Abstract
This study was aimed at defining the histogenesis of the pathologic spectrum of acquired immunodeficiency syndrome–related non-Hodgkin's lymphomas (AIDS-NHL), including AIDS-related small noncleaved cell lymphoma (AIDS-SNCCL), AIDS-related large noncleaved cell lymphoma (AIDS-LNCCL), AIDS-related large cell immunoblastic lymphoma plasmacytoid (AIDS-IBLP), and AIDS-related primary effusion lymphoma (AIDS-PEL). Forty-six cases of AIDS-NHL were investigated for the expression pattern of BCL-6, a protein specifically expressed by germinal center (GC) B-cells, and CD138/syndecan-1 (syn-1), a marker of post-GC B-cell differentiation. Expression of BCL-6 and syn-1 segregated two major phenotypic patterns among AIDS-NHL: (1) the BCL-6+/syn-1− pattern associated with AIDS-SNCCL and AIDS-LNCCL; (2) the BCL-6−/syn-1+ pattern associated with AIDS-IBLP and AIDS-PEL. Among systemic AIDS-NHL infected by Epstein-Barr virus (EBV), expression of the EBV-encoded latent membrane protein-1 (LMP-1) preferentially associated with the BCL-6−/syn-1 + profile. Analysis of nonneoplastic lymph nodes showed that the two phenotypic patterns detected in AIDS-NHL correspond to physiologic stages of B-cell development, ie, GC B-cells (BCL-6+/syn-1−) and preterminally differentiated post-GC B-cells (BCL-6−/syn-1+). Thus, BCL-6+/syn-1− AIDS-NHL reflects a GC stage of differentiation, whereas AIDS-NHL which are BCL-6−/syn-1+, and LMP-1+when infected by EBV, derive from B cells that have entered post-GC plasmacell differentiation. These findings are relevant for the pathogenesis and differential diagnosis of AIDS-NHL.
ACQUIRED IMMUNODEFICIENCY syndrome–related non-Hodgkin's lymphomas (AIDS-NHL) represent a markedly heterogeneous group of lymphomas derived from mature B cells.1-3 The pathologic spectrum of AIDS-NHL includes systemic NHL, primary central nervous system lymphomas (PCNSL), and primary effusion lymphoma (PEL). Systemic AIDS-NHL are histologically classified into AIDS-related small noncleaved cell lymphoma (AIDS-SNCCL) and AIDS-related diffuse large cell lymphoma (AIDS-DLCL).2 Depending on the presence of immunoblastic features, AIDS-DLCL may be further distinguished into large noncleaved cell lymphoma (LNCCL) and large cell immunoblastic lymphoma plasmacytoid (IBLP).1,2,4 AIDS-related PCNSL (AIDS-PCNSL) are represented in all cases by IBLP or LNCCL,5 whereas AIDS-related PEL (AIDS-PEL) morphologically bridges immunoblastic and anaplastic features.6-8
The pathologic heterogeneity of AIDS-NHL correlates with the heterogeneity of the molecular lesions associated with these lymphomas.2,3,9-11 AIDS-SNCCL selectively associates with activation of c-MYC, whereas rearrangements of BCL-6 are restricted to a fraction of AIDS-DLCL. Infection by Kaposi's sarcoma–associated herpesvirus (KSHV) clusters selectively with AIDS-PEL,6-8 whereas infection by Epstein-Barr virus (EBV) occurs at different rates in different AIDS-NHL types.12-15
The histogenetic derivation of the various types of AIDS-NHL has not been elucidated, although it has been suggested that a fraction of AIDS-NHL may be related to germinal center (GC) B cells.16More recently, the study of the histogenesis of mature B-cell neoplasms has been facilitated by the availability of biologic markers of distinct subsets of mature B cells. Two such markers are represented by BCL-6 and CD138/syndecan-1 (syn-1). BCL-6 is a proto-oncogene product coding for a zinc finger transcriptional repressor which, in the B-cell lineage, is expressed selectively in GC B-cells.17-21Notably, animal models have shown that expression of BCL-6 is an absolute requirement for GC formation and function.22,23Syn-1 is a proteoglycan belonging to the syndecan family.24,25 Within the mature B-cell compartment, syn-1 is not expressed in GC B cells whereas it is expressed in specific subsets of post-GC B cells, including immunoblasts and plasma cells.26-28
In an attempt to elucidate the histogenesis of AIDS-NHL, we have investigated the expression pattern of BCL-6 and syn-1 throughout the pathologic spectrum of these lymphomas as well as in nonneoplastic lymphoid tissues. The results indicate that AIDS-NHL may be subdivided into two main phenotypic categories corresponding to the GC (BCL-6+/syn-1−) and the post-GC (BCL-6−/syn-1+) stage of physiologic B-cell differentiation. In systemic AIDS-NHL carrying EBV infection, expression of LMP-1 clusters with the BCL-6−/syn-1+ phenotype. Overall, these results have implications for the histogenesis and diagnosis of AIDS-NHL.
MATERIALS AND METHODS
Neoplastic samples.
This study included AIDS-NHL samples from 40 patients (27 systemic AIDS-NHL, 8 AIDS-PCNSL, and 5 AIDS-PEL). Pathological specimens were classified according to the Working Formulation for NHL and the revised European-American classification of lymphoid neoplasms (REAL).4 AIDS-PEL were classified on the basis of their clinico-pathological and virological (eg, KSHV positivity) characteristics.6,7 Tissues from systemic AIDS-NHL and AIDS-PCNSL were fixed in Bouin solution or neutral buffered formalin. In most cases, a portion of unfixed tissue was snap frozen in liquid nitrogen and stored at −80°C. Samples of AIDS-PEL were collected under sterile conditions during standard diagnostic procedures and treated according to a standard regimen used at the Division of Pathology of the Centro di Riferimento Oncologico.7 29 All tissue-based AIDS-NHL and AIDS-PEL were B-cell monoclonal proliferations according to their immunophenotypic and immunogenotypic characteristics.
Cell lines.
In vitro established AIDS-NHL cell lines were also studied. The detailed characterization of these cell lines has been reported previously. The cell lines HBL-6, BC-1 (purchased from American Type Culture Collection [ATCC], Rockville, MD), BC-2 (purchased from ATCC), BC-3, BCBL-1, and CROAP-2 were derived from AIDS-PEL.29-33 Four AIDS-PEL cell lines (HBL-6, BC-1, BC-2, and CROAP-2) carry EBV infection, whereas two AIDS-PEL cell lines (BC-3 and BCBL-1) are EBV−.
Nonneoplastic samples.
Nonneoplastic lymph node samples from seven human immunodeficiency virus (HIV)-seropositive patients with persistent generalized lymphadenopathy (PGL) were also included in the study. All PGL samples carried EBV DNA sequences without evidence of monoclonal EBV episomes, as assessed by Southern blot analysis. The histopathologic pattern of PGL samples was predominantly represented by hyperplastic changes of the lymphoid follicles.
Immunohistochemical studies and analysis of BCL-6 and syn-1 expression.
Deparaffinized and cryostat sections were used for immunophenotyping and lineage assignment of AIDS-NHL and PGL samples. Sources and specificities of the antibodies used in this study have been reported in detail previously.29 Immunohistochemistry was performed by the avidin-biotin-peroxidase complex (ABC-px) or alkaline phosphatase anti alkaline phosphatase (APAAP) methods as previously described.34 35
The BCL-6 protein was detected by using the PG-B6 monoclonal antibody (MoAb) that has been recently generated in the laboratory of one of the investigators (B.F.) by immunizing BALB/c mice with a glutathione S-transferase-BCL-6 fusion protein.36 The antibody is directed against the aminoterminal portion of the human BCL-6 gene product. Immunostaining for BCL-6 was performed on frozen or formalin-fixed, paraffin-embedded sections and cytospin preparations by the APAAP method.35 37
Anti–B-B4 MoAb (Serotec, Oxford, UK), which specifically recognizes the syn-1 antigen,28 was applied to paraffin-embedded tissues from all 35 tissue-based AIDS-NHL and all 7 PGL samples included in this study. The MoAb was also applied to frozen tissues from a representative subset of AIDS-NHL cases for control purposes. The MoAb was applied to cytospin preparations from all AIDS-PEL cases as well as from the AIDS-PEL cell lines. Air-dried frozen sections were kept under vacuum for 3 hours and then fixed in a 1:1 solution of acetone and chloroform for 10 minutes; sections were hydrated with phosphate-buffered saline (PBS), pre-incubated with normal rabbit serum (1:50 for 20 minutes at room temperature) and subsequently incubated with anti-B-B4 MoAb for 1 hour at room temperature. Paraffin-embedded tissue sections were pretreated in a microwave oven (Jet 900 W; Philips, Eindhoven, The Netherlands) twice for 5 minutes at 650 W in citrate buffer (pH 6); immunostaining was performed by incubating anti–B-B4 MoAb, with the addition of 3% normal human serum, for 1 hour at room temperature. Cytospin preparations of AIDS-PEL and of AIDS-PEL–derived cell lines were fixed in acetone-chloroform at room temperature for 10 minutes and immunostained with anti–B-B4 MoAb by the APAAP (alkaline phosphatase anti-alkaline phosphatase) method.35
The percentage of BCL-6+ or syn-1+ neoplastic cells was assigned to one of the following categories: 0, less than 10%, 10% to 25%, 25% to 50%, 50% to 75%, and greater than 75%.
Two-color staining.
Multiple immunohistochemical staining was performed to detect BCL-6 plus syn-1 in selected PGL samples. Formalin fixed paraffin-embedded tissue sections were first treated twice for 5 minutes in 1 mmol/L EDTA buffer pH 8.0 and immunostained with anti–BCL-6 MoAb by the APAAP method35 37 using naphthol AS-MX phosphate along with Fast Blue BB salt (Sigma Chemical Co, St Louis, MO) for the development of alkaline phosphatase; subsequently, sections were treated twice for 5 minutes in citrate buffer (pH 6) in a microwave oven to denature bound antibody molecules and to inactivate alkaline phosphatase present in the APAAP complex. Finally, sections were incubated overnight at 4°C with anti–syn-1 MoAb and immunostained by the APAAP method using naphthol AS-MX phosphate along with Fast Red TR salt (Sigma) for the development of alkaline phosphatase.
Analysis of viral infection.
All samples of AIDS-NHL and PGL included in this study were subjected to determination of tumor infection by EBV and KSHV, according to previously reported strategies.7,29 EBER in situ hybridization (ISH) studies were performed on AIDS-NHL and PGL samples to identify the nature and distribution of EBV-infected cells. ISH studies were performed on Bouin or formalin fixed paraffin-embedded tissues or cell block sections, as previously described.16
In the case of EBER+ samples, immunostaining for LMP-1 was performed with an LMP-1 specific antibody (Dakopatts A/S, Glostrup, Denmark) on Bouin or formalin-fixed paraffin-embedded tissue sections and cytospin preparations, as described above. The percentage of LMP-1+ neoplastic cells was assigned to one of the following categories: 0, less than 10%, 10% to 25%, 25% to 50%, 50% to 75%, and greater than 75%.
Analysis of c-MYC genetic lesions.
Genetic studies of BCL-6.
The configuration of the BCL-6 locus was investigated by Southern blot analysis using previously reported assays.10The presence of mutations of BCL-6 5′ noncoding regions was tested in a 740-bp fragment within BCL-6 intron 1 and inBCL-6 exon 1 (fragments E1.10, E1.11, E1.12 according to Migliazza et al39), which contains ≥95% of mutations detected in B-cell NHL of the immunocompetent host and AIDS-NHL.11 39
RESULTS
Expression of BCL-6, syn-1, and LMP-1 among systemic AIDS-NHL.
The panel of 27 systemic AIDS-NHL biopsies included 11 cases of AIDS-SNCCL and 16 cases of AIDS-DLCL (Table1). Within the AIDS-DLCL group, 7 cases were classified as AIDS-LNCCL and 9 cases were classified as AIDS-IBLP. Immunologic/genotypic analyses showed a B-cell origin in all cases (data not shown). Consistent with previous reports,9-16infection of the tumor clone by EBV was detected in 6 of 11 AIDS-SNCCL, 2 of 7 AIDS-LNCCL, and 6 of 9 AIDS-IBLP cases (Table 1). As previously observed,6 9-11 all samples were devoid of KSHV infection, whereas structural alterations of c-MYC were detected in 100% (8 of 8) of AIDS-SNCCL and in a minority (2 of 8) of AIDS-DLCL cases (Table 1).
Expression of BCL-6 Protein, CD138/syndecan-1 (syn-1), and LMP-1 in AIDS-Related Systemic NHLs
| Case-150 . | Diagnosis . | Immunohistochemistry-151 . | Viral Infections . | c-MYC . | BCL-6 . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Working Formulation . | REAL Classification . | BCL-6 (%) . | syn-1 (%) . | LMP-1 (%) . | EBV-152 . | KSHV-152 . | Genetic Lesions . | Rearrangements . | Mutations . | |
| 1 | LNCCL | DLCL | >75 | 0 | 0 | + | − | ND | ND | ND |
| 2 | LNCCL | DLCL | >75 | 0 | 0 | − | ND | ND | ND | ND |
| 3 | LNCCL | DLCL | 50-75 | 0 | 0 | + | ND | ND | ND | ND |
| 4 | LNCCL | DLCL | 50-75 | 0 | 0 | − | − | − | − | + |
| 5 | LNCCL | DLCL | >75 | 0 | 0 | − | − | ND | ND | ND |
| 6 | LNCCL | DLCL | >75 | 0 | 0 | − | − | − | ND | + |
| 7 | LNCCL | DLCL | 10-25 | 0 | 0 | − | − | − | − | + |
| 8 | IBLP | DLCL | 10-25 | 0 | 0 | + | − | − | ND | + |
| 9 | IBLP | DLCL | 0 | <10 | <10 | + | − | − | − | + |
| 10 | IBLP | DLCL | 0 | 50-75 | 10-25 | + | − | + | − | − |
| 11 | IBLP | DLCL | 0 | 10-25 | 10-25 | + | − | ND | ND | ND |
| 12 | IBLP | DLCL | 0 | <10 | 25-50 | + | ND | ND | ND | ND |
| 13 | IBLP | DLCL | 0 | 10-25 | 25-50 | + | − | + | − | ND |
| 14 | IBLP | DLCL | 50-75 | 0 | 0 | − | − | − | ND | − |
| 15 | IBLP | DLCL | 0 | 50-75 | 0 | − | ND | ND | ND | ND |
| 16 | IBLP | DLCL | 0 | 0 | 0 | − | − | ND | ND | ND |
| 17 | SNCCL | Burkitt's | >75 | 0 | 0 | + | − | + | − | + |
| 18 | SNCCL | Burkitt's | >75 | 0 | 0 | + | − | + | − | + |
| 19 | SNCCL | Burkitt's | >75 | 0 | 0 | + | − | ND | ND | ND |
| 20 | SNCCL | Burkitt's | >75 | 0 | 0 | + | − | + | − | − |
| 21 | SNCCL | Burkitt's | 50-75 | 0 | 0 | + | − | + | − | − |
| 22 | SNCCL | Burkitt's | 25-50 | 0 | 0 | + | − | + | ND | + |
| 23 | SNCCL | Burkitt's | >75 | 0 | 0 | − | − | ND | ND | − |
| 24 | SNCCL | Burkitt's | 10-25 | 0 | 0 | − | − | + | − | + |
| 25 | SNCCL | Burkitt's | >75 | 0 | 0 | − | − | + | − | + |
| 26 | SNCCL | Burkitt's | >75 | 0 | 0 | − | − | + | − | + |
| 27 | SNCCL | Burkitt's | 50-75 | 0 | 0 | − | ND | ND | ND | ND |
| Case-150 . | Diagnosis . | Immunohistochemistry-151 . | Viral Infections . | c-MYC . | BCL-6 . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Working Formulation . | REAL Classification . | BCL-6 (%) . | syn-1 (%) . | LMP-1 (%) . | EBV-152 . | KSHV-152 . | Genetic Lesions . | Rearrangements . | Mutations . | |
| 1 | LNCCL | DLCL | >75 | 0 | 0 | + | − | ND | ND | ND |
| 2 | LNCCL | DLCL | >75 | 0 | 0 | − | ND | ND | ND | ND |
| 3 | LNCCL | DLCL | 50-75 | 0 | 0 | + | ND | ND | ND | ND |
| 4 | LNCCL | DLCL | 50-75 | 0 | 0 | − | − | − | − | + |
| 5 | LNCCL | DLCL | >75 | 0 | 0 | − | − | ND | ND | ND |
| 6 | LNCCL | DLCL | >75 | 0 | 0 | − | − | − | ND | + |
| 7 | LNCCL | DLCL | 10-25 | 0 | 0 | − | − | − | − | + |
| 8 | IBLP | DLCL | 10-25 | 0 | 0 | + | − | − | ND | + |
| 9 | IBLP | DLCL | 0 | <10 | <10 | + | − | − | − | + |
| 10 | IBLP | DLCL | 0 | 50-75 | 10-25 | + | − | + | − | − |
| 11 | IBLP | DLCL | 0 | 10-25 | 10-25 | + | − | ND | ND | ND |
| 12 | IBLP | DLCL | 0 | <10 | 25-50 | + | ND | ND | ND | ND |
| 13 | IBLP | DLCL | 0 | 10-25 | 25-50 | + | − | + | − | ND |
| 14 | IBLP | DLCL | 50-75 | 0 | 0 | − | − | − | ND | − |
| 15 | IBLP | DLCL | 0 | 50-75 | 0 | − | ND | ND | ND | ND |
| 16 | IBLP | DLCL | 0 | 0 | 0 | − | − | ND | ND | ND |
| 17 | SNCCL | Burkitt's | >75 | 0 | 0 | + | − | + | − | + |
| 18 | SNCCL | Burkitt's | >75 | 0 | 0 | + | − | + | − | + |
| 19 | SNCCL | Burkitt's | >75 | 0 | 0 | + | − | ND | ND | ND |
| 20 | SNCCL | Burkitt's | >75 | 0 | 0 | + | − | + | − | − |
| 21 | SNCCL | Burkitt's | 50-75 | 0 | 0 | + | − | + | − | − |
| 22 | SNCCL | Burkitt's | 25-50 | 0 | 0 | + | − | + | ND | + |
| 23 | SNCCL | Burkitt's | >75 | 0 | 0 | − | − | ND | ND | − |
| 24 | SNCCL | Burkitt's | 10-25 | 0 | 0 | − | − | + | − | + |
| 25 | SNCCL | Burkitt's | >75 | 0 | 0 | − | − | + | − | + |
| 26 | SNCCL | Burkitt's | >75 | 0 | 0 | − | − | + | − | + |
| 27 | SNCCL | Burkitt's | 50-75 | 0 | 0 | − | ND | ND | ND | ND |
Abbreviations: syn-1, CD138/syndecan-1; REAL, revised European-American Classification of Lymphoid Neoplasms; SNCCL, small noncleaved cell lymphoma; LNCCL, large noncleaved cell lymphoma; IBLP, immunoblastic lymphoma plasmacytoid; DLCL, diffuse large cell lymphoma; LMP-1, latent membrane protein-1; EBV, Epstein-Barr virus; KSHV, Kaposi's sarcoma associated herpesvirus; ND, not done; +, positive; −, negative.
Pathologic samples derived from patients are indicated by numbers.
The percentage of BCL-6, syn-1, and LMP-1+neoplastic cells was assigned to one of the following categories: 0, less than 10%, 10% to 25%, 25% to 50%, 50% to 75%, and greater than 75%.
Tumor cell infection as defined by a combination of in situ hybridization, Southern blot analysis, and PCR studies.
The immunoreactivity of anti–BCL-6, anti–syn-1, and anti–LMP-1 MoAbs was scored using the criteria described in Materials and Methods. The data are summarized in Tables 1 and 2 and representative results are shown in Fig1.
Expression of BCL-6 Protein and CD138/syndecan-1 (syn-1) Throughout the Pathologic Spectrum of AIDS-Related NHLs
| Samples . | . | Phenotype* . | ||
|---|---|---|---|---|
| BCL-6+/syn-1− . | BCL-6−/syn-1+ . | BCL-6−/syn-1− . | ||
| Primary samples | ||||
| Systemic AIDS-NHL | ||||
| AIDS-SNCCL | (n = 11) | 11 | 0 | 0 |
| AIDS-LNCCL | (n = 7) | 7 | 0 | 0 |
| AIDS-IBLP | (n = 9) | 2 | 6 | 1 |
| AIDS-PCNSL | ||||
| AIDS-LNCCL | (n = 4) | 4 | 0 | 0 |
| AIDS-IBLP | (n = 4) | 0 | 2 | 2 |
| AIDS-PEL | (n = 5) | 0 | 5 | 0 |
| Cell lines | ||||
| AIDS-PEL | (n = 6) | 0 | 6 | 0 |
| Samples . | . | Phenotype* . | ||
|---|---|---|---|---|
| BCL-6+/syn-1− . | BCL-6−/syn-1+ . | BCL-6−/syn-1− . | ||
| Primary samples | ||||
| Systemic AIDS-NHL | ||||
| AIDS-SNCCL | (n = 11) | 11 | 0 | 0 |
| AIDS-LNCCL | (n = 7) | 7 | 0 | 0 |
| AIDS-IBLP | (n = 9) | 2 | 6 | 1 |
| AIDS-PCNSL | ||||
| AIDS-LNCCL | (n = 4) | 4 | 0 | 0 |
| AIDS-IBLP | (n = 4) | 0 | 2 | 2 |
| AIDS-PEL | (n = 5) | 0 | 5 | 0 |
| Cell lines | ||||
| AIDS-PEL | (n = 6) | 0 | 6 | 0 |
Abbreviations: syn-1, CD138/syndecan-1; SNCCL, small noncleaved cell lymphoma; LNCCL, large noncleaved cell lymphoma; IBLP, immunoblastic lymphoma plasmacytoid; PCNSL, primary central nervous system lymphoma; PEL, primary effusion lymphoma.
None of the AIDS-NHL samples displayed the BLC-6+/syn-1+ phenotype.
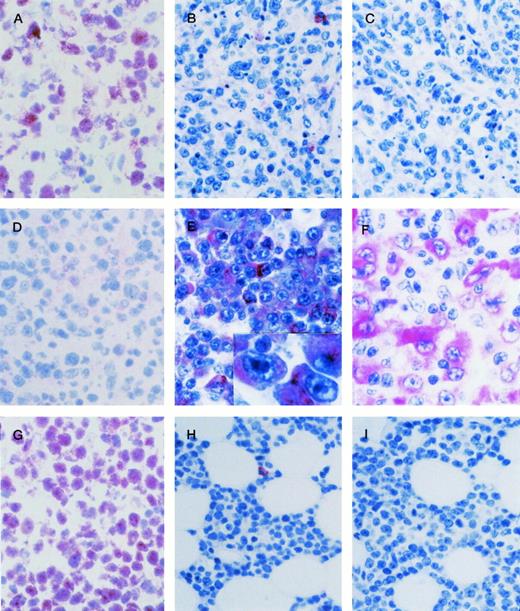
AIDS-related systemic NHLs. (A through C) AIDS-LNCCL displaying the BCL-6+ (A), CD138/syndecan-1 (syn-1)− (B), LMP-1− (C) phenotype. (A) Large tumor cells show a nuclear staining pattern with anti–BCL-6 MoAb. (B) A residual plasma cell shows cytoplasmic staining for anti–syn-1 MoAb. (C) No LMP-1 expression is detectable. (D through F) AIDS-IBLP displaying the BCL-6− (D), syn-1+ (E), LMP-1+ (F) phenotype. (D) No BCL-6 expression is detectable. (E) Most immunoblastic-plasmacytoid tumor cells show strong cytoplasmic immunoreactivity with the anti–syn-1 MoAb. (E inset) A higher-power photograph showing the cytoplasmic staining pattern on large immunoblasts-plasmacytoid. (F) LMP-1 positivity is manifested as cytoplasmic or membrane staining on several large tumor cells displaying immunoblastic-plasmacytoid morphology. (G through I) AIDS-SNCCL displaying the BCL-6+ (G), syn-1− (H), LMP-1− (I) phenotype. (G) Most neoplastic cells show strong nuclear immunoreactivity with the anti–BCL-6 MoAb. (H) A residual plasma cell shows cytoplasmic staining for anti–syn-1 MoAb. (I) No LMP-1 expression is detectable. APAAP immunostaining; (A), (D), (G) frozen section; (B), (C), (E), (E inset), (F), (H), (I) paraffin-embedded tissue section, hematoxylin counterstain. Original magnification ×250 (A through I).
AIDS-related systemic NHLs. (A through C) AIDS-LNCCL displaying the BCL-6+ (A), CD138/syndecan-1 (syn-1)− (B), LMP-1− (C) phenotype. (A) Large tumor cells show a nuclear staining pattern with anti–BCL-6 MoAb. (B) A residual plasma cell shows cytoplasmic staining for anti–syn-1 MoAb. (C) No LMP-1 expression is detectable. (D through F) AIDS-IBLP displaying the BCL-6− (D), syn-1+ (E), LMP-1+ (F) phenotype. (D) No BCL-6 expression is detectable. (E) Most immunoblastic-plasmacytoid tumor cells show strong cytoplasmic immunoreactivity with the anti–syn-1 MoAb. (E inset) A higher-power photograph showing the cytoplasmic staining pattern on large immunoblasts-plasmacytoid. (F) LMP-1 positivity is manifested as cytoplasmic or membrane staining on several large tumor cells displaying immunoblastic-plasmacytoid morphology. (G through I) AIDS-SNCCL displaying the BCL-6+ (G), syn-1− (H), LMP-1− (I) phenotype. (G) Most neoplastic cells show strong nuclear immunoreactivity with the anti–BCL-6 MoAb. (H) A residual plasma cell shows cytoplasmic staining for anti–syn-1 MoAb. (I) No LMP-1 expression is detectable. APAAP immunostaining; (A), (D), (G) frozen section; (B), (C), (E), (E inset), (F), (H), (I) paraffin-embedded tissue section, hematoxylin counterstain. Original magnification ×250 (A through I).
Overall, a positive BCL-6 staining was detected in 20 of 27 (74%) systemic AIDS-NHL (Table 1). Expression of BCL-6 clustered with AIDS-SNCCL (11 of 11, 100%) and AIDS-LNCCL (7 of 7, 100%), whereas it was restricted to 2 of 9 (22.2%) AIDS-IBLP cases (Table 1). As previously observed,16 expression of BCL-6 protein among systemic AIDS-NHL patients occurred both in the presence and in the absence of structural alterations of the BCL-6 gene (Table 1).
Expression of syn-1 was detected in 6 of 27 (22%) cases of systemic AIDS-NHL (Table 1). The syn-1+ phenotype preferentially associated with cases displaying the AIDS-IBLP morphology (6 of 9, 66%), whereas it was not detected in any case of AIDS-SNCCL or AIDS-LNCCL (Table 1). All systemic AIDS-NHL cases which scored positive for syn-1 were found to be negative for BCL-6 expression (Tables 1 and2).
Among systemic AIDS-NHL carrying EBV infection (n = 14), expression of the EBV encoded LMP-1 antigen was detected in 5 cases (Table 1). All cases of LMP-1+ systemic AIDS-NHL were morphologically classified as AIDS-IBLP, expressed syn-1, and stained negative for BCL-6 (Table 1). Conversely, expression of LMP-1 was consistently negative in all cases of EBV+ systemic AIDS-NHL expressing BCL-6 (Table 1).
Expression of BCL-6, syn-1, and LMP-1 among AIDS-PCNSL.
The panel of AIDS-PCNSL biopsies (n = 8) displayed in all cases a morphology consistent with AIDS-DLCL (Table 3). Four cases were classified as AIDS-LNCCL and four cases were classified as AIDS-IBLP. All AIDS-PCNSL were EBV+ and KSHV− (Table3). Immunologic/genotypic analyses showed a B-cell origin in all cases (not shown).
Expression of BCL-6 Protein, CD138/syndecan-1 (syn-1), and LMP-1 in AIDS-PCNSL and AIDS-PEL
| Case* . | Diagnosis . | Immunohistochemistry† . | Viral Infections . | c-MYC . | BCL-6 . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| BCL-6 (%) . | Syn-1 (%) . | LMP-1 (%) . | EBV‡ . | KSHV‡ . | Genetic Lesions . | Rearrangements . | Mutations . | |||
| 28 | AIDS-PCNSL | LNCCL | 50-75 | 0 | 0 | + | − | − | ND | − |
| 29 | AIDS-PCNSL | LNCCL | >75 | 0 | 0 | + | − | − | ND | + |
| 30 | AIDS-PCNSL | LNCCL | 50-75 | 0 | 0 | + | − | − | ND | + |
| 31 | AIDS-PCNSL | LNCCL | 50-75 | 0 | 0 | + | − | − | ND | − |
| 32 | AIDS-PCNSL | IBLP | 0 | 10-25 | 0 | + | − | − | ND | − |
| 33 | AIDS-PCNSL | IBLP | 0 | <10 | 10-25 | + | − | − | ND | − |
| 34 | AIDS-PCNSL | IBLP | 0 | 0 | 50-75 | + | − | − | ND | − |
| 35 | AIDS-PCNSL | IBLP | 0 | 0 | 10-25 | + | − | − | ND | + |
| 36 | AIDS-PEL | 0 | >75 strong | 0 | + | + | − | − | + | |
| 37 | AIDS-PEL | 0 | >75 strong | <10 | + | + | − | − | + | |
| 38 | AIDS-PEL | 0 | >75 strong | 0 | − | + | − | − | + | |
| 39 | AIDS-PEL | 0 | >75 strong | 0 | − | + | − | − | + | |
| 40 | AIDS-PEL | 0 | >75 strong | 0 | + | + | − | − | − | |
| HBL-6 | AIDS-PEL | 0 | >75 strong | <10 | + | + | − | − | + | |
| BC-1 | AIDS-PEL | 0 | >75 strong | 0 | + | + | − | − | + | |
| BC-2 | AIDS-PEL | 0 | >75 strong | 0 | + | + | − | − | + | |
| CRO-AP/2 | AIDS-PEL | 0 | >75 strong | 10-25 | + | + | − | − | + | |
| BC3 | AIDS-PEL | 0 | >75 strong | 0 | − | + | − | − | − | |
| BCBL-1 | AIDS-PEL | 0 | >75 strong | 0 | − | + | − | − | + | |
| Case* . | Diagnosis . | Immunohistochemistry† . | Viral Infections . | c-MYC . | BCL-6 . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| BCL-6 (%) . | Syn-1 (%) . | LMP-1 (%) . | EBV‡ . | KSHV‡ . | Genetic Lesions . | Rearrangements . | Mutations . | |||
| 28 | AIDS-PCNSL | LNCCL | 50-75 | 0 | 0 | + | − | − | ND | − |
| 29 | AIDS-PCNSL | LNCCL | >75 | 0 | 0 | + | − | − | ND | + |
| 30 | AIDS-PCNSL | LNCCL | 50-75 | 0 | 0 | + | − | − | ND | + |
| 31 | AIDS-PCNSL | LNCCL | 50-75 | 0 | 0 | + | − | − | ND | − |
| 32 | AIDS-PCNSL | IBLP | 0 | 10-25 | 0 | + | − | − | ND | − |
| 33 | AIDS-PCNSL | IBLP | 0 | <10 | 10-25 | + | − | − | ND | − |
| 34 | AIDS-PCNSL | IBLP | 0 | 0 | 50-75 | + | − | − | ND | − |
| 35 | AIDS-PCNSL | IBLP | 0 | 0 | 10-25 | + | − | − | ND | + |
| 36 | AIDS-PEL | 0 | >75 strong | 0 | + | + | − | − | + | |
| 37 | AIDS-PEL | 0 | >75 strong | <10 | + | + | − | − | + | |
| 38 | AIDS-PEL | 0 | >75 strong | 0 | − | + | − | − | + | |
| 39 | AIDS-PEL | 0 | >75 strong | 0 | − | + | − | − | + | |
| 40 | AIDS-PEL | 0 | >75 strong | 0 | + | + | − | − | − | |
| HBL-6 | AIDS-PEL | 0 | >75 strong | <10 | + | + | − | − | + | |
| BC-1 | AIDS-PEL | 0 | >75 strong | 0 | + | + | − | − | + | |
| BC-2 | AIDS-PEL | 0 | >75 strong | 0 | + | + | − | − | + | |
| CRO-AP/2 | AIDS-PEL | 0 | >75 strong | 10-25 | + | + | − | − | + | |
| BC3 | AIDS-PEL | 0 | >75 strong | 0 | − | + | − | − | − | |
| BCBL-1 | AIDS-PEL | 0 | >75 strong | 0 | − | + | − | − | + | |
Abbreviations: syn-1, CD138/syndecan-1; LNCCL, large noncleaved cell lymphoma; IBLP, immunoblastic lymphoma plasmacytoid; LMP-1, latent membrane protein-1; EBV, Epstein-Barr virus; KSHV, Kaposi's sarcoma associated herpesvirus; ND, not done; +, positive; −, negative.
Pathologic samples derived from patients are indicated by numbers; cell lines are indicated by their conventional denomination.
The percentage of BCL-6, CD138/syndecan-1, and LMP-1+ neoplastic cells was assigned to one of the following categories: 0, less than 10%, 10% to 25%, 25% to 50%, 50% to 75%, and greater than 75%.
Tumor cell infection as defined by a combination of in situ hybridization, Southern blot analysis, and PCR studies.
Results of immunoreactivity of anti–BCL-6, anti–syn-1, and anti–LMP-1 MoAbs in AIDS-PCNSL are summarized in Tables 2 and 3. Representative results are shown in Fig 2.
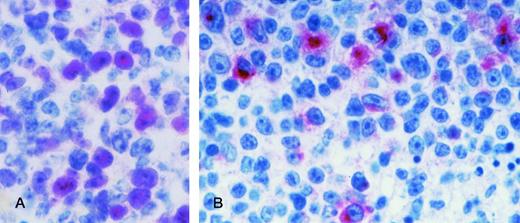
AIDS-PCNSL. (A) BCL-6 protein expression in a case of AIDS-PCNSL (diffuse large cell lymphoma). The microphotograph shows that the tumor is relatively monomorphous and consists of large tumor cells displaying a large noncleaved cell morphology. In this field, nuclear positivity for BCL-6 is present on several tumor cells. Paraffin-embedded tissue section, APAAP immunostaining, hematoxylin counterstain. (B) LMP-1 expression in a case of AIDS-PCNSL (diffuse large cell lymphoma). The microphotograph shows that the tumor is polymorphous and consists of large tumor cells displaying an immunoblastic-plasmacytoid morphology. LMP-1 positivity is manifested as cytoplasmic or membrane staining on some large tumor cells. Paraffin-embedded tissue section, APAAP immunostaining, hematoxylin counterstain. Original magnification ×400 (A) and (B).
AIDS-PCNSL. (A) BCL-6 protein expression in a case of AIDS-PCNSL (diffuse large cell lymphoma). The microphotograph shows that the tumor is relatively monomorphous and consists of large tumor cells displaying a large noncleaved cell morphology. In this field, nuclear positivity for BCL-6 is present on several tumor cells. Paraffin-embedded tissue section, APAAP immunostaining, hematoxylin counterstain. (B) LMP-1 expression in a case of AIDS-PCNSL (diffuse large cell lymphoma). The microphotograph shows that the tumor is polymorphous and consists of large tumor cells displaying an immunoblastic-plasmacytoid morphology. LMP-1 positivity is manifested as cytoplasmic or membrane staining on some large tumor cells. Paraffin-embedded tissue section, APAAP immunostaining, hematoxylin counterstain. Original magnification ×400 (A) and (B).
Overall, expression of BCL-6 was detected in 4 of 8 (50%) cases of AIDS-PCNSL (Table 3). All BCL-6+ AIDS-PCNSL displayed a morphology consistent with AIDS-LNCCL. Conversely, expression of BCL-6 was scored negative in all AIDS-PCNSL classified as AIDS-IBLP (Table3). Expression of BCL-6 protein among AIDS-PCNSL occurred both in the presence and in the absence of structural alterations of theBCL-6 gene (Table 3).
Expression of the syn-1 antigen was detected in 2 of 8 (25%) AIDS-PCNSL cases (Table 3). The two syn-1+ AIDS-PCNSL cases displayed a morphology consistent with AIDS-IBLP and failed to express BCL-6 (Tables 2 and 3).
Expression of LMP-1 was detected in 1 of the 2 BCL-6−, syn-1+ AIDS-PCNSL cases (Table 3). Conversely, LMP-1 stained consistently negative in all BCL-6+, syn-1− AIDS-PCNSL cases (n = 4).
Expression of BCL-6, syn-1, and LMP-1 in AIDS-PEL.
The panel of AIDS-PEL, all positive for KSHV, included 5 primary cases and 6 AIDS-PEL–derived cell lines (Table 3). Three primary AIDS-PEL and 4 AIDS-PEL cell lines carried the EBV genome (Table 3). Immunologic/genotypic analyses showed a B-cell origin in all primary samples and cell lines (not shown). Structural alterations of c-MYC were absent in all cases (Table 3).
Results of immunoreactivity of anti–BCL-6, anti–syn-1, and anti–LMP-1 MoAbs in AIDS-PEL are summarized in Tables 2 and 3. Representative data are shown in Fig 3.
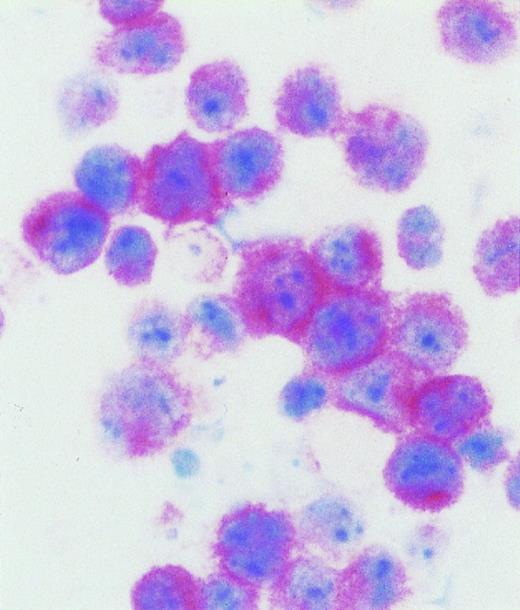
AIDS-related primary effusion lymphoma. The majority of tumor cells show strong cytoplasmic and membrane staining for B-B4 antibody, that recognizes the plasma cell specific CD138/syndecan-1 antigen. Cytospin preparation, APAAP immunostaining, hematoxylin counterstain. Original magnification ×400.
AIDS-related primary effusion lymphoma. The majority of tumor cells show strong cytoplasmic and membrane staining for B-B4 antibody, that recognizes the plasma cell specific CD138/syndecan-1 antigen. Cytospin preparation, APAAP immunostaining, hematoxylin counterstain. Original magnification ×400.
All AIDS-PEL scored negative for BCL-6 protein expression (Tables 2 and3). Conversely, all samples of AIDS-PEL were found to express the syn-1 antigen (Tables 2 and 3). Out of the 7 AIDS-PEL carrying EBV infection, 3 cases displayed a small proportion of cells expressing the LMP-1 antigen (Table 3).
Expression of BCL-6 and syn-1 in PGL.
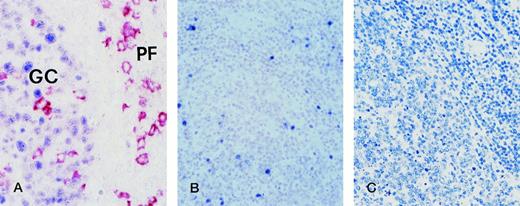
A panel of 7 PGL samples was used to define the expression pattern of BCL-6 and syn-1 in nonneoplastic lymph nodes. In all PGL samples tested, a strong and specific reactivity for BCL-6 was detectable within the follicular GC (see Fig 4 for representative results). The mantle and paracortical zones were mostly negative with the exception of several small lymphoid cells and rare isolated large cells, presumably represented by T cells.21In the same lymph node samples, the anti–syn-1 MoAb showed a strong cytoplasmic and membrane staining of plasma cells, but no reactivity in other cell populations. Syn-1+ plasma cells were consistently present in the interfollicular areas. In addition, a variable number of plasma cells was also found in follicular GC and their surrounding mantle zones. Thus, in the context of PGL, individual lymphoid cells expressed selectively either BCL-6 or syn-1. In particular, when considering PGL areas in which BCL-6+cells and syn-1+ cells were simultaneously detectable, double-labelling experiments ruled out the co-expression of BCL-6 and syn-1 by the same cell (Fig 4).
Hyperplastic lymph node from an HIV-infected person with persistent generalized lymphadenopathy (PGL). (A) Two-color staining. Within a follicle numerous germinal center (GC) cells exhibit nuclear staining (blue) for BCL-6. In the same follicle, large cells with a plasma cell morphology show a strong cytoplasmic and membrane staining (reddish) with the anti-CD138/syndecan-1 MoAb. They are present within the GC and in the perifollicular (PF) zone. No co-expression of both markers by the same GC cell is detectable. Paraffin-embedded tissue section, no counterstain. (B) EBER in situ hybridization. EBER+ cells are localized around the hyperplastic follicle (right) and within the expanded germinal center (left). Paraffin-embedded tissue section, nuclear fast red counterstain. (C) Immunostaining for LMP-1. No LMP-1 expression by lymph node cells is detectable. Paraffin-embedded tissue section, APAAP method, hematoxylin counterstain. Original magnification ×250 (A), ×100 (B) and (C).
Hyperplastic lymph node from an HIV-infected person with persistent generalized lymphadenopathy (PGL). (A) Two-color staining. Within a follicle numerous germinal center (GC) cells exhibit nuclear staining (blue) for BCL-6. In the same follicle, large cells with a plasma cell morphology show a strong cytoplasmic and membrane staining (reddish) with the anti-CD138/syndecan-1 MoAb. They are present within the GC and in the perifollicular (PF) zone. No co-expression of both markers by the same GC cell is detectable. Paraffin-embedded tissue section, no counterstain. (B) EBER in situ hybridization. EBER+ cells are localized around the hyperplastic follicle (right) and within the expanded germinal center (left). Paraffin-embedded tissue section, nuclear fast red counterstain. (C) Immunostaining for LMP-1. No LMP-1 expression by lymph node cells is detectable. Paraffin-embedded tissue section, APAAP method, hematoxylin counterstain. Original magnification ×250 (A), ×100 (B) and (C).
EBV-infected cells were detected in all PGL samples by EBER ISH studies (see Fig 4 for representative results) and Southern blot analysis (not shown). EBV-carrying cells were usually restricted to the interfollicular zone. However, in 2 of 7 PGL samples they were also present within the follicular GC (Fig 4). EBER+ B cells within the GC were consistently negative for LMP-1 expression (Fig 4).
DISCUSSION
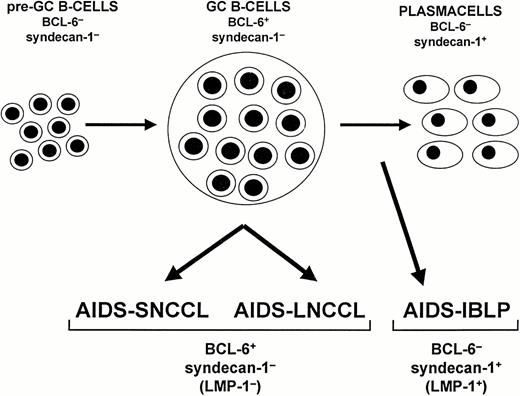
The results presented in this study indicate that expression of BCL-6 and syn-1 segregates two major phenotypic subsets of AIDS-NHL, ie, BCL-6+/syn-1− and BCL-6−/syn-1+ (Fig5). These two phenotypic patterns were confirmed in nonneoplastic B cells, indicating that they correspond to two distinct differentiation programs of normal B cells. These findings bear implications for the histogenesis and diagnosis of AIDS-NHL.
Model of systemic AIDS-NHL histogenesis. The proposed model is based on the expression pattern of BCL-6 and CD138/syndecan-1 (syn-1) throughout physiologic B-cell differentiation. B cells within the germinal center (GC) display the BCL-6+/syn-1− phenotype, whereas B cells that have exited the GC and have undergone further maturation toward the plasma cell stage exhibit the BCL-6−/syn-1+ phenotype. On these bases, systemic AIDS-NHL displaying the BCL-6+/syn-1− phenotype, ie, AIDS-SNCCL and AIDS-LNCCL, are postulated to originate from GC B cells. Conversely, systemic AIDS-NHL displaying the BCL-6−/syn-1+ phenotype, ie, AIDS-IBLP, are postulated to derive from preterminally differentiated B cells. In the case of AIDS-NHL infected by EBV, the BCL-6−/syn-1+ phenotype is permissive for expression of the EBV encoded LMP-1 antigen. Conversely, LMP-1 expression is consistently absent among AIDS-NHL displaying the BCL-6+/syn-1− phenotype.
Model of systemic AIDS-NHL histogenesis. The proposed model is based on the expression pattern of BCL-6 and CD138/syndecan-1 (syn-1) throughout physiologic B-cell differentiation. B cells within the germinal center (GC) display the BCL-6+/syn-1− phenotype, whereas B cells that have exited the GC and have undergone further maturation toward the plasma cell stage exhibit the BCL-6−/syn-1+ phenotype. On these bases, systemic AIDS-NHL displaying the BCL-6+/syn-1− phenotype, ie, AIDS-SNCCL and AIDS-LNCCL, are postulated to originate from GC B cells. Conversely, systemic AIDS-NHL displaying the BCL-6−/syn-1+ phenotype, ie, AIDS-IBLP, are postulated to derive from preterminally differentiated B cells. In the case of AIDS-NHL infected by EBV, the BCL-6−/syn-1+ phenotype is permissive for expression of the EBV encoded LMP-1 antigen. Conversely, LMP-1 expression is consistently absent among AIDS-NHL displaying the BCL-6+/syn-1− phenotype.
In normal lymphoid tissues, the phenotypic patterns identified by BCL-6 and syn-1 map to lymph node areas that are populated by B cells at different stages of differentiation21 26-28 (and this report). In particular, B cells within the GC are BCL-6+/syn-1−, whereas plasma cells, which predominate in interfollicular areas, are BCL-6−/syn-1+. This pattern of expression confirms that post-GC terminal differentiation of B cells is coupled to downregulation of BCL-6 and upregulation of syn-1 (Fig 5).
These observations suggest a histogenetic model for AIDS-NHL development (Fig 5). AIDS-NHL displaying the BCL-6+/syn-1− phenotype originate from GC-related B cells, whereas cases displaying the BCL-6−/syn-1+ phenotype derive from B cells which have exited from the GC and are maturing toward the plasma cell stage. Because AIDS-NHL categories that are pathologically and molecularly distinct (eg, AIDS-SNCCL and AIDS-LNCCL) share the same phenotypic pattern (eg, BCL-6+/syn-1−), it is conceivable that the same progenitor cell may give rise to different types of AIDS-NHL depending on the molecular pathway that is being activated.
Among EBV-infected AIDS-NHL, expression of the EBV-encoded LMP-1 antigen can be found only in BCL-6−/syn-1+cases. Furthermore, all AIDS-DLCL expressing LMP-1 exhibit morphological (IBPL) and/or phenotypic (BCL-6−/syn-1+) features consistent with an advanced stage of B-cell maturation. This suggests that the GC stage is not permissive for LMP-1 expression and that maturation beyond the GC is required for successful LMP-1 expression in B cells. This hypothesis was previously suggested based on the pattern of LMP-1 expression in an in vitro model of EBV-infected Burkitt's lymphoma (BL).40-44 Expression of LMP-1 is absent in EBV-infected BL cell lines retaining the GC phenotype characteristic of BL in vivo (conventionally denominated as group I BL cell lines), whereas it is upregulated in EBV+ BL cell lines which have acquired immunoblastic features after in vitro culture (conventionally denominated as group III BL cell lines). Once the cell becomes permissive for LMP-1 expression, LMP-1 may directly downregulate the level of BCL-6 protein. In fact, recent results indicate that the genetically induced expression of LMP-1, as well as activation of CD40, induces downregulation of BCL-6 expression, suggesting that it may be associated with a critical step of post-GC differentiation of B cells.45
Finally, the expression pattern of BCL-6 and syn-1 bears implications for AIDS-NHL diagnosis. In fact, distinct pathologic and molecular categories of AIDS-NHL selectively associate with different patterns of BCL-6 and syn-1 expression. Remarkably, the association between phenotype and histology appears to be independent of the primary site of the disease, as exemplified by the fact that both systemic AIDS-LNCCL and AIDS-PCNSL with LNCCL morphology share the same BCL-6+/syn-1− phenotype. On these bases, it is conceivable that the expression pattern of BCL-6 and syn-1 may contribute to the morphological diagnosis of these lymphomas.
ACKNOWLEDGMENT
The following reagent was obtained through the AIDS Research and reference Reagent Program, Division of AIDS, NIAID, NIH, Bethesda, MD: BCBL-1 SP from Drs Michael McGrath and Don Ganem. BC-3 was the kind gift of Dr E. Cesarman (Cornell University, New York, NY). LAM C3+ was the kind gift of Dr S. Roncella (IST, Genova, Italy).
Supported in part by the Instituto Superiore di Sanità, AIDS project 1996 and 1997, Rome, Italy; by the Associazione Italiana per la Ricerca sul Cancro, Milan, Italy; by Fondazione “Piera Pietro e Giovanni Ferrero,” Alba, Italy; and by National Institutes of Health Grant No. CA-37295
Address reprint requests to Antonino Carbone, MD, Division of Pathology, Centro di Riferimento Oncologico, IRCCS, via Pedemontana Occidentale, Aviano I-33081, Italy.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be here-by marked “advertisement” in accordance with 18 U.S.C. section 17.34 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal