Abstract
Activation of human peripheral blood neutrophils by pathogens or by phorbol myristate acetate (PMA), fMLP, or myeloid growth factors generates a respiratory burst in which superoxide production plays an important role in killing invading microorganisms. Although the increased energy demands of activated neutrophils would be expected to be associated with increased glucose uptake and utilization, previous studies have shown that PMA inhibits 2-deoxyglucose (2-DOG) uptake. In this study, we show that PMA activation of neutrophils, isolated by methods not involving hypotonic lysis, increases the rate of 2-DOG uptake and results in a 1.6-fold to 2.1-fold increase in transporter affinity for glucose without changing Vmax. Increased transporter affinity in response to PMA was also observed with 3-O-methyglucose, which is not phosphorylated, and inclusion of glucose in the activation medium further increased respiratory burst activity. Increased 2-DOG uptake and increased transporter affinity for glucose were also observed with the peptide activator, fMLP, and with granulocyte-macrophage colony-stimulating factor (GM-CSF). The protein kinase C (PKC) inhibitor, calphostin C, and the tyrosine kinase inhibitor, genistein, inhibited both PMA- and fMLP-stimulated 2-DOG uptake. In contrast, genistein inhibited fMLP-induced superoxide production, but had little effect on the PMA-induced response, while staurosporine differentially inhibited PMA-induced superoxide production. These results show that neutrophil activation involves increased glucose transport and intrinsic activation of glucose transporter molecules. Both tyrosine kinases and PKC are implicated in the activation process.
THE INFLAMMATION THAT is associated with infection is characterized by recruitment of professional phagocytes and secretory cells including neutrophils, eosinophils, mast cells, and mononuclear cells to the affected area. Several interrelated defense mechanisms are deployed that include the release of highly toxic secretory granule components, activation of the respiratory burst, and phagocytosis. Although the respiratory burst activity of human peripheral blood neutrophils has been studied intensively,1and many components of the membrane-bound respiratory burst oxidase responsible for superoxide production identified,2knowledge of the way that neutrophils use endogenous and exogenous energy supplies to fuel the respiratory burst is poorly understood.
Increased superoxide production that is associated with phagocytosis and phorbol myristate acetate (PMA) treatment of neutrophils involves increased glucose-C-1 metabolism via the hexose monophosphate (HMP) shunt.3-6 With neutrophils, uptake of 2-deoxyglucose (2-DOG) and certain amino acids has been shown to be inhibited by PMA along with the uptake of radiolabelled bacteria,4 but with peritoneal macrophages and macrophage cell lines, superoxide production in response to PMA and colony-stimulating factor (CSF)-1 was dependent on glucose in the culture media.6,7 These results suggest that neutrophils may use endogenous energy supplies to facilitate the respiratory burst, whereas macrophages rely on exogenously supplied energy. In the short-term, simple sugar phosphates in neutrophils could provide the energy needed for respiratory burst activity, but in the longer term, stored glycogen is used.8
Transport of glucose across the plasma membrane is a passive process involving a family of structurally related ‘facilitative’ glucose transporter molecules, which shift glucose down its concentration gradient without expending energy.9 These transporters are often expressed in a tissue or cell-specific manner, and in some cases, their expression is regulated by extracellular signals such as hormones and growth factors.10 In adipose and muscle cells in the short-term, increased glucose transport in response to insulin occurs by translocation of Glut-4 from an intracellular pool to the plasma membrane.11,12 Glut-1 is also recruited to the plasma membrane in response to insulin, although this occurs to a much lesser extent than Glut-4. In addition to increased plasma membrane expression, there is also evidence that the intrinsic activity of Glut-1 and Glut-4 are modulated in response to insulin.13-16
Regulation of glucose transport in systems not involving insulin is poorly understood. Stress induced by a variety of reagents stimulates glucose transport by translocating transporters from intracellular sites to the plasma membrane17 or by increasing transporter expression.18 On the other hand, changes in the intrinsic activity of Glut-1 have been associated with glucose deprivation19 or cadmium treatment20 of 3T3L1 cells and azide treatment of rat liver clone 9 cells.21,22In hematopoietic cells, we and others have shown that interleukin (IL)-3 and other growth factors stimulate glucose transport in growth factor-dependent cells by increasing the affinity of glucose transporters for glucose without a change in transporter expression or Vmax.23,24 In other studies, the malignant phenotype and acutely transforming viruses and oncogenes have been associated with increased transporter affinity for glucose,25,26 while apoptosis induced in human Jurkat cells by an antibody against CD95 dramatically reduced the affinity of Glut-1 for glucose.27
To further investigate a possible relationship between glucose uptake and respiratory burst activation, human peripheral blood neutrophils were purified using Polymorphprep and their activation by PMA and fMLP studied. Contrary to previous reports,4 28 we show that increased superoxide production in response to PMA is associated with increased 2-DOG uptake. Furthermore, a 1.6-fold to 2.1-fold increase in affinity of glucose transporters for glucose was observed, and physiologic concentrations of glucose in the incubation medium doubled superoxide production. Increased transporter affinity for glucose was also observed with fMLP and with the myeloid growth factor, granulocyte-macrophage colony-stimulating factor (GM-CSF). Both tyrosine kinase activity and protein kinase C (PKC) were shown to be involved in PMA and fMLP stimulation of 2-DOG uptake, although differential effects of these inhibitors were observed on superoxide production. These results show that neutrophil activation involves acute regulation of glucose transporter function.
MATERIALS AND METHODS
Collection and preparation of neutrophils.
Human peripheral blood neutrophils were collected from venous blood of healthy donors using EDTA as an anticoagulant. Blood was layered over an equal volume of Polymorphprep (Nycomed Pharma, Oslo, Norway) and centrifuged at 500g for 30 minutes at 20°C. The lower band containing polymorphonuclear leukocytes was collected, washed twice with phosphate-buffered saline (PBS), and resuspended at a concentration of 107/mL. Purity and viability were greater than 95%.
Materials.
Calphostin C was obtained from Kamiya Biomedical Company (Thousand Oaks, CA) and 2-DOG from Fluka (Buchs, Switzerland). All other chemicals and enzymes including PMA, fMLP, cytochrome c, catalase, genistein, and staurosporine were from Sigma Chemical Company (St Louis, MO). Superoxide dismutase (SOD) was from Boehringer Mannheim (Mannheim, Germany). Recombinant human GM-CSF was obtained from Dr J.D. Watson, Genesis Research and Development Corporation, Auckland and was sourced from Immunex, Seattle, WA.
Superoxide production.
Superoxide production was determined by measuring SOD-inhibitable reduction of ferricytochrome c to the ferrous form. Briefly, 1 to 1.5 × 106 cells were incubated in 1 mL PBS, pH 7.4 containing 40 μmol/L cytochrome c and 20 μg/mL catalase with or without 20 μg/mL SOD. Samples were equilibrated for 5 minutes at 37°C and the reaction initiated by adding PMA (100 ng/mL), fMLP (0.5 μmol/L), or GM-CSF (100 ng/mL). All incubations were performed at 37°C for 10 minutes unless stated otherwise. Reactions were stopped by placing the tubes on ice for 5 minutes, after which the cells were removed by centrifugation at 10,000g for 1 minute. The absorbance of the supernatant containing reduced cytochrome c was measured at 550 nm against a blank containing all reagents except the cells. Superoxide production was calculated using a millimolar extinction coefficient of 21.1.
[3H]-2–DOG uptake.
2-DOG uptake was measured by the zero-trans method using [3H]-2-deoxy-D-glucose (2-DOG, 100 μmol/L, 1 μCi, Amersham, UK) as described previously.24 Neutrophils (106) were preincubated in 1 mL PBS for 5 minutes at 37°C before adding PMA, fMLP, or GM-CSF for the times indicated. Cells were recovered by centrifugation and suspended in 0.25 mL PBS containing [3H]-2–DOG. Uptake was stopped by adding ice-cold PBS containing 0.3 mmol/L phloretin and cells collected by microcentrifugation at 10,000g for 1 minute through a cushion of 10% bovine serum albumin (BSA). The cell pellet was washed, lysed in 0.1 mL 1% Triton X-100, and radioactivity determined. Kinetic analysis of 2-DOG uptake used 0.1 to 4 mmol/L 2-DOG in the extracellular medium and uptake was determined over 3 minutes. Where inhibitors were used, cells were treated with the inhibitors for 20 minutes before addition of PMA, fMLP, or growth factor.
[3H]-3-O methylglucose uptake.
3-O-methylglucose uptake was measured by the zero-trans method described above, using [3H]-3-O-methylglucose (3-O-MG, 100 μmol/L, 1 μCi, Amersham). Uptake was determined at 37°C in 50 μL glucose-free RPMI instead of PBS and was performed over 3 seconds, which approached the linear range of the uptake curve.
RESULTS
Effects of PMA on superoxide production and [3H]-2–DOG uptake by neutrophils.
Human peripheral blood neutrophils were prepared by a one-step density fractionation procedure using Polymorphprep. Neutrophils were washed twice in PBS before stimulation with 100 ng/mL PMA for the times indicated. This concentration of PMA had previously been shown to be optimum for both superoxide production and for [3H]-2–DOG uptake. Figure 1A shows that superoxide production, determined as SOD-inhibitable cytochrome c reduction, increased linearly over 30 minutes. In contrast, the rate of [3H]-2–DOG uptake, determined over 3-minute intervals at the times indicated, continued to increase over 60 minutes (Fig 1B). An approximate twofold increase in the rate of [3H]-2–DOG uptake was observed at 60 minutes.
Effect of PMA on superoxide production by neutrophils and on [3H]-2–DOG uptake. (A) SOD-inhibitable cytochrome c reduction. Neutrophils (106) were treated with 100 ng/mL PMA for increasing times in the presence and absence of SOD. Control absorbance (A550 + SOD) was 0.364 ± 0.009. (B) [3H]-2–DOG uptake determined over 3-minute intervals at the times indicated after exposure to PMA. Each value represents the mean of duplicate determinations obtained from two separate experiments. [3H]-2–DOG uptake at zero time was 1619 ± 41 cpm.
Effect of PMA on superoxide production by neutrophils and on [3H]-2–DOG uptake. (A) SOD-inhibitable cytochrome c reduction. Neutrophils (106) were treated with 100 ng/mL PMA for increasing times in the presence and absence of SOD. Control absorbance (A550 + SOD) was 0.364 ± 0.009. (B) [3H]-2–DOG uptake determined over 3-minute intervals at the times indicated after exposure to PMA. Each value represents the mean of duplicate determinations obtained from two separate experiments. [3H]-2–DOG uptake at zero time was 1619 ± 41 cpm.
Effects of PMA on the kinetics of 2-DOG uptake by neutrophils.
To determine whether the increased 2-DOG uptake observed after activation of neutrophils with PMA was associated with changes in transport kinetics, cells were treated with or without 100 ng/mL PMA for 10 minutes or 30 minutes before determining [3H]-2–DOG uptake over 3-minute intervals using a range of 2-DOG concentrations (0.1 to 4 mmol/L). Lineweaver-Burk analysis of the results of several experiments is summarized in Table 1. At both time points, PMA treatment significantly reduced transporter Km for glucose without affecting Vmax. Other methods of analysis, eg, Eadie-Hofstee plots gave similar results. The possibility that posttransport phosphorylation of 2-DOG may influence uptake kinetics was explored using the nonphosphorylatable glucose analogue 3-O-methylglucose. With this sugar, an uptake time of 3 seconds was used, as this was as close as we could get to the linear range of the uptake curve without compromising reproducibility. With 3-O-MG, PMA treatment for 10 minutes increased the affinity of glucose transporters for glucose by 21% without changing Vmax. Thus, neutrophils treated with PMA for 10 minutes exhibited a Km(mmol/L) of 2.79 ± 0.12 compared with 3.54 ± 0.04 for untreated controls (n = 2), whereas Vmax(nmol/106cells/minute) was 10.1 ± 0.1 and 10.37 ± 0.27, respectively.
Effect of PMA, fMLP, and GM-CSF on Kinetics of [3H]-2-DOG Uptake by Human Peripheral Blood Neutrophils
| Cell Treatment . | No. . | Km (mmol/L) . | Vmax (nmol/106 cells/min) . |
|---|---|---|---|
| None | 4 | 0.99 ± 0.03 | 2.91 ± 0.09 |
| PMA (10 min) | 4 | 0.61 ± 0.08* | 2.78 ± 0.19 |
| PMA (30 min) | 2 | 0.47 ± 0.01† | 2.99 ± 0.02 |
| fMLP (10 min) | 3 | 0.20 ± 0.02† | 1.65 ± 0.20 |
| GM-CSF (60 min) | 3 | 0.22 ± 0.04† | 1.40 ± 0.33 |
| Cell Treatment . | No. . | Km (mmol/L) . | Vmax (nmol/106 cells/min) . |
|---|---|---|---|
| None | 4 | 0.99 ± 0.03 | 2.91 ± 0.09 |
| PMA (10 min) | 4 | 0.61 ± 0.08* | 2.78 ± 0.19 |
| PMA (30 min) | 2 | 0.47 ± 0.01† | 2.99 ± 0.02 |
| fMLP (10 min) | 3 | 0.20 ± 0.02† | 1.65 ± 0.20 |
| GM-CSF (60 min) | 3 | 0.22 ± 0.04† | 1.40 ± 0.33 |
Peripheral blood neutrophils were stimulated with PMA (100 ng/mL), fMLP (0.5 μmol/L), or GM-CSF (100 ng/mL) for the times indicated before measuring [3H]-2-DOG uptake at increasing 2-DOG concentrations (0.1 to 4.0 mmol/L). Data were analyzed using Lineweaver Burk plots. Values are the mean ± SEM of the indicated number of experiments involving duplicate or triplicate determinations.
Significantly different from control, P < .01.
P < .002.
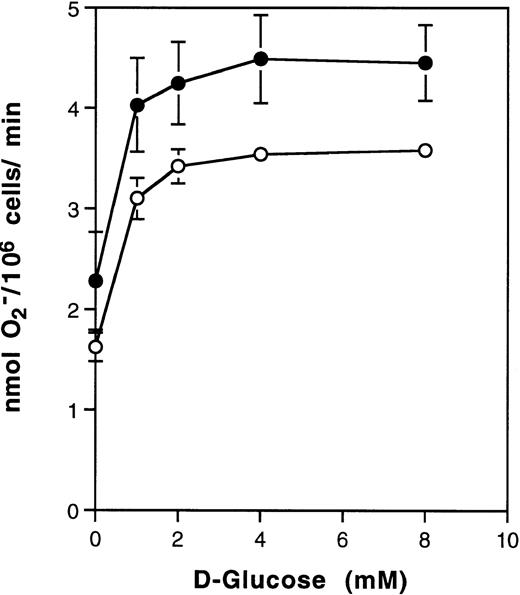
Superoxide production by neutrophils is facilitated by exogenous glucose.
The effects of PMA on 2-DOG uptake by neutrophils described above were determined in PBS without added glucose. The possibility that glucose may promote PMA-stimulated superoxide production was therefore determined. Figure 2 shows that PMA-stimulated superoxide production increased with the concentration of D-glucose to a plateau 2.5-fold at 5 to 10 mmol/L glucose. Thus, at physiologic concentrations of glucose, near maximum effects of PMA on superoxide production were observed. Figure 2 also shows the effects of glucose on superoxide production in the presence of divalent cations. Although the response curve was shifted, the fractional effect on 2-DOG uptake remained similar to that in the absence of Ca2+/Mg2+.
Effect of D-glucose on superoxide production by human neutrophils. Neutrophils (106) were treated with 100 ng/mL PMA in the presence of increasing concentrations of D-glucose for 20 minutes at 37°C and SOD-inhibitable cytochrome c reduction determined. Result are the average of two experiments involving duplicate determinations made in the presence (•) or absence (○) of 1.4 mmol/L Ca2+ and Mg2+. Control absorbances at A550 (no glucose) were 0.722 ± 0.19 and 0.605 ± 0.017, respectively and in the presence of SOD, 0.376 ± 0.007 and 0.364 ± 0.013, respectively.
Effect of D-glucose on superoxide production by human neutrophils. Neutrophils (106) were treated with 100 ng/mL PMA in the presence of increasing concentrations of D-glucose for 20 minutes at 37°C and SOD-inhibitable cytochrome c reduction determined. Result are the average of two experiments involving duplicate determinations made in the presence (•) or absence (○) of 1.4 mmol/L Ca2+ and Mg2+. Control absorbances at A550 (no glucose) were 0.722 ± 0.19 and 0.605 ± 0.017, respectively and in the presence of SOD, 0.376 ± 0.007 and 0.364 ± 0.013, respectively.
Effects of fMLP and the myeloid growth factor, GM-CSF on [3H]-2–DOG uptake by neutrophils.
Figure 3 compares the effects of fMLP on superoxide production and [3H]-2–DOG uptake by neutrophils. Maximum effects of fMLP were observed within 4 to 8 minutes at 10-7 to 10-8 M fMLP. Interestingly, [3H]-2–DOG uptake showed slightly greater sensitivity to fMLP than did superoxide production (Fig 3A and C). Increased 2-DOG uptake was also observed after treatment with GM-CSF for 60 minutes. At optimum times of stimulation by fMLP and GM-CSF, increased 2-DOG uptake was associated with a 4.5-fold to fivefold increase in transporter affinity for glucose, but with these activators, some reduction in Vmax was also observed (Table 1). With GM-CSF, only a small (30%) increase in superoxide production was observed consistent with its role in neutrophil priming (compared with 1.6-fold to threefold increase with PMA and fMLP).
Effect of fMLP on superoxide production and [3H]-2–DOG uptake by human neutrophils. Neutrophils (106) were incubated with 0.5 μmol/L fMLP for the times indicated (B and D) or for 5 minutes at the concentrations indicated (A and C) and SOD-inhibitable cytochrome c reduction (A and B) and [3H]-2–DOG uptake (C and D) determined. The results are the average of duplicate determinations. For (A) and (B), control absorbances (A550) in the presence of SOD were 0.342 ± 0.003 and 0.305 ± 0.003, respectively, and for (C) and (D), control uptake was 2,613 ± 32 cpm and 2,068 ± 94 cpm, respectively.
Effect of fMLP on superoxide production and [3H]-2–DOG uptake by human neutrophils. Neutrophils (106) were incubated with 0.5 μmol/L fMLP for the times indicated (B and D) or for 5 minutes at the concentrations indicated (A and C) and SOD-inhibitable cytochrome c reduction (A and B) and [3H]-2–DOG uptake (C and D) determined. The results are the average of duplicate determinations. For (A) and (B), control absorbances (A550) in the presence of SOD were 0.342 ± 0.003 and 0.305 ± 0.003, respectively, and for (C) and (D), control uptake was 2,613 ± 32 cpm and 2,068 ± 94 cpm, respectively.
Effects of protein kinase inhibitors on PMA and fMLP stimulation of [3H]-2–DOG uptake and superoxide production.
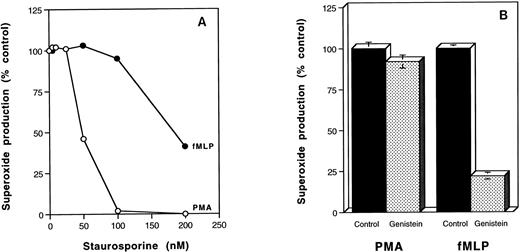
The PKC inhibitor, calphostin C, and the tyrosine kinase inhibitor, genistein, inhibited 2-DOG uptake stimulated by PMA and fMLP by 60% to 70%, and this was similar to the level of inhibition observed in the absence of the activating molecules (Fig4). These results contrast with the effects of PKC and tyrosine kinase inhibitors on superoxide production stimulated by PMA and fMLP (Fig 5). Genistein extensively inhibited fMLP-stimulated superoxide production, but had little effect on the PMA-induced response (Fig 5B). In contrast, PMA-stimulated superoxide production showed fourfold greater sensitivity to the protein kinase inhibitor, staurosporine, than the fMLP response (Fig 5A).
Effects of genistein and calphostin C on [3H]-2–DOG uptake by unstimulated and stimulated human neutrophils. Neutrophils (106) were pretreated without (solid bars) or with 100 μmol/L genistein (patterned bars) or 1 μmol/l calphostin C (shaded bars) for 20 minutes before stimulation with 100 ng/mL PMA for 20 minutes or 0.5 μmol/L fMLP for 5 minutes and [3H]-2–DOG uptake determined over 3 minutes in the absence of inhibitor. Results are the average of duplicate determinations.
Effects of genistein and calphostin C on [3H]-2–DOG uptake by unstimulated and stimulated human neutrophils. Neutrophils (106) were pretreated without (solid bars) or with 100 μmol/L genistein (patterned bars) or 1 μmol/l calphostin C (shaded bars) for 20 minutes before stimulation with 100 ng/mL PMA for 20 minutes or 0.5 μmol/L fMLP for 5 minutes and [3H]-2–DOG uptake determined over 3 minutes in the absence of inhibitor. Results are the average of duplicate determinations.
Effects of genistein and staurosporine on PMA and fMLP stimulation of human neutrophils. (A) Neutrophils (106) were pretreated without or with increasing concentrations of staurosporine for 20 minutes before stimulation with 100 ng/mL PMA for 20 minutes (○) or 0.5 μmol/L fMLP for 5 minutes (•) and determination of SOD-inhibitable cytochrome c reduction. (B) Neutrophils (106) were pretreated with or without 100 μmol/L genistein before stimulation with 100 ng/mL PMA for 20 minutes or 5 × 10-7 mol/L fMLP for 5 minutes and determination of SOD-inhibitable cytochrome c reduction. Results are the average of duplicate determinations. Control absorbances were: (A) unstimulated cells, 0.336 ± 0.013; PMA-treated, 0.931 ± 0.005; fMLP-treated, 0.754 ± 0.008. (B) Unstimulated cells, 0.386 ± 0.005; PMA-treated, 0.606 ± 0.015; fMLP-treated 0.540 ± 0.007.
Effects of genistein and staurosporine on PMA and fMLP stimulation of human neutrophils. (A) Neutrophils (106) were pretreated without or with increasing concentrations of staurosporine for 20 minutes before stimulation with 100 ng/mL PMA for 20 minutes (○) or 0.5 μmol/L fMLP for 5 minutes (•) and determination of SOD-inhibitable cytochrome c reduction. (B) Neutrophils (106) were pretreated with or without 100 μmol/L genistein before stimulation with 100 ng/mL PMA for 20 minutes or 5 × 10-7 mol/L fMLP for 5 minutes and determination of SOD-inhibitable cytochrome c reduction. Results are the average of duplicate determinations. Control absorbances were: (A) unstimulated cells, 0.336 ± 0.013; PMA-treated, 0.931 ± 0.005; fMLP-treated, 0.754 ± 0.008. (B) Unstimulated cells, 0.386 ± 0.005; PMA-treated, 0.606 ± 0.015; fMLP-treated 0.540 ± 0.007.
DISCUSSION
The phagocytic respiratory burst of human peripheral blood neutrophils can be activated by the phorbol ester, PMA, by the peptide, fMLP, and to a lesser extent by the myeloid growth factor, GM-CSF. In this study, we show coordinate stimulation of superoxide production and glucose uptake by neutrophils after activation by PMA and fMLP. With GM-CSF, enhanced 2-DOG uptake was associated with a relatively small increase in superoxide production. Thus, previous reports that PMA inhibits 2-DOG uptake by neutrophils4,28 were not substantiated. Rather, activated neutrophils showed enhanced glucose transporter activity and were able to use extracellular glucose, as well as intracelluar energy supplies, to fuel the respiratory burst. The reason why we have been able to show that the respiratory burst and glucose uptake are coordinately regulated most likely relates to the gentle one-step procedure currently used to isolate neutrophils. In the earlier studies, density separation was followed by hypotonic lysis in deionized or distilled water to remove residual erythrocytes. Comparison of respiratory burst activation of these neutrophils with those prepared by the Polymorphprep procedure used in the present study, showed an approximate fivefold greater ability to produce superoxide when hypotonic lysis was not used. Reduced glucose transporter function in neutrophils subjected to hypotonic lysis is indicated by lower Vmax values for 2-DOG uptake in a study by Potashnik et al29 (0.51 ± 0.11 nmol/106 cells/minute) compared with 2.7 to 3.2 nmol/106 cells/minute in the present study. It is also interesting to note that the Km values reported in the study of Potashnik et al are those of PMA-activated neutrophils (Fig3), explaining perhaps, the failure of De Chatelet et al4to further increase 2-DOG uptake after PMA activation. A more recent study of the effect of fMLP on hexose transport in polymorphonuclear leukocytes30 showed a twofold to fivefold increase in 2-DOG and 3-O-MG transport in response to fMLP, while another brief report31 indicated increased transport in response to PMA, contradicting previous reports.4 28
Increased 2-DOG uptake by human peripheral blood neutrophils after treatment with PMA was associated with functional activation of glucose transporter molecules on the cell surface. Thus, a twofold increase in glucose transporter affinity for glucose was observed at both 10 minutes and 30 minutes after PMA activation without a significant change in Vmax. However, the increasing rate of 2-DOG uptake shown in Fig 1B cannot be fully explained in terms of transporter affinity changes alone, as the increased affinity remained unchanged between 10 minutes and 30 minutes, during which time the rate of 2-DOG uptake increased by about 25%. Attempts to analyze the kinetics of 2-DOG uptake after 60 minutes treatment with PMA failed to show any further increase in transporter affinity for 2-DOG (results not shown). Therefore, it appears that additional mechanisms need to be invoked to explain the increased rate of 2-DOG uptake in response to PMA at times greater than 10 minutes. Although a possible contribution of 2-DOG phosphorylation to the observed kinetic changes cannot be ruled out, transporter activation was also observed when the nonphosphorylatable hexose, 3-O-MG, was used adding support to the activation model. Our results differ from those obtained with the chemotactic peptide, fMLP, where a fivefold increase in Vmax was observed without a change in Km, both with 2-DOG and 3-O-MG.30
Increased 2-DOG uptake and intrinsic activation of glucose transporters on neutrophils in response to PMA was initially demonstrated in PBS. However, addition of D-glucose increased PMA-induced superoxide production 2.5-fold, showing that increased glucose transport and transporter activation may be physiologically relevant. These results suggest that acute activation of the respiratory burst is contributed to about equally by extracellular glucose and intracellular energy sources. In the longer term, neutrophil glycogen reserves would be mobilized.8
The respiratory burst can also be activated in bone marrow and peritoneal macrophages and macrophage cell lines in response to PMA and myeloid growth factors,6,7,32 but in these situations, extracellular glucose is mandatory for respiratory burst activation and for the associated increase in 2-DOG uptake. With rat peritoneal macrophages stimulated with CSF-1 or PMA, increased 2-DOG uptake was associated with a 40% increase in transporter affinity for glucose,6,33 and this correlated with hexokinase translocation to the plasma membrane and coupling to sugar transport. Although Kiyotaki et al7 failed to detect a change in transporter affinity after PMA stimulation of the murine macrophage cell line, J774.16, we have consistently observed twofold to fourfold increased affinity of Glut-3 for glucose in RAW 264.7 cells activated by PMA, fMLP, and GM-CSF, and with IL-3 despite its inability to promote superoxide production.34
Human peripheral blood neutrophils express the glucose transporter subtype, Glut-1, no detectable Glut-3 being observed (N. Ahmed and M.V. Berridge, unpublished results). Therefore, regulation of the glucose transport activity of Glut-1 is implicated. Although the rapid neutrophil responses and the kinetic parameters observed are inconsistent with increased glucose transport being explained by an increase in gene and protein expression, we cannot exclude the possibility that transporter translocation from an internal pool may contribute to the observed increase in transport. However, with PMA, the fact that Vmax did not change significantly suggests that translocation contributes little to the changes in glucose transport described in this study.
Inhibitor studies showed that glucose uptake into unstimulated neutrophils is dependent on both tyrosine kinase and PKC activity. Likewise PMA and fMLP-stimulated 2-DOG uptake was inhibited by genistein and calphostin C (Fig 4). These results contrast with the effects of the inhibitors on superoxide production in that fMLP-stimulated superoxide production was differentially inhibited by genistein, whereas PMA-stimulated superoxide production exhibited greater sensitivity to staurosporine. Differential effects of staurosporine on PMA-stimulated superoxide production by neutrophils have previously been noted,35,36 whereas genistein has been shown to differentially inhibit fMLP-induced respiratory burst activity.37
ACKNOWLEDGMENT
We thank Dr Jim Watson for providing GM-CSF, Dr Gwyn Gould for providing the antisera against human Glut-3, and Maya Kansara for initial analysis of glucose transporter expression.
Supported by the Cancer Society of New Zealand and its Wellington Division and by the Health Research Council of New Zealand.
Address reprint requests to Michael V. Berridge, PhD, Malaghan Institute of Medical Research, PO Box 7060, Wellington South, New Zealand.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

![Fig. 1. Effect of PMA on superoxide production by neutrophils and on [3H]-2–DOG uptake. (A) SOD-inhibitable cytochrome c reduction. Neutrophils (106) were treated with 100 ng/mL PMA for increasing times in the presence and absence of SOD. Control absorbance (A550 + SOD) was 0.364 ± 0.009. (B) [3H]-2–DOG uptake determined over 3-minute intervals at the times indicated after exposure to PMA. Each value represents the mean of duplicate determinations obtained from two separate experiments. [3H]-2–DOG uptake at zero time was 1619 ± 41 cpm.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/2/10.1182_blood.v91.2.649/3/m_blod4020901.jpeg?Expires=1769140022&Signature=5BVX7yjEP2qoW-ON4D5vutxjlT4GRhu4jJxP9EDR4yYq6XXO6MXZXvoEwcyydNpyOw8cCZxJQH3lHX66yK9ElynbUAAvgJFWEZARdyXv~praqNWE7fZrad8sQS6uqykEKi0jqnHfmL5-t7vyuzCR6IQUMI84OtPXPcGP1gNkC-P8IZKo4DChH1t11Ozrh9YkJLna8wI0vrvXwGdoSmIoQpr6VaHtVhEcVTQhNrj8h0vWMeA9jgk3ckBXDSev7lM--vGGF0H-A3BO8GYEXLAe0HMXb-z-ZA-6xsh8ALV8yBpLkJzTFOr0A9RldB1C2eqnry-ZtNJT6mpa5dI-2Yp0pQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. Effect of fMLP on superoxide production and [3H]-2–DOG uptake by human neutrophils. Neutrophils (106) were incubated with 0.5 μmol/L fMLP for the times indicated (B and D) or for 5 minutes at the concentrations indicated (A and C) and SOD-inhibitable cytochrome c reduction (A and B) and [3H]-2–DOG uptake (C and D) determined. The results are the average of duplicate determinations. For (A) and (B), control absorbances (A550) in the presence of SOD were 0.342 ± 0.003 and 0.305 ± 0.003, respectively, and for (C) and (D), control uptake was 2,613 ± 32 cpm and 2,068 ± 94 cpm, respectively.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/2/10.1182_blood.v91.2.649/3/m_blod4020903.jpeg?Expires=1769140022&Signature=38f-U4pnLIvHvPcXJuMNAHeafu01mB8bJsbhOp6H9PGjx~JkMP15VgnMr-lWAa~fLgsLZU09MbCeAd~GW6LCVdGgexyLDxwlKDQgQE6oWz3rSjZFfw5gHosBQEFx8WCmyrRexptIpQVwYgE8JA9hzo5Lg1QVOLyNb8ID5LXdZdL~sMbvtb8CEAzkujli-qNwmO0t93SwvFRA3e5VJloQapZFaC0pqjtVdXrX3isFD2doNOAs-xBM0Sb5hepx~y0V~CRgtIDdhVbZ3xYlsZ2Goyq9VGQYVPYcRDF7vKWA7e-tAmsIcAIEKq2yNG06Tf2pRx11NgQK7cOm7gDZ3ikukQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. Effects of genistein and calphostin C on [3H]-2–DOG uptake by unstimulated and stimulated human neutrophils. Neutrophils (106) were pretreated without (solid bars) or with 100 μmol/L genistein (patterned bars) or 1 μmol/l calphostin C (shaded bars) for 20 minutes before stimulation with 100 ng/mL PMA for 20 minutes or 0.5 μmol/L fMLP for 5 minutes and [3H]-2–DOG uptake determined over 3 minutes in the absence of inhibitor. Results are the average of duplicate determinations.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/2/10.1182_blood.v91.2.649/3/m_blod4020904.jpeg?Expires=1769140022&Signature=W3L-WKWx6yFfHvpyjRvvE5R6s~H2Ay~wPbCqKym45y2n8caDw5wCs0sD~sk~J-x0lqPVB0NkhreICvmnyJU8thQjI43LGiz92zdyN8uMQNIiNnfRYQyoAd5WpUcrVtg3F162MoJD30rKyEIKkn1SBDQj-4qUkcUrvz7q2eFqy5kcUvkD-c3ThOxtr4TUMyz9o5Jex8J~pH5ulyS5Zlfu6w~sthXK7kyFtFr-YHoSt~8noceEFTDHgYyF0D-Lrubx8g-1DS11CKJjumC69UQtvufAWP4R3VCefDvlTXGsupMJXisfyAJdaTxSqWSyjxTL7JgBxXiXh7GztbcUEv4~3g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal