Abstract
An additional decay-accelerating factor (DAF) mutation, designated as Inab phenotype in the Cromer blood group system, was recently identified in a 28-year-old Japanese woman (H.A.). The red blood cells of H.A., like those of other Inab phenotype individuals, were negative for Cromer system antigens, Cra, Tca, Dra, UMC, and IFC. The deficiency of DAF on the red blood cells of H.A. has been shown by immunoblotting with a murine monoclonal antibody to DAF. Molecular analysis has shown that H.A. is homozygous for a single nucleotide substitution, C1579→A, at the position 24 bp upstream of the 3′-end of exon 2 of the DAF gene. This substitution causes the activation of a novel cryptic splice site and results in the production of mRNA with a 26 bp deletion. The deletion introduces a reading frame shift and creates a stop codon immediately downstream of the deletion. Translation of mRNA would be terminated at the first amino acid residue of the second short consensus repeat (SCR2) domain (exon 3) of DAF. The functional domains of DAF's complement regulatory activity and the carboxy-terminal signal domains for glycosylphosphatidylinositol (GPI) anchoring are predicted to be lacking in H.A. Thus, there would be no DAF present on the cell surface.
DECAY-ACCELERATING factor (DAF) is a 70-kD glycophospholipid-anchored membrane protein that protects cells from complement-mediated damage.1-5 The deficiency of DAF and CD59, together with other glycosylphosphatidylinositol (GPI)-anchored proteins, causes the acquired hematopoietic stem cell disorder, paroxysmal nocturnal hemoglobinuria (PNH).6-8 The single copy of the human DAF gene is located on the long arm of chromosome 1, q322 and organized into four short consensus repeats (SCRs) that are homologous to each other. The third SCR of DAF is responsible for complement regulatory activity and signaling.9-11
The Cromer system antigens are located on DAF.12,13 The system includes at least 10 individual antigens, of which 7 are very high frequency antigens and three are low frequency antigens.14 The Inab phenotype is a very rare one in which the red blood cells (RBCs) lack all Cromer system antigens.14-17 The RBCs of individuals with Inab phenotype have a deficiency of DAF but these individuals are not known to have any associated hematologic abnormalities.
The molecular analysis of the DAF gene of the original Inab phenotype propositus showed a single nucleotide substitution in codon 53 of exon 2: TGG, a tryptophan codon, to TGA, a stop codon.18 19 This truncation near the amino terminus explains the complete absence of surface DAF in this propositus.
In this report, we present the case of a fourth propositus (H.A.) with Inab phenotype. Like the third Inab propositus and her brother, H.A. had no history of intestinal disease. The present analysis of the Inab phenotype in H.A. at both cDNA and genomic DNA levels showed that a molecular mechanism underlay the Inab phenotype in H.A., which was different from that in the case of the original Inab phenotype propositus.
MATERIALS AND METHODS
Serological testing.
Standard serological techniques were used throughout. Antiglobulin tests were performed in tubes after centrifugation using antihuman IgG or antimouse IgG. Human polyclonal red cell typing reagents were from our in-house collection. A murine monoclonal antibody to DAF, CBC98, was produced in our laboratory. Murine monoclonal anti-DAF, BRIC110, BRIC128, and BRIC230, were obtained from Dr D.J. Anstee (IBGRL, Bristol, UK). Anti-Dra and Escherichia coli OX75 were supplied by Dr C. Levene (Government Central Laboratory, Jerusalem, Israel) and Mr J.J. Mould (Gamma Biological Inc, Houston, TX), respectively.
Immunoblotting.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed on a 10% separating gel with a 3% stacking gel. Separated proteins were electroblotted and immunostained with anti-DAF antibody (CBC98).
In vitro DNA amplification.
Total cellular RNAs were extracted from Epstein-Barr virus-transformed lymphoblastoid cell lines (EBV-LCL.) from H.A. and normal control using the guanidinium thiocyanate-phenol-chloroform extraction method.20 The cDNA synthesis was performed following the manufacturer's instructions (RNase H-M-MLV reverse transcriptase, Superscript; GIBCO-BRL, Gaithersburg, MD). Based on the published DAF cDNA sequence,21 the oligonucleotide primers flanking 1,152 bp of DAF mRNA sequence (from base 51 to 1,202) were designed: the sense primer DAF1F (5′-TGCTGCTGGTGCTGTTGT-3′) and the antisense primer DAF4 (5′-TAGCAGATAAGTCTAAGAAACTAGG-3′). Genomic DNAs were obtained from the EBV-LCLs of H.A. and normal control using a conventional method. Primers flanking 220 bp of the exon 2/intron 2 boundary region (from base 1,531 to 1,780) were synthesized based on the published sequences of the DAF gene22: the sense primer DAF6 (5′-TTCCTGGCGAGAAGGACT-3′) and the antisense primer DAF7 (5′-TAGCTGGAGTGTGACGTG-3′). The polymerase chain reaction (PCR) was performed in a DNA thermal cycler (Perkin Elmer-Cetus, Emeryville, CA) for 30 cycles using 2.5 U of Taq DNA polymerase (Perkin Elmer-Cetus) in a solution containing 200 μmol/L each of dNTPs; 50 mmol/L KCl; 10 mmol/L Tris-HCl (pH 8.3); 2.5 mmol/L MgCl2 in a final volume of 100 μL. The amplification cycles consisted of denaturation for 30 seconds at 95°C, annealing for 30 seconds at 59°C, and extension for 60 seconds at 72°C.
Cloning and sequencing of amplified fragments.
The amplified products were cloned into T vectors (TA Cloning System; Invitrogen, San Diego, CA). Individual colonies containing plasmids with inserts were selected and the plasmid DNAs were purified using a plasmid DNA purification kit (Wizard Minipreps, Madison, WI). The sequence reaction was performed according to the manufacturer's instructions (Taq DyeDeoxy Terminator Cycle Sequencing Kit; Applied Biosystems, Foster City, CA). The sequence electrophoresis was performed on an 8% polyacrylamide gel using an automated DNA sequencer (373A; Applied Biosystems, Tokyo, Japan).
Analysis of single-strand conformation polymorphisms (SSCP).
The 220-bp PCR products of exon 2/intron 2 boundary region of genomic DNA from H.A. and normal controls were mixed with a denaturing solution (95% formamide, 20 mmol/L EDTA, 0.05% bromophenol blue, and 0.05% xylene cyanol FF) and denatured at 95°C for 5 minutes. Electrophoresis was performed in a neutral 10% polyacrylamide gel at 38°C. Single-strand DNA fragments in the gel were detected by silver-staining.
RESULTS
Serological testing.
H.A.'s RBCs were of the following phenotypes: A, D+C+c−E−e+, M+N+S−s+, P1+, Le(a−b−), Fy(a+b−), Jk(a+b+), Di(a−b+), Xg(a+) and positive for the high frequency antigens H, P, Kpb, Jsb, k, Ku, U, Ena, I, Lub, Sc1, Ve1, Coa, Lan, Kna, McCa, Ch, Rg, Ge2, Ge3, AnWj, Jra, Era, LWa, Inb, Rh17, Rh29, and Yka. RBCs of H.A. failed to react with Cromer-related antibodies, anti-Cra, -Tca, -Dra, -IFC, and -UMC. H.A.'s RBCs showed no reactivity with the monoclonal anti-DAF antibodies and E coli OX75.23 Like the other Inab phenotypes, H.A.'s RBCs were also shown to carry other GPI-linked proteins, Yta, Gya, and JMH blood group antigens, which have been reported to reside on GPI-linked proteins24 and CD59 by hemagglutination test.
Immunoblotting.
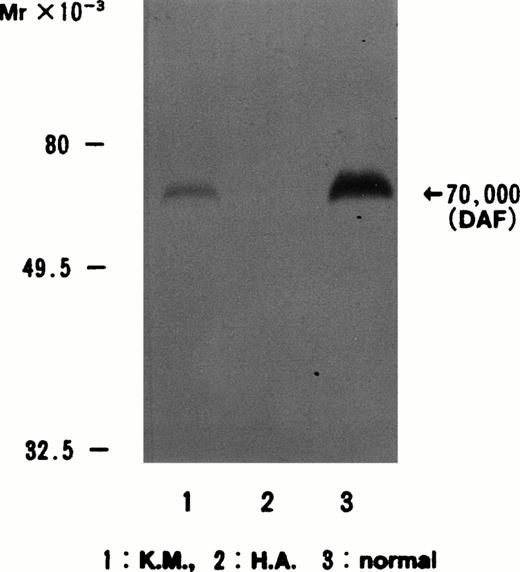
When anti-DAF antibody (CBC98) was used to stain immunoblots of normal control, Dr(a−), and H.A. RBC membranes, a strong band in the case of the normal control, a weak band in that of the Dr(a−), and no band in the case of H.A. were detected, respectively (Fig 1).
Immunoblotting of erythrocyte membranes with anti-DAF antibody CBC98. The DAF band at 70 kD is present in normal controls and in Dr(a−) (weaker than normal) but not in H.A. Lane 1, Dr(a−); lane 2, H.A.; lane 3, normal control.
Immunoblotting of erythrocyte membranes with anti-DAF antibody CBC98. The DAF band at 70 kD is present in normal controls and in Dr(a−) (weaker than normal) but not in H.A. Lane 1, Dr(a−); lane 2, H.A.; lane 3, normal control.
Nucleotide sequence analysis of DAF cDNA from HA.
A molecular study of the DAF gene of the original Inab phenotype propositus has been performed. The nucleotide sequence analysis of genomic DNA and cDNA showed a single nucleotide substitution in exon 2 of the DAF gene. This substitution introduced a Bcl I restriction site. To determine whether the same substitution was present in the DAF gene of H.A., the amplified DAF cDNA from H.A. was digested with Bcl I and analyzed by PAGE. The results showed that the DAF gene of H.A. did not have this single nucleotide substitution (data not shown).
Analysis of the sequences of the entire DAF coding region from 10 independent DAF cDNA clones of H.A. showed a 26-bp deletion in the DAF mRNA from H.A., which corresponded to bases 246 to 271 of the published DAF cDNA sequence21 (Fig 2).
Sequencing analysis of DAF cDNA in H.A. Ten clones with identical sequence were obtained from the cDNA of H.A., and parts of the cDNA and deduced amino acid sequences of DAF from normal control and H.A. were compared with the sequence of normal control and the original Inab phenotype propositus. A 26-bp nucleotide deletion was identified in the cDNA derived from H.A. at the 3′ end of exon 2. The deletion resulted in a reading frame shift and generation of a stop codon immediately downstream from the deletion.
Sequencing analysis of DAF cDNA in H.A. Ten clones with identical sequence were obtained from the cDNA of H.A., and parts of the cDNA and deduced amino acid sequences of DAF from normal control and H.A. were compared with the sequence of normal control and the original Inab phenotype propositus. A 26-bp nucleotide deletion was identified in the cDNA derived from H.A. at the 3′ end of exon 2. The deletion resulted in a reading frame shift and generation of a stop codon immediately downstream from the deletion.
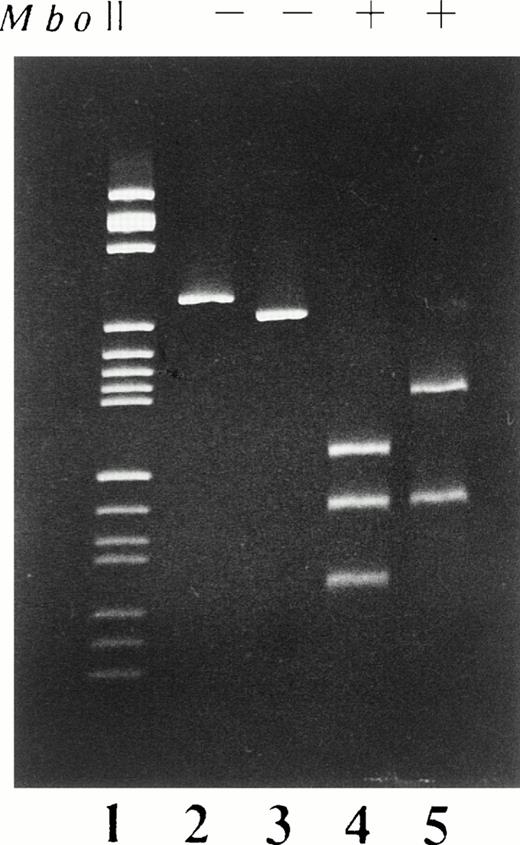
Computer analysis of the DAF cDNA sequence from H.A. showed that the deletion of these 26 bp resulted in the loss of an Mbo II restriction site. PCR amplification of cDNA from base 200 to base 495 and restriction fragment length polymorphism (RFLP) analysis were used to confirm the deletion in the mRNA. The differences in the restriction pattern between the PCR products of H.A. and normal control are shown in Fig 3. The restriction fragments of PCR products obtained from H.A. were 160 bp and 110 bp long, in contrast with the 130 bp, 110 bp, and 53 bp fragments of those from normal controls.
PCR-RFLP analysis of the cDNA derived from the EBV-LCL of H.A. cDNA fragments extending from base 200 to base 495 were enzymatically amplified from mRNA of the EBV-LCL of H.A. and normal individuals. The PCR products, both before (−) and after (+) digestion with the restriction enzyme Mbo II were analyzed on 5% polyacrylamide gel stained with ethidium bromide. Lane 1, pBR322HaeIII digest size marker; lanes 2 and 4, normal controls; lanes 3 and 5, H.A.
PCR-RFLP analysis of the cDNA derived from the EBV-LCL of H.A. cDNA fragments extending from base 200 to base 495 were enzymatically amplified from mRNA of the EBV-LCL of H.A. and normal individuals. The PCR products, both before (−) and after (+) digestion with the restriction enzyme Mbo II were analyzed on 5% polyacrylamide gel stained with ethidium bromide. Lane 1, pBR322HaeIII digest size marker; lanes 2 and 4, normal controls; lanes 3 and 5, H.A.
Nucleotide sequence analysis of DAF genomic DNA from H.A.
To determine whether a deletion corresponding to the mRNA from the EBV-LCLs of H.A. was present in the genomic DNA of H.A., the 220 bp of genomic DNA in the exon 2/intron 2 boundary region was cloned. Ten independent clones were sequenced from base 1,531 to base 1,750 corresponded to the published DAF gene sequence.22 The results showed a single nucleotide substitution, C to A, at base 1,579 of exon 2 of the DAF gene from H.A. rather than a 26-bp deletion as detected in the cDNA derived from mRNA of H.A.
To further confirm that this nucleotide substitution was present on both DAF alleles in H.A., 220-bp genomic DNAs in the exon 2/intron 2 boundary region from H.A. and normal controls were subjected to SSCP analysis. In SSCP analysis, samples possessing a single allele showed two-band patterns that corresponded to the sense and antisense strands, whereas heterozygotes showed four-band patterns.25 H.A. was shown to be homozygous for the DAF gene carrying this single nucleotide substitution (Fig 4).
PCR-SSCP analysis of exon 2/intron 2 boundary region of H.A. and normal controls. Bases 1,531 to 1,750 were enzymatically amplified from genomic DNA from H.A. and normal control. Electrophoresis was carried out at 38°C. Lanes 1 and 2, normal controls; lane 3, H.A.
PCR-SSCP analysis of exon 2/intron 2 boundary region of H.A. and normal controls. Bases 1,531 to 1,750 were enzymatically amplified from genomic DNA from H.A. and normal control. Electrophoresis was carried out at 38°C. Lanes 1 and 2, normal controls; lane 3, H.A.
DISCUSSION
Testing for GPI-linked proteins showed that DAF was absent, whereas other GPI-linked proteins were present on RBCs from H.A.
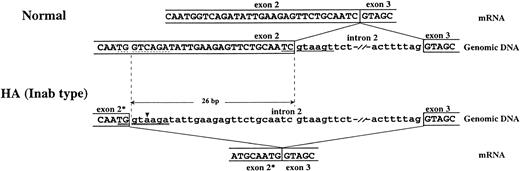
Amplification of the entire coding region of DAF cDNA derived from mRNA of H.A. by reverse transcription-PCR (RT-PCR) and sequence analysis of the products allowed us to identify a 26-bp deletion at the exon 2/intron 2 boundary from base 246 to base 271. This deletion introduced a reading frame shift and generated a stop codon immediately downstream of the deletion (Fig 2). Translation of the DAF mRNA in this individual would not proceed beyond the first short consensus repeat (SCR1) near the amino terminus of the protein. This deletion resulted in the loss of an Mbo II restriction site, providing an RFLP that permits discrimination of the Inab phenotype from the normal one (Fig 3). Furthermore, the results of the genomic DNA sequencing showed the presence of a single nucleotide substitution rather than a 26-bp deletion in the DAF genomic DNA from H.A. This substitution lay 24 bp upstream from the mRNA splicing junction (exon 2/intron 2) and was considered to be responsible for a 26-bp deletion of the mRNA by creating an activated cryptic splice site (Fig 5).
Schematic representation of alternative splicing of the DAF gene in H.A. An alternatively spliced mRNA results from the activation of a cryptic splice site due to a C→A mutation upstream from the normal splice site. This abnormal mRNA lacks the last 26 bp of exon 2, which causes a reading frame shift and creates a stop codon next to the end of the deletion. (▾) base substitution, (—) normal 5′-splice site, (dashed line) cryptic 5′-splice site, (=) mutated 5′-splice site, (*) the truncated exon.
Schematic representation of alternative splicing of the DAF gene in H.A. An alternatively spliced mRNA results from the activation of a cryptic splice site due to a C→A mutation upstream from the normal splice site. This abnormal mRNA lacks the last 26 bp of exon 2, which causes a reading frame shift and creates a stop codon next to the end of the deletion. (▾) base substitution, (—) normal 5′-splice site, (dashed line) cryptic 5′-splice site, (=) mutated 5′-splice site, (*) the truncated exon.
A sequence of eight nucleotides at the boundary between an exon and an intron is known to be highly conserved, as the 5′ donor splice site.26 “Consensus values” (CVs), as defined by Shapiro and Senapathy,26 were introduced to quantify nucleotide sequence homology of mutated splice sites to the splice site consensus sequence. The novel splice sites with higher CVs than those of the normal sites successfully compete with the normal sites for a splicing factor. Almost all novel splice sites are situated upstream of the normal sites.27 Through comparison of the sequences of exon 2/intron 2 boundary region of normal controls and H.A. with the consensus sequence of the 5′ donor splice site, a cryptic splice site (TGgtcaga) 26 bp upstream of the 5′ splice site (TCgtaagt) in the genomic DNA of a normal subject and a mutated cryptic splice site (TGgtaaga) resulting from a C1579→A substitution in H.A. were found. The calculated CVs of the normal and cryptic 5′ splice site in normal subjects and mutated 5′ splice site in H.A. were 0.786, 0.765, and 0.865, respectively. The CV of mutated splice site in H.A. was higher than that of the splice site in normal subject. This single base pair substitution led to activation of the cryptic splice site 26 bp upstream from the original splice site in exon 2, and resulted in substitution of the novel splice site for the original one and a partial deletion in the DAF cDNA in H.A. (Fig5). Moreover, the result of SSCP analysis of amplified genomic DNA indicated that this mutation occurred on both DAF alleles in the propositus. In the Cromer blood group system, the Dr(a−) phenotype was also found due in part to the single point mutation that results in a novel use of alternative splicing.19 28Although a missplicing is caused by a different mechanism, the molecular bases of both Inab and Dr(a−) phenotypes have shown novel forms of mutation leading to use of a cryptic alternative splice site.
The original Inab phenotype propositus was reported to result from a single nucleotide substitution, G314→A in the DNA, which introduces a stop codon in the SCR1 domain. Translation of the DAF mRNA would be terminated at codon 53 of exon 2. It should noted that the DAF deficiency in the original Inab individual and H.A. arose via different mechanisms although both of these individuals are Japanese. Thus, different molecular mechanisms may underlie the other Inab phenotype propositi who are not Japanese.
Interestingly, there is a similarity between the original Inab individual and H.A., although two different molecular defects result in the Inab phenotype. The synthesis of DAF in both individuals would not proceed beyond the SCR1 domain and the functional domains of DAF's complement regulatory activity and the carboxy-terminal signal domains for GPI anchoring. Thus, no DAF would be present on the cell surface. It would be interesting to determine whether this similarity is represented among other Inab phenotype individuals.
ACKNOWLEDGMENT
We thank Dr D.J. Anstee (International Blood Group Reference Laboratory, Bristol, UK) for supplying the monoclonal anti-DAF, Mr J.J. Mould (Gamma Biological Inc, Houston, TX) for EcoliOX75, and Dr C. Levene for the anti-Dra (Government Central Laboratory, Jerusalem, Israel).
Address reprint requests to Makoto Uchikawa, PhD, The Japanese Red Cross Central Blood Center, Hiroo 4-1-31, Shibuya-ku, Tokyo, Japan 150.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal