Abstract
Recently, a new alloantigen on IgG Fc receptor type IIIb (FcγRIIIb), SH, was described (Bux et al, Blood 89:1027, 1997). We identified three healthy individuals whose neutrophils reacted positively with the SH antiserum. The neutrophil antigen (NA) phenotype of all three donors was NA(1+,2+). Analysis of genomic DNA showed that the three donors were positive for the described SH-encoding mutation in the NA2-FcγRIIIB gene, 266C→A. However, NA(1,2) genotyping and nucleotide sequencing of an NA2-specific fragment amplified from the genomic DNA fragment showed that these individuals carried three FcγRIIIBgenes, namely, NA1-FcγRIIIB, NA2-FcγRIIIB, andSH-FcγRIIIB, encoding NA1-FcγRIIIb, NA2-FcγRIIIb, and SH-FcγRIIIb, respectively. Southern blot analysis confirmed these findings. Furthermore, all three transcripts were isolated from neutrophil mRNA. To investigate whether the presence of threeFcγRIIIB genes resulted in a higher membrane expression of FcγRIIIb, we measured the reactivity of neutrophils from NA(1+,2+)SH(+) individuals with a panel of CD16 monoclonal antibodies (MoAbs) in comparison with neutrophils from NA(1+,2+)SH(−) controls. Reactivity of four different anti–pan-FcγRIII MoAbs and NA2-specific MoAb GRM1 was higher with SH(+) neutrophils compared with controls, whereas that of NA1-specific MoAbs was similar, which is in concordance with the results from the genomic analysis. We observed that reactivity with NA2-specific CD16 MoAb PEN1 was sixfold higher in SH(+) individuals compared with controls. Apparently, the 60Ala→Asp substitution in SH-FcγRIIIb influences the epitope recognized by PEN1. In conclusion, we identified three NA(1+,2+)SH(+) individuals carrying three FcγRIIIB genes and observed a clear gene-dosage effect on the level of expression of neutrophil FcγRIIIb.
NEUTROPHIL ANTIGENS (NAs) are involved in several clinical conditions, such as blood transfusion reactions and immune-mediated neutropenia.1 The NA system has been extensively investigated and is located on IgG Fc receptor type IIIb (FcγRIIIb; CD16).2,3 NA1- and NA2-FcγRIIIb differ by four amino acids in the membrane-distal Ig-like domain.4These differences lead to distinct glycosylation patterns of NA1- and NA2-FcγRIIIb.2,3 Although the amino acid differences are not located in the membrane-proximal, ligand-binding domain of the receptor, the FcγRIIIb-NA(1,2) isoforms differ in their capacity to interact with IgG.5-8 Alloantibodies against NA1-FcγRIIIb and, to a lesser extent, NA2-FcγRIIIb can be detected in a large proportion of patients suffering from neonatal immune-mediated neutropenia.9
Recently, Bux et al10 described four cases of alloimmune neutropenia in which alloantibodies recognizing a thus far unknown antigen on FcγRIIIb were identified. The newly identified alloantigen was termed SH and has a gene frequency of 4% in the German population.10 Nucleotide sequence analysis showed that theSH-FcγRIIIB gene differed from NA2-FcγRIIIB by a single base pair (266C→A), encoding an Ala→Asp substitution at amino acid position 60. The authors concluded that SH-FcγRIIIb is the product of an NA2-FcγRIIIBpolymorphism.10
In this report, we analyzed three individuals whose neutrophils were phenotyped as NA(1+,2+)SH(+). Genomic analysis showed that in these individuals, an FcγRIIIB gene encoding SH-FcγRIIIb exists alongside an NA1- and an NA2-FcγRIIIB gene. All threeFcγRIIIB genes were transcribed into mRNA. Furthermore, in all three donors we observed a clear gene-dosage effect with regard to the neutrophil membrane expression of FcγRIIIb, attributable to the equal expression of the three isoforms.
MATERIALS AND METHODS
Antibodies and antisera.
Anti–pan-FcγRIII (CD16) monoclonal antibodies (MoAbs) used were CLBFcRgran1 (mIgG2a), 3G8 (mIgG1), BW209/2 (mIgG2a), and MEM154 (mIgG1). CLBgran11 (mIgG2a) and MG38 (mIgG1) recognize NA1-FcγRIIIb, whereas GRM1 (mIgG2a) recognizes NA2-FcγRIIIb and FcγRIIIa. PEN1 (mIgG2a) reacts with a small oligosaccharide moiety of NA2-FcγRIIIb and with FcγRIIIa.11 BW209/2 was a generous gift from Dr Kurrle (Behring Werke, Marburg, Germany) and GRM1 was provided by Dr Garrido (Hospital des Nieves, Granada, Spain). CLBFcRgran1, CLBgran11, and irrelevant control MoAbs were from our own institute. The other CD16 MoAbs were obtained via the Fifth International Workshop on Leukocyte Antigens (Boston, MA; November 1993). A human antiserum recognizing SH-FcγRIIIb was obtained via the Second International Granulocyte Serology Workshop (Helsinki, Finland; May 1996). Sera from healthy AB-positive individuals were used as controls. Fluorescein isothiocyanate (FITC)-labeled F(ab′)2 fragments of goat-antimouse–Ig and FITC–goat-antihuman–Ig from our institute were used to detect MoAb and human antibody binding, respectively.
Isolation of cells.
EDTA-anticoagulated blood was centrifuged over a Ficoll-Hypaque gradient with a specific gravity of 1.076 g/mL (Pharmacia Fine Chemicals, Uppsala, Sweden). Mononuclear cells were harvested from the interphase for DNA isolation and the pellet was treated with ice-cold NH4Cl solution (155 mmol/L NH4Cl, 10 mmol/L KHCO3, 0.1 mmol/L EDTA, pH 7.4) to lyse erythrocytes. The remaining cells were more than 95% neutrophils.
Flow cytometry.
Neutrophils were tested for reactivity with a panel of CD16 MoAbs and with the SH antiserum by indirect immunofluorescence.12Briefly, neutrophils were fixed with 1% paraformaldehyde (PFA; wt/vol) and were incubated with MoAb or human antiserum for 30 minutes at room temperature. After washing with phosphate-buffered saline (PBS) containing 0.2% bovine serum albumin (wt/vol) the cells were stained with FITC-labeled F(ab′)2 fragments of goat-antimouse–Ig or FITC–goat-antihuman–Ig. Binding of the conjugate was assessed with a FACScan flowcytometer (Becton Dickinson, San Jose, CA).
Soluble FcγRIII enzyme-linked immunosorbent assay (ELISA).
The plasma level of sFcγRIII was measured by a sandwich ELISA, essentially as previously described.13 Briefly, ELISA plates were incubated with CD16 MoAb CLBFcRgran1 and blocked with PBS containing 2% (vol/vol) milk. The plates were incubated with plasma that was diluted in High Performance ELISA (HPE) buffer (CLB, Amsterdam, The Netherlands). Subsequently, a biotinylated polyclonal rabbit-antihuman–FcγRIII antibody diluted in HPE buffer was added. After incubation with horse radish peroxidase-labeled streptavidin, a substrate was added to measure the amount of bound antibody. Plasma from 90 healthy individuals was pooled and used to construct a calibration curve. The level of sFcγRIII in this pool was set at 100 arbitrary units (AU).
FcγRIIIB-NA(1,2) genotyping assays.
Genotyping for the FcγRIIIB-NA(1,2) polymorphism was performed as described before.14 In brief, two sets of primers specifically annealing to either an NA1-FcγRIIIB or an NA2-FcγRIIIB fragment were used. NA1-FcγRIIIB– and NA2-FcγRIIIB–specific fragments were separately amplified from gDNA in a Perkin Elmer Cetus Cycler (Norwalk, CT) in a total volume of 50 μL. Amplification of a fragment of thep22-phox (CYBA) gene served as an internal control in each polymerase chain reaction (PCR). The nucleotide sequence ofFcγRIIIB-NA2–specific fragments was determined by cycle sequencing of purified PCR products with 33P-labeled terminators with the Thermo Sequenase kit, according to the manufacturer's instructions (Amersham Life Sciences, Cleveland, OH).
Southern blot-based restriction fragment length polymorphism (RFLP) assay.
Southern blot-based restriction fragment analysis was performed as previously described.14 Briefly, 10 μg of genomic DNA was digested overnight with BamH1 and EcoR1 (Promega, Madison, WI). After gel electrophoresis and transfer to nylon sheets, the blot was hybridized with 32P-labeled pGP5, a probe that contains the entire coding region of NA1-FcγRIIIb (Dr G. Peltz, DNAX Research Institute of Molecular and Cellular Biology, Palo Alto, CA).15 This probe hybridizes to both isoforms of FcγRIIIB, as well as to FcγRIIIA. The double digestion resulted in an FcγRIIIB- and anFcγRIIIA-specific fragment of 2.0 and 5.5 kb, respectively. The labeling intensities of the two fragments were measured and compared by phospho-imaging (Fuji, London, UK). NA(1+,2+) genotyped donors and known hemizygous FcγRIIIB gene-deficient donors served as controls.
Sequence analysis of FcγRIIIB-encoding cDNA.
Messenger RNA was isolated from purified neutrophils with a CsCl2 gradient and was reverse transcribed into cDNA. The entire coding region of FcγRIIIB was amplified by PCR,16 after which the products were cloned into a pGEM-T vector according to the manufacturer's instructions (Promega). After transformation of Escherichia coli, inserts were amplified by PCR and sequenced with 32P-end-labeled primers (Amersham) with the BRL cycle sequencing kit (BRL, Gaithersburg, MD).
RESULTS
Genomic analysis.
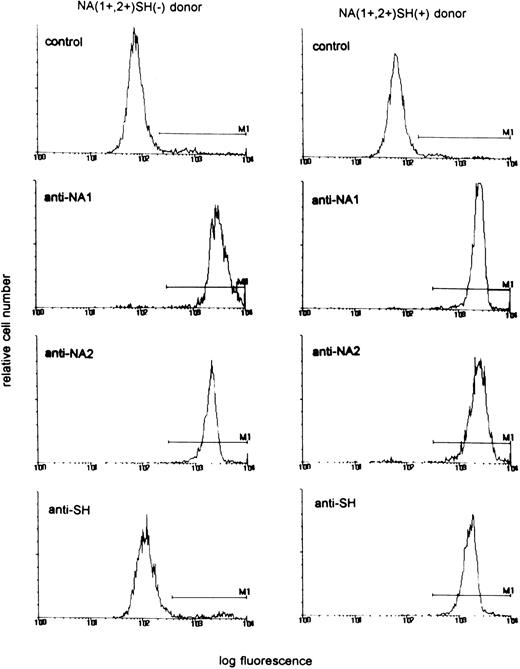
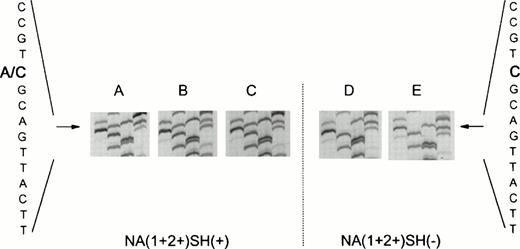
In a population of 55 healthy laboratory workers, we identified three healthy donors whose neutrophils reacted positively with the SH antiserum giving a phenotype frequency of approximately 5%, in concordance with previous findings.10 Neutrophils from all three donors were phenotyped as NA(1+,2+)SH(+). Figure 1 shows a representative experiment with neutrophils from an SH(−) and an SH(+) donor. To determine whether the NA2-FcγRIIIB gene contained the reported 266C→A mutation,10 we specifically amplified a fragment of the NA2-FcγRIIIB gene from gDNA by an allele-specific primer-annealing (ASPA) PCR. As shown in Fig 2A to C, direct sequencing of these products showed that the individuals were heterozygous at nucleotide position 266. At this position, a C as well as an A were detected, suggesting that two different fragments were amplified with theNA2-FcγRIIIB–specific primer set. Sequence analysis ofNA2-FcγRIIIB–specific fragments amplified from genomic DNA from two NA(1+,2+)SH(−) controls showed a single band at position 266 (Fig 2D to E).
Reactivity of neutrophils from an NA(1+,2+)SH(+) donor and an NA(1+,2+)SH(−) control with human anti–NA1- and anti–NA2-FcγRIIIb antisera and the SH antiserum. AB serum was used as control.
Reactivity of neutrophils from an NA(1+,2+)SH(+) donor and an NA(1+,2+)SH(−) control with human anti–NA1- and anti–NA2-FcγRIIIb antisera and the SH antiserum. AB serum was used as control.
Nucleotide sequence (GATC) ofFcγRIIIB-NA2–specific genomic DNA fragments. (A to C) Fragments from three NA(1+2+)SH(+) individuals. Two bands (A and C) are visible at nucleotide position 266, indicating the presence of a normal (266C) as well as of a mutated (266A) FcγRIIIB-NA2 gene. (D and E) Sequences of NA(1+2+)SH(−) controls.
Nucleotide sequence (GATC) ofFcγRIIIB-NA2–specific genomic DNA fragments. (A to C) Fragments from three NA(1+2+)SH(+) individuals. Two bands (A and C) are visible at nucleotide position 266, indicating the presence of a normal (266C) as well as of a mutated (266A) FcγRIIIB-NA2 gene. (D and E) Sequences of NA(1+2+)SH(−) controls.
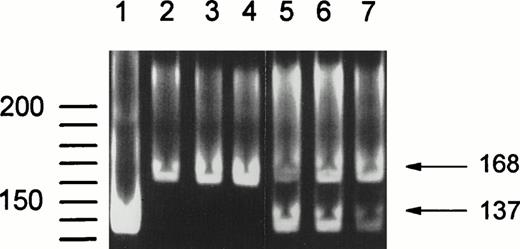
Additionally, NA2-FcγRIIIB–specific PCR products were digested with SfaNI, which recognizes a 5′-GATGC-3′ sequence present only in the SH-FcγRIIIB–derived fragment.10 Figure 3 shows the results of this digestion. Lane 1 shows SfaNI treatment of a fragment amplified from a plasmid containing SH-FcγRIIIb–encoding cDNA, resulting in a single band of 137 bp, confirming that complete digestion is obtained by SfaNI analysis. Lanes 2 to 4 containSfaNI-treated DNA fragments from NA(1+,2+)SH(−) controls. Only the undigested fragment of 168 bp is present. In lanes 5 to 7 DNA fragments from three NA(1+,2+)SH(+) individuals are shown. Digested (137 bp) and undigested (168 bp) fragments are visible, indicating the presence of an SH-FcγRIIIB and an NA2-FcγRIIIBsequence.
SfaNI digestion of the 168-bpFcγRIIIB-NA2–specific gDNA fragment containing the SH mutation at nucleotide position 266. SfaNI recognizes a 5′-GATGC-3′ sequence, which is only present in theFcγRIIIB-SH gene. Lane 1 shows digestion of a fragment amplified from a plasmid containing SH-FcγRIIIb–encoding cDNA, confirming that complete digestion is obtained by Sfa NI treatment. DNA fragments from NA(1+,2+)SH(−) individuals were not digested (lanes 2 through 4), whereas an additional band of 137 bp is visible after digestion of fragments from SH(+) individuals (lanes 5 through 7).
SfaNI digestion of the 168-bpFcγRIIIB-NA2–specific gDNA fragment containing the SH mutation at nucleotide position 266. SfaNI recognizes a 5′-GATGC-3′ sequence, which is only present in theFcγRIIIB-SH gene. Lane 1 shows digestion of a fragment amplified from a plasmid containing SH-FcγRIIIb–encoding cDNA, confirming that complete digestion is obtained by Sfa NI treatment. DNA fragments from NA(1+,2+)SH(−) individuals were not digested (lanes 2 through 4), whereas an additional band of 137 bp is visible after digestion of fragments from SH(+) individuals (lanes 5 through 7).
To confirm the hypothesis that more than two FcγRIIIB genes are present in the genome of NA(1+,2+)SH(+) individuals, a Southern blot-based RFLP assay was performed. The number of FcγRIIIBgenes was determined by comparing the labeling intensities of anFcγRIIIB-specific and an FcγRIIIA-specific fragment. Genomic DNA from two of the three individuals was available for testing. Table 1 shows the quantitative results of the Southern blot, obtained with a phospho-imager. For these two NA(1+,2+)SH(+) individuals, the ratio between the FcγRIIIB- and FcγRIIIA-specific band is 1.16 and 1.14, respectively. This is approximately three times higher than the ratio obtained for three individuals with only one FcγRIIIB gene (0.37 ± 0.17), and 1.5-fold the ratio found for six NA(1+,2+)SH(−) controls (0.69 ± 0.12).
Quantitative Results of Southern Blot-Based RFLP Assay as Measured by Phospho-Imaging
| . | No. . | Ratio of Band Intensities FcγRIIIB:FcγRIIIA . | Ratio Relative to 0.37 . |
|---|---|---|---|
| One FcγRIIIB gene | 3 | 0.37 ± 0.17 | 1 |
| Two FcγRIIIB genes | 6 | 0.69 ± 0.12* | 1.9 |
| Donor 1† | 1.16 | 3.1 | |
| Donor 2 | 1.14 | 3.1 |
| . | No. . | Ratio of Band Intensities FcγRIIIB:FcγRIIIA . | Ratio Relative to 0.37 . |
|---|---|---|---|
| One FcγRIIIB gene | 3 | 0.37 ± 0.17 | 1 |
| Two FcγRIIIB genes | 6 | 0.69 ± 0.12* | 1.9 |
| Donor 1† | 1.16 | 3.1 | |
| Donor 2 | 1.14 | 3.1 |
*Mean value ± standard deviation.
Neutrophils of donor 1 and donor 2 were phenotyped as NA(1+,2+)SH(+).
cDNA analysis.
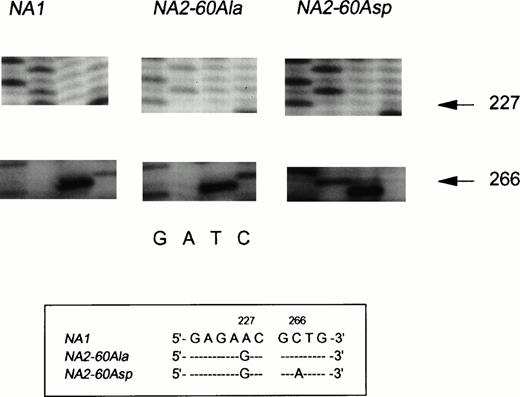
Messenger RNA was isolated from purified neutrophils from one NA(1+,2+)SH(+) individual and reversely transcribed into cDNA to investigate whether all three FcγRIIIB genes were transcribed. The entire coding region of FcγRIIIB was amplified by PCR and cloned into E coli. Figure4, panel 1, shows part of the sequence of an NA1 transcript, whereas the nucleotide sequence shown in panel 2 is derived from anNA2 transcript. The NA1-NA2 difference at nucleotide position 227 is depicted (A→G), and all other described nucleotide differences were normally present (not shown).4The sequence shown in panel 3 only differed from a normal NA2sequence at nucleotide position 266 (C→A). This substitution predicts the Ala→Asp substitution in SH-FcγRIIIb, described by Bux et al.10 Sequence analysis of the complete coding region showed no other nucleotide substitutions. In a total of 30 sequenced clones with an FcγRIIIb-encoding insert from this NA(1+,2+)SH(+) individual, 6 were found to carry theNA1-FcγRIIIB insert, 21 carried the NA2-FcγRIIIBinsert, and 3 carried the SH-FcγRIIIB insert.
Sequence analysis of cDNA, derived from NA(1+2+)SH(+) neutrophils. The nucleotide differences between the three transcripts are shown in the box below the figure. Panel 1 shows the normal NA1-FcγRIIIB–derived sequence as indicated by the presence of an A at nucleotide position 226. The sequence shown in panel 2 is derived from an NA260Ala-FcγRIIIBclone (226G and 266C). The third distinct transcript is derived from anNA2SH-FcγRIIIB gene. The sequence is identical to the NA260Ala-FcγRIIIB sequence except for the A at nucleotide position 266, encoding an Asp at amino acid position 60.
Sequence analysis of cDNA, derived from NA(1+2+)SH(+) neutrophils. The nucleotide differences between the three transcripts are shown in the box below the figure. Panel 1 shows the normal NA1-FcγRIIIB–derived sequence as indicated by the presence of an A at nucleotide position 226. The sequence shown in panel 2 is derived from an NA260Ala-FcγRIIIBclone (226G and 266C). The third distinct transcript is derived from anNA2SH-FcγRIIIB gene. The sequence is identical to the NA260Ala-FcγRIIIB sequence except for the A at nucleotide position 266, encoding an Asp at amino acid position 60.
Neutrophil FcγRIIIb expression.
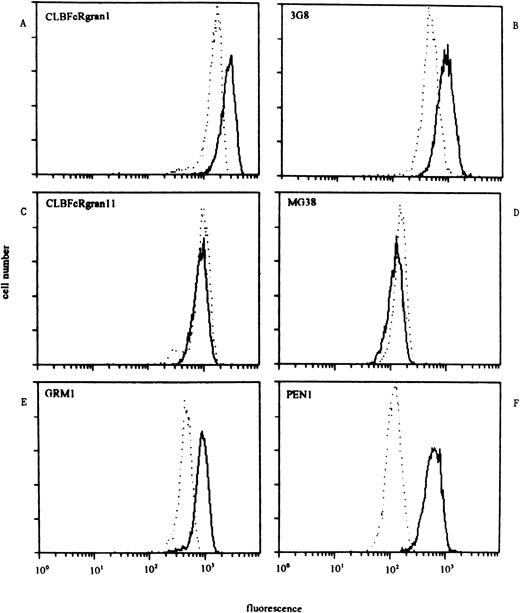
We determined the reactivity of NA(1+,2+)SH(+) neutrophils with a panel of CD16 MoAbs in comparison with neutrophils from donors who were NA(1+,2+)SH(−) in three separate experiments (Table 2). Neutrophils from SH(−) and either homozygous NA1- or NA2-positive individuals were simultaneously analyzed in two of these experiments. Compared with neutrophils from NA(1+,2+)SH(−) individuals, SH(+) neutrophils showed a higher reactivity with anti–pan-FcγRIII MoAbs CLBFcRgran1, 3G8, MEM154, and BW209/2 (170%, 119%, 140%, and 163%, respectively). The NA(1+,2+)SH(+) neutrophils reacted approximately twice as strong with the NA2-specific MoAb GRM1 as did control neutrophils (Table 2). However, the reactivity of the NA(1+,2+)SH(+) neutrophils was comparable to that of NA(1−,2+)-genotyped donors, who carried twoNA2 genes, as determined by Southern blot analysis,13 with mean fluorescent intensities (mfis) of 1,480 and 1,585 ± 568, respectively. However, reactivity of the NA2-specific MoAb PEN1 was about sixfold higher for NA(1+,2+)SH(+) neutrophils compared with NA(1+,2+)SH(−) neutrophils and threefold higher compared with NA(1−,2+)SH(−) neutrophils. Reactivity with NA1-specific MoAbs CLBFcRgran11 and MG38 was similar between SH(−) and SH(+) NA(1+,2+) neutrophils, suggesting an equal membrane expression of NA1-FcγRIIIb (Table 2). Figure 5 shows the results of a representative experiment.
Reactivity of SH-Positive and SH-Negative Neutrophils With a Panel of CD16 MoAbs.
| MoAb . | SH-Negative . | SH-Positive . | |||
|---|---|---|---|---|---|
| NA1NA1 (n = 2) . | NA1NA2 (n = 3) . | NA2NA2 (n = 2) . | NA1NA2 (n = 3) . | ||
| mfi . | mfi ± SD . | mfi . | mfi ± SD . | Relative to NA(1+,2+)SH(−) . | |
| Control | 14 | 15 ± 5 | 12 | 19 ± 7 | — |
| Anti-pan FcγRIII | |||||
| CLBFcRgran1 | 1,764 | 1,970 ± 609 | 2,409 | 3,345 ± 324 | 170% |
| 3G8 | 1,427 | 1,309 ± 820 | 1,426 | 1,555 ± 535 | 119% |
| BW209/2 | 767 | 989 ± 298 | 956 | 1,611 ± 563 | 163% |
| MEM154 | 1,075 | 1,117 ± 568 | 1,198 | 1,636 ± 445 | 140% |
| Anti-NA1 FcγRIII | |||||
| MG38 | 415 | 311 ± 130 | 16 | 248 ± 95 | 80% |
| CLBgran11 | 1,934 | 1,141 ± 317 | 17 | 994 ± 149 | 87% |
| Anti-NA2 FcγRIII | |||||
| GRM1 | 30 | 914 ± 439 | 1,480 | 1,585 ± 568 | 173% |
| PEN1 | 24 | 149 ± 153 | 270 | 834 ± 153 | 560% |
| MoAb . | SH-Negative . | SH-Positive . | |||
|---|---|---|---|---|---|
| NA1NA1 (n = 2) . | NA1NA2 (n = 3) . | NA2NA2 (n = 2) . | NA1NA2 (n = 3) . | ||
| mfi . | mfi ± SD . | mfi . | mfi ± SD . | Relative to NA(1+,2+)SH(−) . | |
| Control | 14 | 15 ± 5 | 12 | 19 ± 7 | — |
| Anti-pan FcγRIII | |||||
| CLBFcRgran1 | 1,764 | 1,970 ± 609 | 2,409 | 3,345 ± 324 | 170% |
| 3G8 | 1,427 | 1,309 ± 820 | 1,426 | 1,555 ± 535 | 119% |
| BW209/2 | 767 | 989 ± 298 | 956 | 1,611 ± 563 | 163% |
| MEM154 | 1,075 | 1,117 ± 568 | 1,198 | 1,636 ± 445 | 140% |
| Anti-NA1 FcγRIII | |||||
| MG38 | 415 | 311 ± 130 | 16 | 248 ± 95 | 80% |
| CLBgran11 | 1,934 | 1,141 ± 317 | 17 | 994 ± 149 | 87% |
| Anti-NA2 FcγRIII | |||||
| GRM1 | 30 | 914 ± 439 | 1,480 | 1,585 ± 568 | 173% |
| PEN1 | 24 | 149 ± 153 | 270 | 834 ± 153 | 560% |
Results of three separate experiments with neutrophils of different donors are shown.
Staining pattern of NA(1+2+)SH(−) (dashed lines) and NA(1+2+)SH(+) neutrophils (continuous lines) with a panel of CD16 MoAbs. One representative experiment of three is shown. (A and B) The reactivity of SH(+) neutrophils with anti–pan-FcγRIII MoAbs (CLBFcRgran1 and 3G8) was higher than that of SH(−) neutrophils. (C and D) The reactivity of both types of neutrophils with NA1-FcγRIIIb–specific MoAbs CLBFcRgran11 and MG38 was comparable. (E) Reactivity of NA2-FcγRIIIb–specific MoAb GRM1 was approximately twice as high with SH(+) neutrophils compared with SH(−) neutrophils. (F) NA2-FcγRIIIb–specific MoAb PEN1 showed a reactivity with SH(+) neutrophils that was sixfold higher compared with the reactivity of SH(−) neutrophils.
Staining pattern of NA(1+2+)SH(−) (dashed lines) and NA(1+2+)SH(+) neutrophils (continuous lines) with a panel of CD16 MoAbs. One representative experiment of three is shown. (A and B) The reactivity of SH(+) neutrophils with anti–pan-FcγRIII MoAbs (CLBFcRgran1 and 3G8) was higher than that of SH(−) neutrophils. (C and D) The reactivity of both types of neutrophils with NA1-FcγRIIIb–specific MoAbs CLBFcRgran11 and MG38 was comparable. (E) Reactivity of NA2-FcγRIIIb–specific MoAb GRM1 was approximately twice as high with SH(+) neutrophils compared with SH(−) neutrophils. (F) NA2-FcγRIIIb–specific MoAb PEN1 showed a reactivity with SH(+) neutrophils that was sixfold higher compared with the reactivity of SH(−) neutrophils.
Because the level of soluble (s)FcγRIII in plasma correlates with the number of FcγRIIIB genes,13 we determined the amount of sFcγRIII in plasma from the three SH(+) individuals. In concordance with the high membrane expression levels, we found plasma sFcγRIII levels of 197, 279, and 137 AU (mean, 204 ± 71 AU). However, this value was not significantly different from the mean sFcγRIII level in 24 NA(1+,2+)SH(−) individuals (106 ± 24 AU13; Welch's approximate t-test, P = .07).
DISCUSSION
Recently, Bux et al identified a new alloantigen on FcγRIIIb.10 Sequence analysis showed that this antigen, termed SH, is encoded by a point mutation in the NA2-FcγRIIIBgene. We amplified both a normal NA2-encoding sequence and an NA2 sequence containing the SH mutation from genomic DNA from three NA(1+,2+)SH(+) individuals by means of anNA2-FcγRIIIB–specific PCR. Digestion of theseNA2-FcγRIIIB–specific fragments with SfaNI confirmed the presence of two NA2 genes. Furthermore, a Southern blot-based RFLP assay showed that three FcγRIIIB genes are present in the genome of these NA(1+,2+)SH(+) individuals. All threeFcγRIIIB genes were transcribed, because three distinct transcripts encoding either NA1-, NA2-, or SH-FcγRIIIb were isolated from neutrophil mRNA. These data indicate that these three donors each carry three FcγRIIIB genes, namely, NA1-FcγRIIIB,NA2-FcγRIIIB, and SH-FcγRIIIB. Six of 14 SH(+) individuals described by Bux et al were phenotyped as NA(1−,2+), and the remaining eight were NA(1+,2+).10 Moreover, three NA(1+,2+)SH(+) donors were reanalyzed and were found to carry threeFcγRIIIB genes as well (J. Bux, personal communication). Only confirmation by PCR and/or Southern blotting can settle the question as to whether any SH positivity without gene duplication exists. However, with these methods the possibility of oneFcγRIIIB gene-deficient chromosome and two FcγRIIIBgenes on the other one cannot be ruled out.14 If we assume that the SH-FcγRIIIB gene is only present in association with a second FcγRIIIB gene on the same chromosome, then the presence of NA(1−,2+)SH(+) phenotyped donors in the study of Bux et al might indicate that NA2-FcγRIIIB is located on the same chromosome as SH-FcγRIIIB. It is conceivable that before or after the SH mutation occurred, an unequal crossing-over event between two chromosomes carrying FcγRIIIBgenes has led to the supposed NA2-SH allele. The counterpart of this unequal crossing-over is an FcγRIIIB gene deletion, which has been described.14,17-20 The genotype frequencies of SH-FcγRIIIB and FcγRIIIB gene deletion, being 4% and 3% to 9%, respectively, are not contradicting this theory.10,18 19 Theoretically, SH(−) donors with three FcγRIIIB genes (gene duplication without mutation) should exist. Southern blot analysis of gDNA from a large group of donors could settle this question.
Neutrophils from healthy individuals carry 100,000 to 300,000 copies of FcγRIIIb per cell.21 Previously, we described that the amount of FcγRIIIb on the neutrophil membrane correlates with the number of FcγRIIIB genes.13 Individuals who are hemizygous FcγRIIIB-gene–deficient have approximately half the neutrophil FcγRIIIb expression and half the plasma soluble (s)FcγRIII level of individuals carrying two FcγRIIIBgenes. Moreover, reactivity of neutrophils from NA-homozygous individuals with NA-specific MoAbs is twice as high compared with NA(1+,2+) neutrophils.13 The three NA(1+,2+)SH(+) individuals that we tested had a higher neutrophil FcγRIIIb expression as measured with anti–pan-FcγRIII MoAbs, with the exception of 3G8. Furthermore, the reactivity with the NA2-specific MoAb GRM1 was approximately twice as high compared with SH(−) neutrophils, whereas the reactivity with NA1-specific MoAbs was similar. MoAb 3G8 recognizes an epitope in the membrane-proximal domain of FcγRIII,22 whereas binding of GRM1 is dependent on the presence of 47Ser.22,23 Whether the 60Ala→Asp substitution influences the affinity of 3G8 will have to be investigated in studies with SH-FcγRIIIb–transfected cells or with neutrophils from donors at the genomic level proven to carry only theSH-FcγRIIIB gene and no NA2-FcγRIIIB. Our data suggest that the substitution of the hydrophobic alanine to the negatively charged aspartic acid in SH-FcγRIIIb influences the epitope recognized by PEN1, which might be located in the membrane-distal Ig-like domain because of its NA2 specificity.11 Finally, SH(+) individuals seemed to have higher levels of plasma sFcγRIII compared with NA(1+,2+)SH(−) control donors. This suggests that the gene-dosage effect observed for plasma sFcγRIII levels also holds for individuals with three FcγRIIIB genes. Therefore, our findings imply that the large interindividual variation in neutrophil FcγRIIIb expression and plasma levels of sFcγRIII might be partly caused by differences in the number of FcγRIIIB genes. One could hypothesize that the number of FcγRIIIb copies on the neutrophil membrane influences the effector functions of the cell. However, hemizygous and homozygousFcγRIIIB gene deficiency does not seem to be associated with an increased infection risk.14 Further experiments should elucidate whether the neutrophil response to opsonized particles correlates with the number of FcγRIIIB genes.
It should be further investigated whether the SH mutation influences the ligand-binding capacity of the receptor. The NA1-FcγRIIIb and NA2-FcγRIIIb isoforms have been shown to interact differently with IgG, although the amino acid differences are all in the membrane-distal domain.5-8 Moreover, mutations in the membrane-distal domain of FcγRIIa affected the ligand-binding capacity as well.24
The Second International Granulocyte Serology Workshop agreed for the time being to term the new antigen SH. For the nomenclature of this geno/phenotype it is important to elucidate whether SH positivity will always be accompanied by NA2 positivity, either because of the close homology between the SH and NA2 isoforms, or because of genetic linkage between the two genes. Thus far, only the SH antiserum, genomic analysis, and possibly CD16 MoAb PEN1 can distinguish between NA2- and NA2SH-FcγRIIIb. To underline the close connection of the SH antigen with the NA(1,2) system and to emphasize the similarity toNA2-FcγRIIIB, we propose that the antigen be termed NA2SH-FcγRIIIb or NA3.
In conclusion, we detected the presence of three FcγRIIIBgenes in three individuals whose neutrophils were phenotyped as NA(1+,2+)SH(+). These three genes, NA1-FcγRIIIB,NA2-FcγRIIIB, and SH-FcγRIIIB, were all transcribed and a clear gene-dosage effect regarding neutrophil FcγRIIIb expression was observed. Our data indicate that it may be possible that a chromosomal locus exists on which NA2-FcγRIIIB is located in tandem with SH-FcγRIIIB.
ACKNOWLEDGMENT
We thank the Department of Leukocyte and Thrombocyte Serology of the CLB for neutrophil phenotyping and R. Dee for helpful technical suggestions.
Supported by Grant No. 900-012-092 of the Netherlands Organization for Scientific Research (NWO).
Address reprint requests to Albert E.G.Kr. Von dem Borne, Central Laboratory of the Netherlands Red Cross Blood Transfusion Service, Department of Experimental Immunohematology, Plesmanlaan 125, 1066 CX Amsterdam, The Netherlands.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal