domain split by a kinase insert sequence. An essential early step in signal transduction by this class of receptors is homodimerization and intermolecular tyrosine phosphorylation. The present report shows that the FRET technique offers one way to examine this proximal step in signal transduction by the c-kit receptor.
Advantages of the FRET technique are that receptor homodimerization or heterodimerization can be rapidly assessed in a population of living cells.19-21,23,24 Small changes in energy transfer can be accurately quantitated by flow cytometric analysis of thousands of cells, and receptor dimerization can be investigated in the absence of ligand. Thus, FRET can be used to study dynamic processes such as assembly of receptor subunits within the cell membrane. Affinity cross-linking, an alternative way to analyze receptor dimerization, is usually performed in the presence of radiolabeled ligand to facilitate identification of the cross-linked species.11,12 In at least one situation, affinity cross-linking of 125I-Epo to erythroid cells, the molecular weight of the major cross-linked proteins did not correspond to the molecular weight of the recombinant functional Epo receptor protein, and antibodies that recognize the recombinant Epo receptor did not immunoprecipitate the major cross-linked proteins.43,44 The efficiency of affinity cross-linking has been estimated to be in the range of 2% to 15%,22,45 46 and affinity cross-linking is less suitable for kinetic analysis than is FRET.
Disadvantages of the FRET technique as used in this report include the need for a nonneutralizing monoclonal antibody that recognizes the receptor (or pair of antibodies in the case of receptor heterodimerization) and that binds to an epitope that is accessible, whether the receptor is a monomer or a dimer. An additional requirement is that the orientation of the dimerized receptor must permit the antibodies to be within the threshold distance of the fluorochrome pair for FRET to occur. Although the lower limit of receptor display that would permit detection of FRET has not been determined, the MO7e cell line studied in this report displays approximately 35,000 c-kitreceptors per cell.47 A previous FRET study used cells expressing approximately 10,000 IL-1 receptors per cell.21However, the present report suggests that the level of c-kitreceptor display on normal hematopoietic precursor cells may be sufficient for the application of the FRET technique to these populations of cells.
The addition of SCF to human hematopoietic cell lines labeled with 104D2-FITC plus 104D2-Cy3 resulted in changes in mean fluorescence intensity that are in the same range as those detected in prior FRET analysis of IL-1 receptor dimerization or of EGF receptor dimerization.21-23 Maximal IL-1 receptor dimerization required at least 60 minutes after the addition of IL-121; maximal c-kit receptor dimerization occurred more rapidly. When MO7e cells were maintained in the continued presence of SCF, thec-kit receptor remained dimerized for at least 1 hour (Fig 2). Flow cytometric analysis (Fig 4) and confocal microscopy (Fig 5) show that a major portion of the c-kit receptor protein has been internalized by 30 to 60 minutes after the addition of SCF. These results suggest that at least a portion of the c-kit receptors remain dimerized after internalization. The EGF receptor also remains dimerized after endocytosis,48 and it has been suggested that internalized tyrosine phosphorylated EGF receptors or PDGF receptors may participate in signal transduction.48,49Normal endocytic trafficking of the EGF receptor is required to achieve the full spectrum of EGF signal transduction.50 Although the present report suggests that internalized c-kit receptors remain dimerized, whether internalized c-kit receptors contribute to signal transduction remains to be determined.
Prior studies using FRET have shown that ligand binding leads to microclustering of the EGF receptor in the cell membrane.23Recent models of cell membrane structure suggest that certain signal transduction proteins may be concentrated in lipid microdomains that form “rafts” in the cell membrane, and that signaling may be optimized by microclustering of receptors within these lipid microdomains.51 The T-cell receptor forms multimeric complexes after engagement of antigenic peptide bound to major histocompatibility complex (MHC) molecules, and the extent of oligomerization of the T-cell receptor complex may influence signal transduction.52 The confocal images in the present report also suggest that the c-kit receptor aggregates in the presence of SCF. Taken together, these reports suggest that the formation of receptor aggregates is a common feature of a number of transmembrane receptors.
Both SCF and Epo are important for normal erythropoiesis in vivo. Mice that lack cell surface c-kit receptor (W/W mice) show a profound reduction in fetal liver colony-forming unit-erythroid (CFU-E) numbers53 and die in the perinatal period with severe anemia,54 indicating that SCF is critical for normal late erythropoiesis in vivo. Likewise, Epo receptor knock-out mice have BFU-E and CFU-E in the fetal liver, but die in utero around embryonic day 13 with profound anemia.55,56When the Epo receptor was introduced into fetal liver cells obtained from Epo receptor knock-out mice, the cells required both SCF and Epo for CFU-E growth in vitro, in contrast to normal fetal liver cells, which require only Epo for CFU-E growth in vitro.26 These results were interpreted to mean that an essential interaction between the c-kit receptor and the Epo receptor normally occurs in erythroid progenitor cells in vivo.26
Studies in the HCD57 murine hematopoietic cell line also suggest that there is functional interaction between the c-kit receptor and the Epo receptor.25 27 Stimulation of HCD57 cells with SCF induced tyrosine phosphorylation of both the c-kit receptor and the Epo receptor, and physical association of the c-kitreceptor with the Epo receptor cytoplasmic domain. However, these reports do not indicate that Epo can trigger c-kit receptor dimerization or tyrosine phosphorylation. The present report suggests that Epo can induce c-kit receptor dimerization and shows that Epo can induce c-kit receptor tyrosine phosphorylation in the human hematopoietic cell line UT-7/Epo. This supports the concept of bidirectional cross-talk between the c-kit receptor and the Epo receptor.
Epo and Tpo exhibit a number of structural and functional similarities, including approximately 50% amino acid homology between Epo and the N-terminal domain of Tpo and conserved location of cysteine residues and α helices.57 Epo and Tpo can synergistically promote megakaryopoiesis (CFU-Meg colony growth) and erythropoiesis (CFU-E generation) in vitro.58-60 Moreover, the Epo receptor and the Mpl receptor, both members of the hematopoietin receptor superfamily, have amino acid sequence homology.61 Both the Epo and the Mpl receptors are found on megakaryocytes and on erythroid progenitor and precursor cells.34,40 62 Although both Epo and Tpo can synergize with SCF, the mechanisms appear to differ in that Epo, but not Tpo, was able to induce subtle changes in FRET suggestive of c-kit receptor dimerization; the latter finding corresponded to the ability of Epo, but not Tpo, to induce c-kit receptor tyrosine phosphorylation in cytokine-responsive cell lines.
Confocal microscopy showed that the c-kit receptor can cap at one end of the cell within 3 minutes after the addition of SCF, showing that the c-kit receptor protein occupied by soluble SCF is highly mobile within the cell membrane. Whether engagement of thec-kit receptor by the transmembrane form of SCF presented by marrow microenvironmental cells would permit equally rapid redistribution of the c-kit receptor on the hematopoietic cell surface remains to be determined. Capping may be related to internalization via clathrin-coated pits; a number of other cytokine receptors are internalized by the clathrin-coated pit mechanism.63 After internalization, the c-kitreceptor protein is detectable within the cell by confocal microscopy for at least 30 minutes. However, internalized c-kit receptor does not redecorate the cell surface: reappearance of the c-kitreceptor on the cell surface requires new protein synthesis. These results suggest that the fate of internalized c-kit receptor is degradation, rather than recycling to the cell surface. It is likely that both lysosomal proteolysis and the ubiquitin-proteasome pathway contribute to c-kit receptor degradation.40,42 64
Receptor oligomerization is important for signal transduction in the hematopoietin receptor superfamily,65 as well as in the tyrosine kinase receptor family. With the use of distinct monoclonal antibodies to the α and β subunits of receptors, as has been done for the T-cell antigen receptor complex,19 20 the FRET technique could be used to examine heterodimerization of hematopoietic growth factor receptors.
ACKNOWLEDGMENT
We thank Dr Peter Rabinovitch for helpful discussions.
Supported by National Institutes of Health (Bethesda, MD) Grants No. DK44194, DK49855, DK43719, ES07033, and a Faculty Research Award from the American Cancer Society (Atlanta, GA).
Address reprint requests to Virginia C. Broudy, MD, Division of Hematology, University of Washington, Box 357710, Seattle, WA 98195-7710.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

![Fig. 1. Proliferation of MO7e cells in response to SCF correlates with c-kit receptor dimerization detected by FRET. MO7e cells were washed to remove GM-CSF and resuspended at a concentration of 1 × 105 cells/mL in IMDM suppplemented with 10% FCS and various concentrations of SCF (0 to 1,000 ng/mL; •), or 104D2 (0.5 μg/mL). After a 3-day incubation at 37°C, the number of viable cells was counted. The data represent the mean (± standard error of mean [SEM]) of triplicate values from one experiment. The wells supplemented with 104D2 contained 1.1 ± 0.2 × 105 MO7e cells/mL on day 3.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/3/10.1182_blood.v91.3.898/3/m_blod4031801.jpeg?Expires=1765906352&Signature=Fz3ErwiaSXnEaWrtuuMMP1KpEnZ7XgYc3YVBVU8FPitAoXM0225~F0cL0m0yujF2aCQme5pMVWyfrZs3dauxDSkiCMYr7wnzU2f4HZ3QI4rEwVs5Yro38wh72PDM-N-jUTXLzKno2FA4OGn4z3KGdyZ~AmXhZNqxMUdvweaUCOb1LwsZ~CisqgHgix172dZ4YOIoqRHlmO~tiDNJPf6tv-2ZO8m76eNLbYyj1-Kbvo1m0z2CHZvKIqoRD6BAAakmo3Ro09uEqQnrbvDC3EeCwRVsYL8IT06e76VnLOjlXo1lZIMD1YPNbq1De7GpnSR~LZu82jdKhng42hTjRBjRnQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal