Abstract
FcγRIIa is widely expressed on hematopoietic cells. There are two known allelic polymorphic forms of FcγRIIa, FcγRIIa-R131 and FcγRIIa-H131, which differ in the amino acid at position 131 in the second Ig-like domain. In contrast to FcγRIIa-R131, FcγRIIa-H131binds hIgG2 but not mIgG1, and this differential binding has clinical implications for host defense, autoimmune disease, immunohematologic disease, and response to therapeutic monoclonal antibodies. We identified a novel FcγRIIA genotype in a healthy individual homozygous for FcγRIIA R/R131 in whom a C to A substitution at codon 127 changes glutamine (Q) to lysine (K) in one of the two FcγRIIA genes. This individual's homozygosity for FcγRIIA-R/R131 leads to the prediction that the receptors on her cells would not bind hIgG2. Monocyte and neutrophil phagocytosis of hIgG2-opsonized erythrocytes was significantly higher (P < .05) for cells from this K/Q127, R/R131 individual than for Q/Q127, R/R131 donors. Platelet aggregation stimulated by an mIgG1 anti-CD9 antibody in this individual was significantly different (P < .05) from Q/Q127, H/R131 and Q/Q127, H/H131 donors and similar to Q/Q127, R/R131. Our data show that the K127/R131 receptors have a unique phenotype, binding both hIgG2 and mIgG1. Further functionally significant mutations in human Fcγ receptors and possible novel mechanisms for inherited differences in disease susceptibility should be sought with unbiased screening methods.
Fc RECEPTOR IIA for IgG (FcγRIIa, CD32), the most widely distributed Fcγ receptor, is expressed on neutrophils, monocyte/macrophages, and platelets. Unlike the group of multisubunit immune recognition receptors to which other Fcγ receptors belong, FcγRIIa as a single unit possesses both ligand binding and signal transducing activities. Allelic polymorphism of FcγRIIa influences receptor function. There are two known codominantly expressed alleles of FcγRIIA that differ in the amino acid at position 131 in the second Ig-like extracellular domain. The two forms are FcγRIIA-R131 (Arginine, codon CGT) and FcγRIIA-H131 (Histidine, codon CAT). The relative frequency of the R131 and H131 allotypes varies in different ethnic groups.1-3 A second polymorphism in FcγRIIa at position 27 (glutamine or tryptophan) is not linked to the polymorphism at position 131 and does not affect receptor function.4 In contrast to FcγRIIA-R131 and all of the other Fcγ receptors, FcγRIIA-H131 is unique in its efficient binding of the human (h) IgG2subclass.4-7 The clinical consequences of the differential binding of IgG subclasses are profound. Individuals homozygous for R131 are at higher risk for serious infection with encapsulated organisms,8-10 which are cleared via a predominant IgG2 response and for the consequences of impaired immune complex removal associated with renal involvement in systemic lupus erythematosus.11,12 Conversely, individuals homozygous for the H131 allotype may be at higher risk for autoimmune disorders mediated by IgG2, such as heparin-induced thrombocytopenia.13,14 The FcγRIIA allotypes also differ in the binding of specific murine and rat IgG subclasses.15,16 In particular, FcγRIIA-R131binds murine IgG1 (mIgG1) well, but FcγRIIA-H131 does so minimally. Clinically, this differential binding has been noted to affect the therapeutic utility of certain murine monoclonal antibodies (MoAbs).17
In the course of screening an African-American population with a polymerase chain reaction single-stranded conformational polymorphism (PCR-SSCP) method to determine the FcγRIIA-H/R131 genotype distribution,18 we identified a healthy individual with a novel FcγRIIA genotype. She has no history of infectious or immune complex-mediated diseases. In this individual homozygous for FcγRIIA-R/R131, a C to A substitution at codon 127 changes wild-type glutamine (Q) to lysine (K) in one of the two FcγRIIA genes. Cells from this individual would be predicted not to bind hIgG2 via FcγRIIa. We examined the functional consequences of this mutation in neutrophils, monocytes and platelets from this individual compared with those of R/R131, H/R131, and H/H131individuals, all of whom were wild-type (Q/Q) at position 127. We show that the K127 substitution imparts to an FcγRIIA-R131 molecule the ability to interact with the hIgG2 subclass and enhance monocyte and neutrophil phagocytosis in comparison with that of wild-type homozygous Q/Q127, R/R131. In addition, we show that the mutant receptor retains the ability to bind mIgG1 and thus has a unique phenotype.
MATERIALS AND METHODS
Cell preparation and PCR amplification.
Peripheral blood (10 mL) was collected in a heparinized tube. Collection of blood samples from donors was performed with informed consent after obtaining the approval of the Institutional Review Board. The erythrocytes were selectively lysed and genomic DNA was isolated from the resulting white blood cell pellet with an automated nucleic acid extractor according to manufacturer's instructions (Applied Biosystems, Inc, Foster City, CA). Genomic DNA was reconstituted in sterile water (1 mL) and the concentration was determined by optical density at 260 nm on a spectrophotometer.
Oligonucleotide primers were chosen that selectively amplify the FcγRIIA gene and not the highly homologous FcγRIIB and FcγRIIC genes. One of two sense primers (PCR1 or PCR3) from the second extracellular domain was used. The antisense primer (4INM) was in the intron immediately downstream of the second extracellular domain where the sequence for FcγRIIA, FcγRIIB, and FcγRIIC diverge. The resulting PCR product was either 322 bp (using PCR1) or 277 bp (using PCR3). Both products contained the distal portion of the second extracellular FcγRIIA exon (which contained the polymorphism at codon 131 and the mutation at codon 127), the splice junction, and the proximal portion of the downstream intron. The primers are as follows: PCR1, 5′ GGA GAA ACC ATC ATG CTG AG 3′; PCR3, 5′ CTG GTC AAG GTC ACA TTC TTC 3′; and 4INM, 5′ CAA TTT TGC TGC TAT GGG C 3′.
PCR reactions were performed in 100 μL containing buffer (50 mmol/L KCl, 10 mmol/L Tris-HCl, pH 8.3, 0.001% [wt/vol] gelatin, 1.5 mmol/L MgCl2), 200 ng of sense and 200 ng of antisense primer, 130 to 860 ng of genomic DNA, and 400 μmol/L each of dATP, dCTP, dGTP, and TTP. After 5 minutes of incubation at 95°C, 2.5 U of AmpliTaq DNA polymerase (Perkin-Elmer/Cetus, Norwalk, CT) was added. The mixture was amplified for 30 to 35 cycles using a GeneAmp PCR System 9600 (Perkin-Elmer/Cetus). Each cycle consisted of a denaturing step (94°C for 30 seconds), an annealing step (50°C for 30 seconds), and an elongation step (72°C for 30 seconds), in that order. PCR amplification was performed on five separate occasions for the individual with the codon 127 substitution (4 from an initial blood draw and 1 from a second blood draw).
SSCP analysis.
We previously described an SSCP protocol for identification of the FcγRIIA-H/R131 genotype.2 Briefly, a 0.65 ng (typically 5.4 to 6.3 μL) sample of the above-described PCR product and 10 μL of loading buffer (95% [vol/vol] formamide, 0.05% [wt/vol] xylene cyanol, 20 mmol/L EDTA) were heated to 100°C for 10 minutes and then placed immediately on wet ice. All subsequent steps were performed in a cold room at 4°C. Samples were loaded onto a nondenaturing 8% (wt/vol) polyacrylamide-TBE (92 mmol/L Tris, 95 mmol/L borate, 2.5 mmol/L EDTA) gel (18 × 24 cm; SE 600; Hoefer Scientific Instruments, San Francisco, CA) with a 37.5:1 ratio of acrylamide to bisacrylamide. The gel apparatus was further cooled by the Hoefer SE 6160 heat exchanger with a continuous flow of cold water surrounding the chamber. Electrophoresis was performed in a discontinuous buffer (25 mmol/L Tris, 192 mmol/L glycine) at 200 V for 6 hours. Gels were silver stained according to the manufacturer's instructions (silver stain kit; Bio-Rad, Melville, NY).
Automated DNA sequence analysis.
PCR products were purified with Magic PCR Preps DNA Purification System (Promega, Madison, WI), and then automated DNA sequence analysis with dye-labeled dideoxynucleotide chain terminators was performed following the manufacturer's instructions (Taq dideoxy terminator cycle sequencing; Applied Biosystems, Inc). The reaction products were analyzed on a laser-based, fluorescence emission 373A DNA sequencer (Applied Biosystems, Inc). All DNA sequences were determined in both directions using sense (PCR1 or PCR3) and antisense (4INM) primers.
Subcloning and analysis of FcγRIIA PCR products.
The FcγRIIA PCR product containing the substitution at codon 127 was obtained as previously described using PCR1 and 4INM primers. The product was purified with Magic PCR Preps DNA Purification System (Promega) and subcloned directly into the pT7Blue T-Vector using the pT7Blue T-Vector Kit (Novagen, Madison, WI) following the manufacturer's instructions. Individual colonies were isolated and grown at 37°C, and the subcloned FcγRIIA DNA was amplified directly from individual colonies using PCR and vector-based primers that flank the cloning site. The sequence of each PCR product was determined in both directions by automated DNA sequence analysis as described above.
Quantitation of FcγR expression by flow cytometry.
Leukocytes from fresh anticoagulated blood were prepared as previously reported.12,19 Briefly, isolated monocytes or PMNs were incubated with saturating concentrations of the following MoAbs: murine IgG1 or IgG2b (controls), IV.3 (mIgG2b specific for FcγRII; Medarex, Inc, Annandale, NJ), 41H16 (specific for FcγRIIA-R131; generously provided by Dr Theodore Zipf, University of Texas Cancer Center, Houston, TX20), CLB Gran 1 (specific for FcγRIIIB12; Research Diagnostics Inc, Flanders, NJ), and 22 (specific for FcγRI12; Medarex, Inc). This was followed by incubation with phycoerythrin-conjugated goat antimouse IgG F(ab)′2. After washing with phosphate-buffered saline and fixation with 1% (vol/vol) paraformaldehyde, specific cell-associated immunofluorescence was quantitated on a FACScan (Becton Dickinson, San Jose, CA) as previously described.5
Assay of neutrophil (PMN) FcγR-mediated phagocytosis.
Fresh human peripheral blood was collected in a heparinized syringe and separated by centrifugation through a discontinuous two-step Ficoll-Hypaque gradient.5 Neutrophils (PMNs) were isolated from the lower interface and washed with Hanks' balanced salt solution. Bovine erythrocytes were coupled to IV.3 Fab (anti-FcγRII CD32 MoAb), hIgG1 (human IgG1-myeloma protein), hIgG2 (human IgG2-myeloma protein), or mIgG1 (murine IgG1-myeloma protein) by a biotin-avidin technique.5 The resulting E-IV.3, E-hIgG1, E-hIgG2, and E-mIgG1 were used as probes of FcγR-mediated internalization. The density of opsonization was determined by flow cytometry as described previously and corresponded to low opsonization to maximize the capacity to detect differences among the FcγRIIA genotypes.12 Erythrocyte phagocytosis by PMNs and monocytes was quantitated as reported previously.5 Briefly, phagocytes were combined with E-IV.3, E-hIgG2, E-hIgG1, or E-mIgG1, centrifuged at 44g for 3 minutes, and then incubated at 37°C for 15 minutes to allow for maximum internalization. After hypotonic lysis of noninternalized E, phagocytosis was quantitated by light microscopy. At least 400 cells per slide were counted in duplicate without knowledge of the donor FcγRIIA genotype. The data are expressed as the phagocytic index (PI; number of ingested erythrocytes per 100 PMN). To enable simultaneous quantitation of FcγRIIa function in multiple donors with a range of different erythrocyte probes, a flow cytometric assay was used. Erythrocytes coupled to IgG or Fab were labeled with lipophilic red dye PKH-26 and then fluorescence was determined.21 The phagocytosis assay was performed as described above and, after lysis of noninternalized erythrocytes, phagocyte-associated PKH-26 fluorescence was quantitated by flow cytometry.21a To compare individuals of different FcγRIIA genotypes and to control for interexperiment variability using both assays of phagocytosis, data are expressed as the percentage of PI of the R/R131 homozygote studied in each experiment (%PI = [PI-K127 or H131/PI-R131] × 100).
Platelet aggregation assay.
Peripheral blood (20 mL) was collected in a polypropylene tube (Sarstedt, Nuembrecht, Germany) on two occasions from the individual with the K/Q127, R/R131 FcγRIIA genotype for platelet aggregation analysis. This analysis was also performed for individuals from each of the known FcγRIIA genotypes (n = 5 to 7 per genotype). Platelet-rich plasma and platelet-poor plasma were obtained by differential centrifugation (800 rpm [132g] for 15 minutes, followed by 3,000 rpm [1,862g] for 15 minutes), and the platelet count of the platelet-rich plasma was adjusted to 300,000/μL with the platelet-poor plasma. Aggregation studies were performed in one of two aggregometers (PAP 4; Biodata, Hatboro, PA or Chronolog, Havertown, PA) calibrated to each other by parallel analysis of the same samples. All samples were determined to be free of spontaneous aggregation and to have normal aggregation in response to standard agonists (thrombin and collagen). Alb-6, an mIgG1MoAb also directed against CD9 (AMAC, Inc, Westbrook, ME), was diluted to 200 ng/μL and added to 0.5 mL of platelet-rich plasma at a final concentration of 15 μg/mL, and aggregation was monitored. Lag time (the time from the addition of the antibody agonist until the onset of aggregation as manifested by the start of the marked deflection in the light transmission recording) and the final percentage of aggregation were determined. The final antibody concentration chosen, 15 μg/mL, is a potent stimulus, as shown by platelets from Q/Q127, H/R131 heterozygotes, which aggregate but demonstrate a prolonged lag time.
Statistical analysis.
Results of phagocytosis assays for monocytes and neutrophils from donors with different FcγRIIA genotypes were compared using the paired Student's t-test (two-sided, α < .05). Likewise, platelet aggregation lag time data for platelets from donors with different FcγRIIA genotypes were compared using the paired Student'st-test (two-sided, α < .05).
RESULTS
Detection of the Q to K127 change in FcγRIIA.
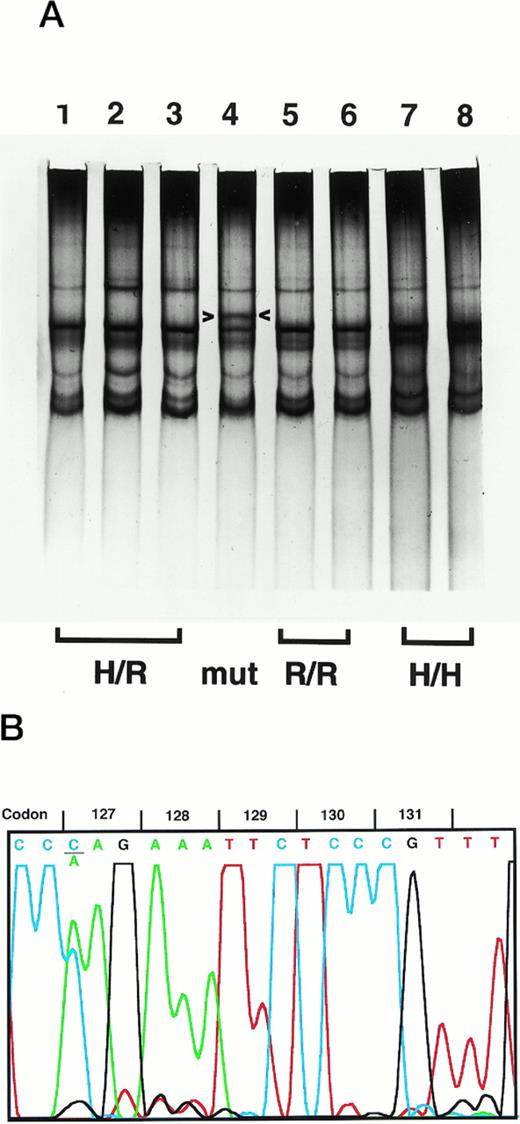
To determine the FcγRIIA-H/R131 genotype distribution in healthy individuals to compare with that in disease populations, we analyzed PCR products from genomic DNA of 50 African-Americans by SSCP.2 This FcγRIIA-specific PCR product includes the known H/R131 polymorphism and extends from the middle of the exon encoding the second Ig-like extracellular domain through the downstream intron. PCR product from one African-American individual had a unique SSCP pattern (Fig 1A) that, when subjected to automated DNA sequence analysis, showed homozygosity for R131 and heterozygosity for a C to A mutation in codon 127 (Fig 1B). This substitution changes the codon from CAG (glutamine, Q) to AAG (lysine, K). To ensure that this was not an artifactual PCR-induced mutation, several steps were taken. Sequence analysis of an independent PCR reaction from the same genomic DNA preparation was confirmatory, as were the results from a second independent preparation of genomic DNA from the same individual. Because codon 127 in the highly homologous FcγRIIB and FcγRIIC genes is AAG (K), we ruled out amplification of the homologous genes by sequence analysis of subcloned PCR products. At every position in the PCR product where FcγRIIA diverges from FcγRIIB/C, the sequence agreed exactly with FcγRIIA. Individually sequenced subclones showed the C to A substitution in a distribution consistent with a heterozygote (6 of 15, A; 9 of 15, C). Thus, we established that this individual has a novel C to A mutation at FcγRIIA codon 127.
Detection of the FcγRIIA K 127 mutation. (A) SSCP analysis of the FcγRIIA PCR product from genomic DNA is shown. In lane 4, a unique SSCP pattern (arrowheads, top band) is seen in comparison with H/R131 (lanes 1 through 3), R/R131 (lanes 5 and 6), and H/H131 (lanes 7 and 8) samples. (B) DNA sequence analysis of the PCR product with the unique SSCP pattern shows heterozygosity for C (blue) and A (green) at the first nucleotide of codon 127. The sequence also indicates R/R131 homozygosity (G, black, at the second nucleotide of codon 131).
Detection of the FcγRIIA K 127 mutation. (A) SSCP analysis of the FcγRIIA PCR product from genomic DNA is shown. In lane 4, a unique SSCP pattern (arrowheads, top band) is seen in comparison with H/R131 (lanes 1 through 3), R/R131 (lanes 5 and 6), and H/H131 (lanes 7 and 8) samples. (B) DNA sequence analysis of the PCR product with the unique SSCP pattern shows heterozygosity for C (blue) and A (green) at the first nucleotide of codon 127. The sequence also indicates R/R131 homozygosity (G, black, at the second nucleotide of codon 131).
The K127 mutation occurs within a 14-amino acid stretch implicated as important in the binding of IgG ligands by the FcγRIIa receptor (Fig 2).22 23 To examine the functional consequences of this change, we performed analysis of cells from the individual with the variant using well-defined antibody reagents that distinguish FcγRIIA-H131 from R131.
Sequence comparison of the amino acids from position 124 to 137 for FcγRIIA wild-type (Q127, green), the described mutation (K127, red diamond), and FcγRIIB/C. Note that NFS (blue, underlined) at 135-137 is a site for N-linked glycosylation in FcγRIIB/C. There is a conservative L (IIA) to S (IIB/C) change at position 132.
Sequence comparison of the amino acids from position 124 to 137 for FcγRIIA wild-type (Q127, green), the described mutation (K127, red diamond), and FcγRIIB/C. Note that NFS (blue, underlined) at 135-137 is a site for N-linked glycosylation in FcγRIIB/C. There is a conservative L (IIA) to S (IIB/C) change at position 132.
Cell surface expression of FcγRIIA K127 mutant is equivalent to wild-type.
To examine the effect of the K127 mutation on phagocyte function, we first assessed receptor expression. Flow cytometry was performed concurrently on monocytes and PMNs from disease-free controls with Q/Q127, H/H131 and Q/Q127, R/R131 genotypes and the individual with the K/Q127, R/R131. Using anti-FcγRII MoAb IV.3, there was similar fluorescence on monocytes (mean channel fluorescence intensity in arbitrary units [MFI]: 526, 600, and 509, respectively) and PMN (MFI, 468, 538, and 547, respectively). IV.3 is a ligand binding site MoAb that recognizes both the Q127/R131 and Q127/H131allotypes of FcγRIIa. Experiments with MoAb 41H16, which specifically recognizes FcγRIIa-R131, supported similar expression of FcγRIIa on K/Q127, R/R131 and Q/Q127, R/R131 PMNs (MFI, 550 v 506). Previous studies using these MoAbs have shown similar expression of FcγRIIa in populations of disease-free individuals of each known genotype.5 20 Although we cannot rule out the possibility of differential binding of the anti-FcγRIIa MoAbs to the K127/R131 variant, our results suggest that the mutant receptor is present at equivalent levels to wild-type receptors. Additionally, recognition by ligand binding site MoAbs supports the possibility that it is a functional receptor.
The Q to K127 change enhances the phagocytosis of erythrocytes coupled to hIgG2 in an R131homozygote.
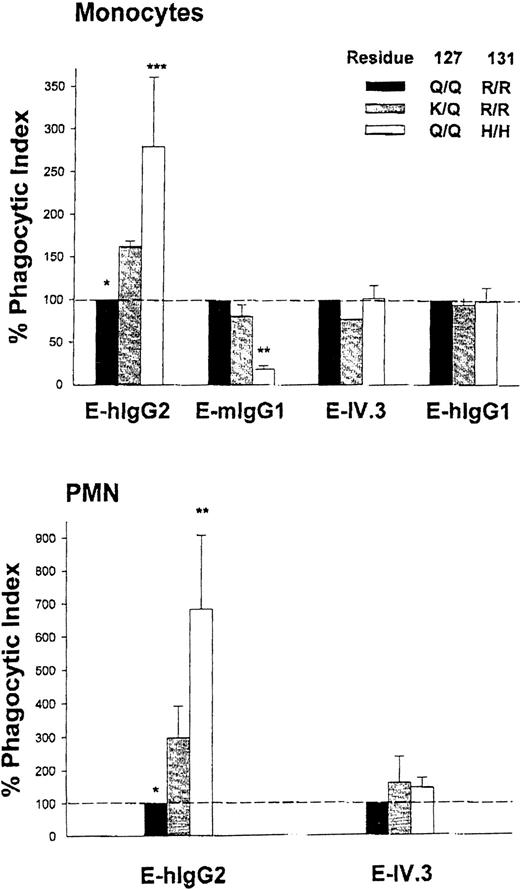
FcγRIIa is a major phagocytic receptor on monocytes and PMNs, and internalization of E-hIgG2 is dependent on FcγRIIA genotype.5,12 PMN and monocytes from H/H131individuals efficiently bind and ingest E-hIgG2, whereas this is minimal or absent in R/R131 homozygotes. H/R131 heterozygotes have an intermediate capacity to recognize hIgG2.12 In contrast, mIgG1 is efficiently bound by R/R131homozygotes and minimally recognized by H/H131 individuals. To examine the effect of the K127 variant on hIgG2 and mIgG1 handling, we simultaneously quantitated E-hIgG2 and E-mIgG1 phagocytosis by monocytes from the individual with the K127 variant and compared those results with results from H/H131 and R/R131 donors. Separate matched triplet experiments were performed, each with a pair of homozygote donors and the variant. Phagocytosis of E-IV.3, an FcγRIIa-specific probe that is not selective for allotypes, was assessed in each experiment. E-hIgG1, which is recognized by both alleles of FcγRIIa and by FcγRI and FcγRIIIa, was a control for general phagocytic potential of donor monocytes. As shown in Fig 3A, phagocytosis of E-hIgG2was higher for the K/Q127, R/R131 variant than for the group of Q/Q127, R/R131 homozygotes (P < .05, n = 3), whereas there was no difference in the capacity to internalize E-IV.3 and E-hIgG1. Although the K/Q127 heterozygote showed enhanced recognition of hIgG2, there was no detectable change in the association with mIgG1. We cannot exclude the possibility that, with a wide range of E-mIgG1 opsonization densities, a difference would be evident, but availability of blood from the variant donor was limited. Formal proof that there is a change in hIgG2binding in the absence of a reciprocal change in mIgG1binding will require phagocytosis experiments using cells transfected with the FcγRIIA-K127/R131.
Monocyte (A) and PMN (B) internalization of erythrocytes coupled with specific human IgG myeloma proteins (E-hIgG1and E-hIgG2), murine IgG1 myeloma protein (E-mIgG1), or anti-FcγRII MoAb IV.3 (E-IV.3). The phagocytic index shown for each erythrocyte probe reflects simultaneous experiments with phagocytes from individuals of each of three FcγRIIA genotypes: Q/Q127, H/H131; Q/Q127, R/R131; and K/Q127, R/R131. The % Phagocytic Index = (PIdonor/ PIQ/Q127, R/R131) × 100. Values represent the mean ± SD of two to five experiments comparing the K127 variant with different H/H131 and R/R131 homozygotes. % PI were compared using the paired Student's t-test. *P< .05, K/Q127, R/R131vQ/Q127, R/R131; **P < .01, K/Q127, R/R131v Q/Q127, H/H131; ***P = .051, K/Q127, R/R131v Q/Q127, H/H131.
Monocyte (A) and PMN (B) internalization of erythrocytes coupled with specific human IgG myeloma proteins (E-hIgG1and E-hIgG2), murine IgG1 myeloma protein (E-mIgG1), or anti-FcγRII MoAb IV.3 (E-IV.3). The phagocytic index shown for each erythrocyte probe reflects simultaneous experiments with phagocytes from individuals of each of three FcγRIIA genotypes: Q/Q127, H/H131; Q/Q127, R/R131; and K/Q127, R/R131. The % Phagocytic Index = (PIdonor/ PIQ/Q127, R/R131) × 100. Values represent the mean ± SD of two to five experiments comparing the K127 variant with different H/H131 and R/R131 homozygotes. % PI were compared using the paired Student's t-test. *P< .05, K/Q127, R/R131vQ/Q127, R/R131; **P < .01, K/Q127, R/R131v Q/Q127, H/H131; ***P = .051, K/Q127, R/R131v Q/Q127, H/H131.
In the experiments with PMN, evidence for differential binding capacity of K/Q127, R/R131 was underscored. Internalization of E-hIgG2 for the heterozygote variant was two to three times greater than that of simultaneously studied R/R131 donors (P < .005, paired t-test, n = 5), whereas handling of E-IV.3 by PMN was comparable for all genotypes in the matched triplet experiments (Fig 3B). Equivalent expression of FcγRIIIb (CD16; MoAb CLB Gran 1) and the absence of FcγRI (CD64; MoAb 22) in the individuals with each of the three genotypes (data not shown) minimizes the possibility of these receptors confounding the analysis of the impact of the Q to K127mutation on PMN function. For the level of opsonization of E-hIgG2 used in these studies, we have previously shown phagocytosis measured by microscopy in a population of wild-type R/R131 donors to be 1.2 ± 2.4 erythrocytes/PMN (range, 0 to 6).12 In the current studies, assessment by microscopy showed R/R131 donors to internalize 4 ± 1 erythrocytes/PMN compared with 14 ± 1 in the K/Q127, R/R131 variant. This difference was confirmed with the flow cytometric assay of phagocytosis, which provided the opportunity to measure the function of a greater number of phagocytes and multiple probes. Given that the individual with the variant is heterozygous for the K127 substitution and thus only half of the FcγRIIa receptor molecules on the PMN or monocyte are the K127/R131 form, our data show enhanced binding of hIgG2 by the variant receptor over the wild-type Q127/R131.
Platelet aggregation mediated by mIgG1 antiplatelet antibody.
The phagocyte data showed clearly that the K127/R131 receptor interacted well with hIgG2. The data also suggested that interaction of K127/R131 receptor proteins with mIgG1 was comparable to that of Q127/R131 and distinct from Q127/H131. We performed experiments with an mIgG1 antiplatelet CD9 antibody to extend these findings in cells (platelets) expressing FcγRIIa as the sole Fc receptor for IgG.24-26 There is a differential ability of platelets to be activated by murine antiplatelet antibodies of the mIgG1subclass depending on the FcγRIIA-H/R131 genotype: R/R131 > H/R131 >> H/H131, as manifested by increases in lag time.2,27 28 We quantitated platelet aggregation from known wild-type FcγRIIA-H/H131, R/R131 and H/R131 donors and the individual with the Q to K127 mutation after stimulation with Alb-6, an mIgG1 anti-CD9 antiplatelet antibody (15 μg/mL). As predicted, platelets from all donors, except Q/Q127, H/H131, underwent aggregation in response to Alb-6 and proceeded to an equivalent final extent. Platelets from all donors also had negligible spontaneous aggregation and equivalent aggregation to standard agonists, including thrombin and collagen. As shown in Fig 4, when stimulated with Alb-6, the lag time for the variant K/Q127, R/R131 platelets was no different from that of Q/Q127, R/R131, but was significantly shorter than that of platelets from both Q/Q127, H/R131 and Q/Q127, H/H131 donors (P < .05). The comparison with Q/Q127, H/R131 is particularly important, because on those platelets 50% of the receptors are of the Q127/H131 form, whereas 50% are Q127/R131. If K127/R131receptors did not interact well with mIgG1, then the platelets from the donor with the variant K/Q127, R/R131 genotype should have behaved like the Q/Q127, H/R131 platelets. Instead, they were significantly different from Q/Q127, H/R131platelets and indistinguishable from Q/Q127, R/R131. These platelet activation results indicate that the FcγRIIA K127/R131 receptors retain significant interaction with mIgG1.
Platelet aggregation triggered by mIgG1antiplatelet CD9 antibody Alb-6. Lag time in minutes (mean ± SEM) is shown for platelets from the variant individual and the three wild-type FcγRIIA genotypes. *Lag time is significantly shorter (P < .05) for K/Q127, R/R131 and Q/Q127, R/R131 than for Q/Q127, H/R131 and Q/Q127, H/H131.
Platelet aggregation triggered by mIgG1antiplatelet CD9 antibody Alb-6. Lag time in minutes (mean ± SEM) is shown for platelets from the variant individual and the three wild-type FcγRIIA genotypes. *Lag time is significantly shorter (P < .05) for K/Q127, R/R131 and Q/Q127, R/R131 than for Q/Q127, H/R131 and Q/Q127, H/H131.
DISCUSSION
We identified an individual with a novel FcγRIIA genotype in which a C to A nucleotide substitution leads to a Q to K127 amino acid change in the setting of homozygosity for R/R131. We showed increased phagocytosis of hIgG2-opsonized erythrocytes by this individual's monocytes and neutrophils in comparison with those of wild-type Q/Q127, R/R131 individuals. On platelets, there was a significant difference in the interaction with mIgG1 in comparison with platelets from Q/Q127, H/R131 and Q/Q127, H/H131 donors, but no difference from Q/Q127, R/R131. Neither surface expression as assessed by MoAb IV.3 nor interaction with hIgG1 were affected by the K127 mutation. These results indicate that this novel mutation converts a receptor with minimal interaction with the hIgG2 subclass (wild-type Q127/R131) into one with clearly detectable interaction (variant K127/R131). In addition, interaction with mIgG1 is preserved. In our studies of phagocytes, we were careful to establish equivalent expression of the Fcγ receptors at physiologic surface densities on cells with the normal phagocytic machinery. Previous experiments have identified critical regions for IgG binding within the extracellular domains of FcγRIIa. The present study confirms their importance in unmanipulated human phagocytes and demonstrates the impact even in the context of other Fcγ receptors (IIIb on PMN and Ia on monocytes). These features of our comparative studies may be an advantage over studies of transfected Fcγ receptor genes in heterologous cells.
The K127/R131 FcγRIIa molecule we describe has a unique phenotype—it recognizes hIgG2-like Q127/H131 and mIgG1-like Q127/R131. The structural basis for ligand binding specificity by this variant and the previously described FcγRIIA allelic isoforms is unknown. The unique phenotype of the FcγRIIA-K127/R131 molecule implies that interactions between amino acids in the ligand binding pocket, such as at 127 and 131, with each other and with specific IgG residues contribute to the differential IgG binding by the receptor isoforms. Chimeric molecules with other Fcγ and Fcε receptors as well as a large number of site-directed mutants have been used in transfected cells in vitro to study FcγRIIA ligand binding, but the 127 position has not been explored by any of these investigators because there was no prior evidence to implicate its importance.23,29-31 Once the FcγRIIa crystal structure has been determined, structure/function studies with wild-type and the mutant K127/R131 receptors will shed light in the future on the nature of differential receptor interactions with various IgG subclasses.32-34
This is the first example of a naturally occurring mutation in a human Fcγ receptor that alters receptor function. Further examples are anticipated as useful genetic screening methods and precise functional studies are more widely applied. Growing evidence supports the view that genetic variation in the Fcγ receptors has functional significance and may be associated with susceptibility to human disease.35-44 Polymorphisms of FcγRIIa and FcγRIIIb and their association with disease underscore their roles as specific genetic risk factors. Rapid determination of the FcγRIIA-H/R131 genotype continues to be important in studies that link human immune, infectious, and inflammatory disorders to this polymorphism.3,9,12,45 Because the K127mutation is not resolved at the level of reactivity with available anti-FcγRII MoAb or with allele-specific genotyping, the current work shows the importance of unbiased genetic screening methods such as SSCP (which we used), DGGE, or direct automated sequence analysis in examining genetic variations in FcγRIIa and other Fcγ receptors.2 46 We have not seen the Q to K127mutation in any other of the approximately 200 healthy individuals screened to date. However, we and others have noted that cells from individuals of known H/R131 genotypes occasionally have anomalous binding, platelet aggregation, or phagocytosis of IgG ligands mediated by FcγRIIa. Thus, in the future, it will be of great interest to more fully examine the prevalence of this or related FcγRIIA mutations in such individuals.
ACKNOWLEDGMENT
The authors thank Donna Patterson for her assistance and Diana Cassel and Margaret Keller for their advice and encouragement.
Supported in part by the following grants from the Public Health Service: NIH T32 HL07150 (C.F.N.), R01 DK16691 (E.S., S.S., S.McK.), P30 HD28815 (S.McK.), P01 HL40387 (S.McK.), and R01 AR38889 (J.E.S.). The flow cytometry Core Facility at the Hospital for Special Surgery is supported in part by the Cornell Multipurpose Arthritis and Musculoskeletal Disease Center (P60 AR 30692).
Address reprint requests to Steven E. McKenzie, MD, PhD, duPont Hospital for Children, 1600 Rockland Rd, Wilmington, DE 19899.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal