Abstract
In an effort to identify novel antileukemic agents that can bypass the mechanisms of multidrug resistance, we found that cyclosporin A ([CyA] 5 μmol/L) produced a median cell kill of 69% (range, 47% to 85%) in seven B-lineage acute lymphoblastic leukemia (ALL) cell lines (OP-1, SUP-B15, KOPN-55bi, RS4;11, NALM6, REH, and 380) and three T-lineage ALL cell lines (MOLT4, CCRF-CEM, and CEM-C7) after 4 days of culture. At 10 μmol/L, median CyA toxicity was 99% (range, 88% to >99%). CyA was equally toxic to both a multidrug-resistant cell line, CEM-VLB100, which overexpresses gp-170 P-glycoprotein, and one resistant to topoisomerase II inhibitors, CEM-VM1-5, which has a mutation in the topoisomerase II gene. CyA was also toxic to primary leukemic cells maintained in stroma-based culture, a system that substantially prolongs in vitro cell survival. Against lymphoblasts from 21 patients with B-lineage ALL, the compound (at 5 μmol/L) reduced the leukemic cell number by a median of 87% (range, 27% to >99%) compared with results for parallel control cultures lacking CyA. Seven of these samples were from cases with unfavorable genetic features (eg, Philadelphia-chromosome orMLL gene rearrangements); three were obtained at relapse. Against T lymphoblasts (from six patients), the median reduction in cell number was 79% (range, 30% to >99%). At 10 μmol/L, the cell kill exceeded 97% in all cases studied. The mechanism of CyA cytotoxicity was found to be the activation of apoptosis, which was suppressed by phorbol myristate acetate but not by inhibitors of ceramide-mediated apoptosis, phosphatidyl inositol-3 kinase activity, or tyrosine kinase activity. These findings demonstrate high levels of CyA-induced toxicity against ALL cells at concentrations achievable in vivo, thus providing a strong rationale for clinical testing of this agent in patients with ALL.
THE FUNGAL undecapeptide cyclosporin A (CyA) is widely used in the treatment of allograft rejection, graft-versus-host disease, and a variety of autoimmune disorders.1,2 Its cellular effects depend on its binding to a ubiquitous cytosolic protein (cyclophilin). The complex formed by CyA and cyclophilin targets the Ca2+- and calmodulin-dependent protein phosphatase calcineurin.1,2 The resuting interference with Ca2+-dependent signaling is accompanied by a block in transcriptional activity mediated by key transcription factors, such as NF-AT, Oct-1, and NF-kB, and suppression of a variety of other regulatory molecules, including interleukin-2, MYC, and tyrosine kinases of the SRC family.1 2 Disruption of the pathways controlled by these molecules leads, in turn, to impairment of normal lymphoid cell activation.
Reports of CyA toxicity against leukemic T-cell lines3-6and, more recently, a complete remission induced by CyA alone in a patient with a lymphoproliferative disorder,7 suggest that this agent can inhibit critical cellular functions not only in normal lymphoid cells but also in their malignant counterparts. In this study, we tested the activity of CyA against childhood acute lymphoblastic leukemia (ALL) cells growing in vitro. To directly assess the effects of this compound on leukemic lymphoblasts from patients, we took advantage of a stromal-layer culture system that promotes long-term survival of ALL cells.8-15 The results reported here demonstrate that CyA triggers apoptosis of both T and B leukemic lymphoblasts, including those with molecular and genetic features of drug resistance, suggesting its potential value as an antileukemic agent.
SUBJECTS AND METHODS
Patients and cell lines.
Bone marrow cells were collected at diagnosis or relapse from 27 patients with ALL (21 B-lineage and six T-lineage cases) aged 7 months to 16 years (median, 8 years; Table 1).Bone marrow cells from three healthy donors were also studied. Approval for these studies was obtained from the St. Jude Institutional Review Board, with informed consent given by the parents or guardians of each child. In all B-lineage cases, more than 80% of the lymphoblasts were positive for CD19, class II antigens, and terminal deoxynucleotidyl transferase (TdT) and lacked surface Igs. Six of 21 cases were classified as pre-B ALL, with lymphoblasts expressing cytoplasmic μ heavy chains. In T-lineage cases, more than 80% of the cells were positive for CD7, cytoplasmic or surface CD3, and TdT. In all T-lineage ALL cases, drug testing was performed immediately after cell collection, whereas B-lineage ALL samples were used either fresh or after cryopreservation; previous experiments have shown that the latter does not affect the ability of these cells to grow in stroma-supported cultures.13 In all experiments, cell viability exceeded 80% by trypan blue dye exclusion.
Immunophenotypic and Karyotypic Features of 27 Patients With ALL
| Patient No. . | Age (yr) . | Immunophenotype-151 . | Karyotype . |
|---|---|---|---|
| 1-150 | 5 | Early B | 46,XY,t(9;22)(q34;q11.2)[19] |
| 2 | 8 | Early B | 46,XY,t(9;22)(q34;q11.2)[23]-152 |
| 3-150 | 8 | Early B | 46,XY,t(9;22)(q34;q11.2)[16]-152 |
| 4-150 | 14 | Pre-B | 46,XX,t(4;11)(q21;q23)[22] |
| 5 | 15 | Pre-B | 47,XY,+X,t(4;11)(q21;q23)[10]/46,idem,−X[9]/46,XY[5]-152 |
| 6 | <1 | Pre-B | 46,XY,t(4;11)(q21;q23)[11]/46,XY[10]-152 |
| 7 | 9 | Early B | 47,XY,+X,t(11;19)(q23;p13.3)[22]/46,XY[4]-152 |
| 8 | 3 | Early B | 46,XX[31]-152 |
| 9 | 2 | Pre-B | 46,XY,add(8)(p21)[10]/46,XY,t(9;11)(p22;q23)[1]/46,XY[4]-152 |
| 10 | 8 | Early B | 46,XY,del(9)(p13),t(9;18)(p13;q11)[12]/46,XY[16] |
| 11 | 4 | Early B | 47,XY,+21[3]/46,XY,del(9)(p21)[2] |
| 12 | 8 | Early B | 29,XY,+4,+8,+18,+21,+mar[5]/57,XY,+X,+?Y,+4,+5,del(5)(p13),?del(6)(q21), +6,+8,+9,+14,+18,+21,+mar[6] |
| 13 | 15 | Pre-B | 46,XY,del(3)(q21q26)[9]/46,XY[5] |
| 14 | 6 | Pre-B | 47,XX,+10,del(11)(q23),t(1;12)(p22;p13),t(4;12)(q21;p13)[7]/45,X,−X, t(4;12)(q21;p13)[2]-152 |
| 15 | 1 | Early B | 45,XX,−7,del(1)(q32),der(12)t(7;12)(q11;p13)[10]/46,XX[8]-152 |
| 16 | 14 | Early B | 46,XX[23] |
| 17 | 14 | Early B | 46,XX,del(11)(q23)[4]/46,XX[24]-152 |
| 18 | 3 | Early B | 46,XX,add(21)(p11)[10]/46,XX[10] |
| 19 | 8 | Early B | 46,XX,t(8;9)(q12-13;p22),t(15;19)(q15;p13)[19]/46,idem,−14,+mar[1] |
| 20 | 3 | Early B | 48,XY,+10,add(12)(p13),+21[22]/46,XY[4]-152 |
| 21 | 3 | Early B | 47,XY,add(19)(p13),+21[8]/46,XY[17] |
| 22 | 5 | T | 46,XY,add(19)(p13)[5]/46,XY[12] |
| 23 | 12 | T | 46,XY[50] |
| 24 | 4 | T | 45,XY,del(11)(q14q23),add(12)(p11.2),−18[11]/45,idem, der(16)t(1;16)(q21;q11.2)[5] |
| 25 | 4 | T | ND |
| 26 | 16 | T | 46,XY,del(6)(q15q21)[5]/46,XY[5] |
| 27 | 12 | T | 46,XX[29] |
| Patient No. . | Age (yr) . | Immunophenotype-151 . | Karyotype . |
|---|---|---|---|
| 1-150 | 5 | Early B | 46,XY,t(9;22)(q34;q11.2)[19] |
| 2 | 8 | Early B | 46,XY,t(9;22)(q34;q11.2)[23]-152 |
| 3-150 | 8 | Early B | 46,XY,t(9;22)(q34;q11.2)[16]-152 |
| 4-150 | 14 | Pre-B | 46,XX,t(4;11)(q21;q23)[22] |
| 5 | 15 | Pre-B | 47,XY,+X,t(4;11)(q21;q23)[10]/46,idem,−X[9]/46,XY[5]-152 |
| 6 | <1 | Pre-B | 46,XY,t(4;11)(q21;q23)[11]/46,XY[10]-152 |
| 7 | 9 | Early B | 47,XY,+X,t(11;19)(q23;p13.3)[22]/46,XY[4]-152 |
| 8 | 3 | Early B | 46,XX[31]-152 |
| 9 | 2 | Pre-B | 46,XY,add(8)(p21)[10]/46,XY,t(9;11)(p22;q23)[1]/46,XY[4]-152 |
| 10 | 8 | Early B | 46,XY,del(9)(p13),t(9;18)(p13;q11)[12]/46,XY[16] |
| 11 | 4 | Early B | 47,XY,+21[3]/46,XY,del(9)(p21)[2] |
| 12 | 8 | Early B | 29,XY,+4,+8,+18,+21,+mar[5]/57,XY,+X,+?Y,+4,+5,del(5)(p13),?del(6)(q21), +6,+8,+9,+14,+18,+21,+mar[6] |
| 13 | 15 | Pre-B | 46,XY,del(3)(q21q26)[9]/46,XY[5] |
| 14 | 6 | Pre-B | 47,XX,+10,del(11)(q23),t(1;12)(p22;p13),t(4;12)(q21;p13)[7]/45,X,−X, t(4;12)(q21;p13)[2]-152 |
| 15 | 1 | Early B | 45,XX,−7,del(1)(q32),der(12)t(7;12)(q11;p13)[10]/46,XX[8]-152 |
| 16 | 14 | Early B | 46,XX[23] |
| 17 | 14 | Early B | 46,XX,del(11)(q23)[4]/46,XX[24]-152 |
| 18 | 3 | Early B | 46,XX,add(21)(p11)[10]/46,XX[10] |
| 19 | 8 | Early B | 46,XX,t(8;9)(q12-13;p22),t(15;19)(q15;p13)[19]/46,idem,−14,+mar[1] |
| 20 | 3 | Early B | 48,XY,+10,add(12)(p13),+21[22]/46,XY[4]-152 |
| 21 | 3 | Early B | 47,XY,add(19)(p13),+21[8]/46,XY[17] |
| 22 | 5 | T | 46,XY,add(19)(p13)[5]/46,XY[12] |
| 23 | 12 | T | 46,XY[50] |
| 24 | 4 | T | 45,XY,del(11)(q14q23),add(12)(p11.2),−18[11]/45,idem, der(16)t(1;16)(q21;q11.2)[5] |
| 25 | 4 | T | ND |
| 26 | 16 | T | 46,XY,del(6)(q15q21)[5]/46,XY[5] |
| 27 | 12 | T | 46,XX[29] |
Abbreviation: ND, not determined.
Studied at relapse.
Early B: CD19+, CD10+ or CD10−; Pre-B: same, but with cytoplasmic μ; T: CD7+, cytoplasmic or surface CD3+.
Karyotypes previously reported.
Mononuclear cells were collected by density-gradient centrifugation (Lymphoprep; Nycomed, Oslo, Norway) and washed three times in phosphate-buffered saline (PBS) and once in AIM-V (GIBCO, Grand Island, NY), a chemically defined, serum-free, cytokine-free tissue culture medium. The ALL cell lines selected for this study (OP-1, SUP-B15, KOPN-55bi, RS4;11, NALM6, REH, 380, CCRF-CEM, CEM-C7, and MOLT4) are maintained in our laboratory, and the cell lines CEM-VLB100 and CEM-VM1-5 were provided by Dr W.M. Beck, University of Illinois Cancer Center (Chicago, IL).16 17All cell lines were maintained in RPMI 1640 tissue culture medium (Whittaker Bioproducts, Walkersville, MD) with 10% fetal calf serum (Whittaker).
Cell culture and treatment with CyA.
Bone marrow stromal cells depleted of T cells by CD6/CD8-mediated rabbit complement lysis were derived from seven normal donors (5 to 26 years old) and prepared in 96-well flat-bottomed plates (Costar, Cambridge, MA) as previously described.12-15 To prepare test cultures, we removed the media from the bone marrow stroma and washed the adherent cells seven times with AIM-V tissue culture medium. Leukemic lymphoblasts or normal bone marrow cells were resuspended in AIM-V, and 1 to 3 × 105 cells were placed on the stromal layer in each well. Continuously growing cell lines were resuspended in RPMI 1640 with additives, and 1 to 3 × 104 cells were plated in each well in the absence of stroma. CyA (Calbiochem Inc, San Diego, CA) was diluted in absolute ethanol (stock solution, 5 mmol/L) and further diluted in AIM-V or RPMI 1640 immediately before it was added to the cultures at the final concentrations indicated in the results. In some experiments, a CyA preparation for intravenous administration (Sandimmune; Sandoz, Basel, Switzerland) was also used. Teniposide was obtained from Bristol Myers Squibb (Wallingford, CT) and daunorubicin from Sigma (St Louis, MO). Phorbol myristate acetate, fumonisin B1, sphingosine 1-phosphate, staurosporine, and genistein were purchased from Sigma. Cultures were incubated at 37°C with 5% CO2 and 90% humidity for the times indicated in the results.
Assessment of drug effects.
At the beginning and end of the cultures, the cell number and immunophenotype were determined by flow cytometry.9,11,13,14 Briefly, cultures were transferred to Falcon tubes (Becton Dickinson, Lincoln Park, NJ). Cells from B-lineage cases were incubated with CD19 (Leu 12) monoclonal antibody conjugated to fluorescein isothiocyanate (FITC), and those from T-lineage cases with CD7-FITC. Normal bone marrow cells were labeled with CD34 conjugated to peridin chlorophyll protein, CD19 PE, as well as with CD13 and CD33 conjugated to FITC. All monoclonal antibodies and isotype-matched unreactive controls were purchased from Becton Dickinson (San Jose, CA) or Dako (Carpinteria, CA). Staining with antibodies was omitted in experiments with cell lines. After two washes in PBS with 0.2% bovine serum albumin and 0.2% sodium azide, the cells were resuspended in 0.5% paraformaldehyde and analyzed with a FACScan flow cytometer and Cell Quest software (Becton Dickinson). In each case, we outlined “gates” surrounding the area of the light-scatter dot plot that comprised all viable leukemic cells, and used them to enumerate cells that were present before and after culture with or without CyA. For primary leukemias and normal bone marrow cells, the results were corrected for the percentage of cells in each sample expressing different antigens. The formula, (no. of cells recovered with CyA/no. of cells recovered without CyA) × 100, was used to calculate relative cell recovery after CyA treatment. All results are reported as the mean of duplicate experiments. Drug concentrations that produced 50% cell killing (LC50) were calculated with the AllFit software from the National Institutes of Health.18
DNA gel electrophoresis to detect apoptotic DNA fragmentation was performed after separating fragmented and intact DNA by centrifugation as previously described.8 We also studied cell labeling by Annexin-V conjugated to FITC (Trevigen, Gaithersburg, MD), which binds to phosphatidylserine exposed on the surface membrane of cells undergoing apoptosis.19 Cell membrane permeabilization was detected by standard propidium iodide labeling.
RESULTS
Toxicity against leukemic cell lines.
CyA at 5 μmol/L was markedly toxic to all of the leukemic cell lines studied (Table 2). After 4 days of culture, the median percentage of cell kill among the seven B-lineage lines (OP-1, SUP-B15, KOPN-55bi, RS4;11, NALM6, REH, and 380) and the three T-lineage lines (MOLT4, CCRF-CEM, and CEM-C7) was 69% (range, 47% to 85%). The extent of CyA cytotoxicity was dose-dependent in each of these cell lines (Table 2). At 10 μmol/L, CyA was highly cytotoxic against all lines, with a median cell kill of 99% (range, 88% to >99%) after 4 days of incubation. With most of the cell lines, CyA-induced toxicity was still appreciable when the drug was added at a concentration of 2 μmol/L (median, 36%; range, 10% to 73%). In cultures containing vehicle only (0.1% ethanol), the number of cells recovered was identical to that of control cultures without additives (data not shown). In comparative experiments, the antileukemic potency of CyA from Calbiochem diluted in ethanol and the CyA preparation for clinical use (Sandimmune) was identical (data not shown).
CyA Toxicity Against ALL Cell Lines
| Cell Line . | % Cell Kill With Increasing Concentration of CyA (μmol/L) . | |||
|---|---|---|---|---|
| 1 . | 2 . | 5 . | 10 . | |
| B-lineage ALL | ||||
| OP-1 | 16 | 34 | 69 | 98 |
| SUP-B15 | 34 | 39 | 78 | >99 |
| KOPN-55bi | 22 | 44 | 59 | >99 |
| RS4;11 | 35 | 35 | 71 | 98 |
| NALM6 | 55 | 73 | 85 | >99 |
| REH | 25 | 25 | 64 | 99 |
| 380 | 20 | 62 | 69 | 90 |
| T-lineage ALL | ||||
| MOLT4 | 32 | 37 | 58 | 88 |
| CCRF-CEM | 6 | 24 | 85 | >99 |
| CEM-C7 | 3 | 10 | 47 | 95 |
| Cell Line . | % Cell Kill With Increasing Concentration of CyA (μmol/L) . | |||
|---|---|---|---|---|
| 1 . | 2 . | 5 . | 10 . | |
| B-lineage ALL | ||||
| OP-1 | 16 | 34 | 69 | 98 |
| SUP-B15 | 34 | 39 | 78 | >99 |
| KOPN-55bi | 22 | 44 | 59 | >99 |
| RS4;11 | 35 | 35 | 71 | 98 |
| NALM6 | 55 | 73 | 85 | >99 |
| REH | 25 | 25 | 64 | 99 |
| 380 | 20 | 62 | 69 | 90 |
| T-lineage ALL | ||||
| MOLT4 | 32 | 37 | 58 | 88 |
| CCRF-CEM | 6 | 24 | 85 | >99 |
| CEM-C7 | 3 | 10 | 47 | 95 |
Data are the mean of duplicate 4-day culture experiments. Intraassay variability was <5% in all experiments.
To determine whether CyA cytotoxicity would bypass the common mechanisms of drug resistance, we tested different concentrations of the compound against the CEM-VLB100 cell line, which overexpresses gp-170 P-glycoprotein and is resistant to several anticancer drugs,16 as well as the CEM-VM1-5 cell line, which carries a mutation in the topoisomerase II gene and is resistant to topoisomerase II inhibitors,17 and compared the results with those obtained with the parent cell line CCRF-CEM. As expected, CEM-VLB100 was relatively resistant to daunorubicin (LC50, 40.1 v 2.2 ng/mL in CCRF-CEM cells), while CEM-VM1-5 showed resistance to teniposide (LC50, 16.8v 0.04 μmol/L in CCRF-CEM cells). By contrast, the concentrations of CyA that were cytotoxic in all three lines were similar (LC50, 2.9 μmol/L in CCRF-CEM, 3.7 in CEM-VLB100, and 5.6 in CEM-VM1-5).
Toxicity against clinical samples of leukemic cells.
To assess the toxic effects of CyA against fresh samples of leukemic lymphoblasts from patients with ALL, we took advantage of a stroma-based culture system that allows prolonged cell culture.8-15 After 7 days of such culture, the median cell recovery among 27 cases studied was 103% (range, 50% to 550%). CyA at 5 μmol/L produced variable levels of cytotoxicity in these cases (Fig 1). Against B-lineage ALL (21 cases), the reduction in leukemic cell number was 27% to more than 99% (median, 87%) of that in parallel 7-day cultures not exposed to the compound but containing 0.1% ethanol (the CyA vehicle). Likewise, CyA at 5 μmol/L was cytotoxic in all six cases of T-cell ALL (range of cell kill, 30% to >99%; median, 79%; Fig 1). CyA cytotoxicity was dose-dependent, and in some cases remained substantial at concentrations as low as 1 μmol/L. At 2 μmol/L CyA, the percentage of cell kill for 20 cases of B-lineage ALL was 12% to 61% (median, 32%). Cytotoxicity was not detectable at this concentration in only one case. Similar levels of cell kill were obtained at this drug concentration in T-cell samples (median, 58%; range, 16% to 83%).
CyA toxicity against ALL cells from patients after 7 days of culture on bone marrow–derived stromal layers. Data are the mean of duplicate experiments. Horizontal bars indicate the median % cell kill for each concentration of CyA. Intraassay variability was <10% in all experiments.
CyA toxicity against ALL cells from patients after 7 days of culture on bone marrow–derived stromal layers. Data are the mean of duplicate experiments. Horizontal bars indicate the median % cell kill for each concentration of CyA. Intraassay variability was <10% in all experiments.
Cells from the seven B-lineage ALL cases with adverse genetic features (no. 1 to 7), including t(9;22), t(4;11), or t(11;19),20were susceptible to CyA (at 5 μmol/L) to the extent that the levels of cell kill did not differ significantly from those determined in other B-lineage cases: median, 95% (range, 67% to >99%) versus 85% (27% to >99%) at 5 μmol/L, and 29% (12% to 59%) versus 33% (<1% to 61%) at 2 μmol/L. Notably, three of the samples with high-risk genetic features (no. 1, 3, and 4) were obtained at relapse. Likewise, the four cell lines with t(9;22) (OP-1, SUP-B15, and KOPN-55bi) or t(4;11) (RS4;11) were as susceptible to CyA cytotoxicity as cell lines without these high-risk features (Table 2).
To determine whether CyA cytotoxicity represented a direct effect on leukemic lymphoblasts or an indirect effect mediated by damage to stromal layers, we incubated the stromal layers with CyA at various concentrations (1 to 10 μmol/L) for 2 days, washed them, and then seeded them with leukemic lymphoblasts. In two independent experiments with samples from patients no. 4 and 8, the percentage of cell recovery after 7 days of culture on CyA-pretreated stroma was identical to that achieved with unmanipulated stromal layers. For example, the percentage of cell recovery after culture on unmanipulated stroma was 111% and 103% in the two cases; after culture on stroma pretreated with CyA 5 μmol/L, it was 111% and 102%, respectively. Thus, any indirect toxicity to stromal layers does not appear to influence the recovery of ALL cells exposed to CyA.
Toxicity against normal immature hematopoietic cells.
Previous studies with colony-forming assays have indicated that CyA has no toxicity against normal hematopoietic cells.21-24 To further address this issue, we prepared stroma-supported cultures of normal bone marrow mononucleated cells from three individuals with and without CyA, and assessed the effects of the compound on the expansion of phenotypically immature cells characterized by low side-scatter, expression of CD34, and absence of markers associated with B-lineage (CD19) or myeloid-lineage (CD13 and CD33) differentiation.25 26 At the beginning of the cultures, cells with these characteristics represented less than 0.1% of bone marrow mononucleated cells in all three samples. After 7 days of culture without CyA, the mean ±SD percentage of CD34+, CD19−, CD13−, CD33− cell recovery was 879% ± 684% of those originally seeded. CyA at 2 μmol/L did not significantly change the expansion of these cells in culture (mean cell recovery, 849% ± 620%). At 5 μmol/L, the number of CD34+, CD19−, CD13−, CD33− cells was lower than in control cultures (percent cell recovery, 233% ± 288%), indicating an inhibitory effect of the compound on the growth of normal hematopoietic cells. Nevertheless, the mean number of CD34+, CD19−, CD13−, CD33− cells in these cultures had doubled despite CyA-mediated inhibition of cell growth.
Molecular mechanism of CyA-induced cell death.
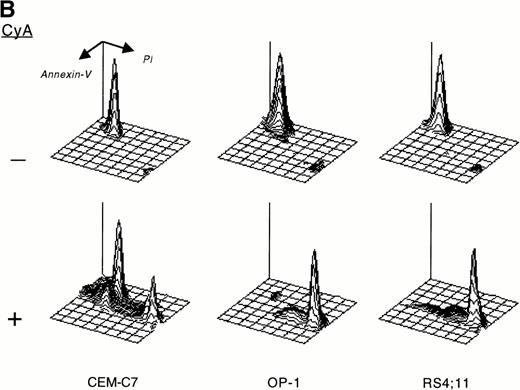
CyA appeared to trigger apoptosis in all cell lines and leukemic cases studied as early as 24 hours after its addition to cultures, as indicated by typical changes in cell morphology observed by inverted microscopy (ie, nuclear fragmentation and cell shrinkage; not shown). CyA-induced morphologic deterioration was reflected by changes in light-scatter properties of the cells determined by flow cytometry, resulting in a reduction in forward light-scatter and an increase in 90° light-scatter (Fig 2A).27 Exposure of phosphatidyl serine residues on the cell membrane (revealed by binding to Annexin-V19; Fig 2B) and massive DNA fragmentation in multiples of 180 base pairs28 (Fig 2C) determined in experiments with cell lines confirmed that CyA induced apoptosis in ALL cells.
CyA induces apoptosis in ALL cells. The T-lineage ALL cell line CEM-C7 and the B-lineage ALL cell lines OP-1 (carrying the Philadelphia chromosome) and RS4;11 (with MLL gene rearrangements) were incubated with CyA (10 μmol/L) or the vehicle (ethanol 0.1%) for 48 hours. (A) Flow-cytometric dot plots illustrate changes in cell light-scatter properties typical of apoptosis caused by exposure to CyA. These consisted of decreased cell size (forward scatter, FSC; x-axis) and increased cell granularity (side scatter, SSC; y-axis). (B) Isometric contour plots illustrate binding of Annexin-V to phosphatidyl serine residues on the cell membrane (a feature of apoptosis) and propidium iodide labeling due to cell membrane permeabilization caused by CyA. (C) In all 3 cell lines, CyA induced a DNA ladder of a multiple of 180 base pairs, characteristic of apoptosis. Molecular mass markers are indicated.
CyA induces apoptosis in ALL cells. The T-lineage ALL cell line CEM-C7 and the B-lineage ALL cell lines OP-1 (carrying the Philadelphia chromosome) and RS4;11 (with MLL gene rearrangements) were incubated with CyA (10 μmol/L) or the vehicle (ethanol 0.1%) for 48 hours. (A) Flow-cytometric dot plots illustrate changes in cell light-scatter properties typical of apoptosis caused by exposure to CyA. These consisted of decreased cell size (forward scatter, FSC; x-axis) and increased cell granularity (side scatter, SSC; y-axis). (B) Isometric contour plots illustrate binding of Annexin-V to phosphatidyl serine residues on the cell membrane (a feature of apoptosis) and propidium iodide labeling due to cell membrane permeabilization caused by CyA. (C) In all 3 cell lines, CyA induced a DNA ladder of a multiple of 180 base pairs, characteristic of apoptosis. Molecular mass markers are indicated.
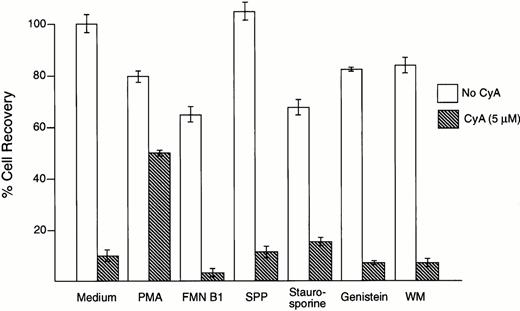
To investigate the signaling pathways leading to apoptosis in leukemic cells exposed to CyA, we used a series of compounds that have been reported to inhibit apoptosis induced by a variety of stimuli. Phorbol myristate acetate (1 to 10 ng/mL), which stimulates protein kinase C activity and inhibits radiation- and glucocorticoid-induced apoptosis,29,30 suppressed the toxic effects of CyA on the pre-B ALL cell line NALM6 (Fig 3) and in the T-cell line MOLT4 (not shown). All other compounds tested at nontoxic concentrations, including the ceramide-mediated apoptosis inhibitors fumonisin B1 (1 to 50 μmol/L) and sphingosine-1-phosphate (0.1 to 10 μmol/L),31-33 the phosphatidyl inositol-3 kinase inhibitor wortmannin (100 nmol/L),34-36 and the tyrosine kinase inhibitors staurosporine (3 to 50 nmol/L) and genistein (1 to 50 nmol/L),37 lacked any discernible effect on CyA-induced cytotoxicity in both cell lines (Fig 3, and not shown).
CyA-induced apoptosis is suppressed by phorbol myristate acetate (PMA). B-lineage ALL cell line NALM6 was incubated for 1 hour with the compounds indicated before addition of CyA (5 μmol/L). After 48 hours of culture, the number of viable cells in cultures with and without CyA was compared. Only preincubation with PMA (1 ng/mL) inhibited CyA-induced apoptosis, while fumonisine B1 (FMN B1, 50 μmol/L), sphingosine 1-phosphate (SPP, 10 μmol/L), staurosporine (3 μmol/L), genistein (10 μmol/L), and wortmannin (WM, 100 nmol/L) had no effect. At higher concentrations, these compounds were markedly toxic. Bars represent the mean ± SD of 4 measurements per test.
CyA-induced apoptosis is suppressed by phorbol myristate acetate (PMA). B-lineage ALL cell line NALM6 was incubated for 1 hour with the compounds indicated before addition of CyA (5 μmol/L). After 48 hours of culture, the number of viable cells in cultures with and without CyA was compared. Only preincubation with PMA (1 ng/mL) inhibited CyA-induced apoptosis, while fumonisine B1 (FMN B1, 50 μmol/L), sphingosine 1-phosphate (SPP, 10 μmol/L), staurosporine (3 μmol/L), genistein (10 μmol/L), and wortmannin (WM, 100 nmol/L) had no effect. At higher concentrations, these compounds were markedly toxic. Bars represent the mean ± SD of 4 measurements per test.
DISCUSSION
In this study, we found that CyA is toxic to leukemic lymphoblasts via induction of apoptosis, which was detectable as early as 24 hours after exposure of the cells to the drug. Although previous studies had indicated that CyA selectively produces toxicity in T cells,3-6 we did not find its effects to be restricted to cells of this lineage. To the contrary, the compound was equally active against B and T lymphoblasts; moreover, both continuously growing cell lines and fresh samples of leukemic cells from patients were susceptible to the toxic effects of CyA. Although the toxicity of CyA observed on primary leukemic cells could be due to an effect of the compound on the stromal layers used to support ALL cell survival in vitro, we think this indirect mechanism unlikely, as our experiments using stroma pretreated with CyA failed to reveal a decrease in its capacity to promote ALL cell survival. In addition, the cytotoxic effects of CyA were also observed in the ALL cell lines growing in vitro without stromal cells.
The suppressive effects of CyA on normal lymphocyte function include inhibition of T-cell activation in vitro in response to mitogenic lectins, CD3 ligation, phorbol ester, and Ca2+ionophore,1,2 as well as suppression of B-cell activation in response to B-cell receptor cross-linking.38 Notably, treatment of murine splenic B cells with CyA induces cell death.39 Our findings indicate that CyA also suppresses the growth of malignant human lymphoid cells. The precise biochemical mechanisms underlying CyA-induced apoptosis in leukemic lymphoblasts remain to be clarified, but appear to be, at least in part, distinct from those operating in cells undergoing apoptosis after exposure to tumor necrosis factor,31,32 daunorubicin,33 and ligation of Fas,32,37 surface Ig,35 or CD38,36 as inhibitors that rescue cells from these agents failed to diminish CyA toxicity. Nevertheless, CyA toxicity was markedly suppressed by exposure to phorbol ester, reminiscent of observations with radiation- and steroid-induced apoptosis in thymocytes.29,30 Interestingly, other compounds known to interfere with Ca2+-mediated signaling and T-cell activation,1,2 such as FK-506 and rapamycin, did not induce apoptosis in ALL cells (our unpublished observation, September 1996). FK-506, which is approximately 100-fold more active than CyA in inhibiting T-cell activation, had no effect on leukemic cell growth or survival,6 while rapamycin had a cytostatic but not cytotoxic activity against ALL cell lines.
Our findings have clear clinical implications. Currently, about 30% of children and 65% of adults with ALL can be expected to relapse one or more times.40 Patients with the Philadelphia-chromosome or 11q23 abnormalities involving the MLL gene, representing approximately 6% of children and 30% of adults with ALL, have an especially dire prognosis.40 Thus, new antileukemic agents capable of overcoming the different mechanisms of drug resistance are urgently needed. It is noteworthy in this regard that all of our cases, including those studied at relapse and/or displaying adverse cytogenetic features, as well as cell lines with molecular features directly linked to multidrug resistance, were susceptible to CyA-induced apoptosis.
The findings of CyA antileukemic activity in this study complement previous observations that CyA increases the sensitivity of multidrug-resistant cells to anticancer agents through a specific interaction of CyA with the 170-kD drug-efflux pump P-glycoprotein.41,42 For example, in experiments with mice engrafted with L1210 lymphoid leukemia and treated with etoposide, the addition of CyA significantly prolonged survival.43 Of note, in our study, the cell line CEM-VLB100, which overexpresses P-glycoprotein, was nevertheless susceptible to CyA-induced apoptosis. Clinical trials of CyA administered intravenously as a modulator of drug resistance have shown that steady-state serum levels of 3 to 5 μmol/L,44,45 which are cytotoxic in vitro, are readily achieved at tolerable administered doses. The unique antileukemic activity of CyA, which may bypass the common mechanisms of drug resistance, and its relatively lower toxicity against immature normal hematopoietic cells21-24 provide a strong rationale for clinical testing of this agent in patients with high-risk ALL.
ACKNOWLEDGMENT
We thank Elaine Coustan-Smith for flow-cytometric analysis of apoptosis and John Gilbert for editorial assistance.
Supported by Grants No. RO1-CA58297, P30-CA21765, and CA20180 from the National Cancer Institute, and by the American Lebanese Syrian Associated Charities.
Address reprint requests to Dario Campana, MD, PhD, Department of Hematology-Oncology, St Jude Children's Research Hospital, 332 N Lauderdale, Memphis, TN 38105.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal