Abstract
Factor VIII (FVIII) is the protein defective in the bleeding disorder hemophilia A. Approximately 5% of hemophilia A patients have normal amounts of a dysfunctional FVIII protein and are termed cross-reacting material (CRM)-positive. The majority of genetic alterations that result in CRM-positive hemophilia A are missense mutations within the A2-domain. To determine the mechanistic basis of the genetic defects within the A2-domain for FVIII function we constructed six mutations within the FVIII cDNA that were previously found in five CRM-positive hemophilia A patients (R527W, S558F, I566T, V634A, and V634M) and one CRM-reduced hemophilia A patient (DeltaF652/3). The specific activity for each mutant secreted into the conditioned medium from transiently transfected COS-1 cells correlated with published data for the patients plasma-derived FVIII, confirming the basis of the genetic defect. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis of immunoprecipitated FVIII protein radiolabeled in COS-1 cells showed that all CRM-positive mutant proteins were synthesized and secreted into the medium at rates similar to wild-type FVIII. The majority of the DeltaF652/3 mutant was defective in secretion and was degraded within the cell. All mutant FVIII proteins were susceptible to thrombin cleavage, and the A2-domain fragment from the I566T mutant had a reduced mobility because of use of an introduced potential N-linked glycosylation site that was confirmed by N-glycanase digestion. To evaluate interaction of FVIII with factor IXa, we performed an inhibition assay using a synthetic peptide corresponding to FVIII residues 558 to 565, previously shown to be a factor IXa interaction site. The concentration of peptide required for 50% inhibition of FVIII activity (IC50) was reduced for the I566T (800 μmol/L) and the S558F (960 μmol/L) mutants compared with wild-type FVIII (>2,000 μmol/L). N-glycanase digestion increased I566T mutant FVIII activity and increased its IC50 for the peptide (1,400 μmol/L). In comparison to S558F, a more conservative mutant (S558A) had a sixfold increased specific activity that also correlated with an increased IC50 for the peptide. These results provided support that the defects in the I566T and S558F FVIII molecules are caused by steric hindrance for interaction with factor IXa.
HEMOPHILIA A is caused by a quantitative or qualitative deficiency of plasma factor VIII (FVIII), a cofactor for factor IXa in the proteolytic activation of factor X to factor Xa.1,2 FVIII is synthesized as a single chain polypeptide of 2,351 amino acids from which a 19–amino acid signal peptide is cleaved, and has a domain organization of A1-A2-B-A3-C1-C2.3,4 The A-domains are homologous to the A-domains of ceruloplasmin,5 a copper binding plasma protein, suggesting a possible role in metal-ion binding. The C-domains are homologous to phospholipid-binding proteins, suggesting a role in phospholipid interaction.6 The B-domain displays no significant homology to any known protein. FVIII is proteolytically processed on secretion from the cell to form heterodimers composed of a series of amino-terminal heavy chain fragments ranging in size from 90 to 220 kD and a carboxy-terminal light chain fragment of 80 kD. Thrombin activates FVIII by cleaving the 90 kD heavy chain (A1-A2) into 50-kD (A1) and 43-kD (A2) fragments, and the 80-kD light chain to a 73-kD (A3-C1-C2) fragment.7 The active form of FVIII is a metal-ion linked heterotrimer of A1, A2, and A3-C1-C2.8 9
Approximately 5% of hemophilia A patients have normal levels of dysfunctional FVIII protein and are termed cross-reacting material (CRM)-positive. CRM-positive patients have considerable amounts of FVIII protein in their plasma (at least 30% of the normal amount), but the protein is nonfunctional; ie, the FVIII activity is much less than the FVIII plasma protein level. In contrast, FVIII antigen is not detected in CRM-negative patients. Patients with CRM-reduced hemophilia A have reduced plasma FVIII antigen levels regardless of activity levels. Some CRM-reduced patients also display lower activity values compared with the plasma antigen levels.10,11 Approximately 40% of the CRM-positive and CRM-reduced hemophilia A patients contain missense mutations within the A2-domain.12 The A2-domain consists of approximately 330 amino acids or approximately 15% of the entire amino acid sequence of FVIII, indicating that a selective clustering of missense mutations occur within this region that result in hemophilia A. Several reports have supported that the A2-domain subunit is required for FVIII procoagulant activity. The thrombin-activated heterotrimer exhibited a pH-dependent dissociation of the A2 subunit from the complex that correlated with a loss in procoagulant activity.13 The difference in stability between porcine and human FVIIIa correlates with increased affinity of the porcine A2-domain compared with the human A2-domain for the A1/A3-C1-C2 heterodimer.8,14 Furthermore, in-frame deletion of the A2-domain in recombinant FVIII yielded a secreted protein that did not exhibit procoagulant activity. However, either cotransfection of this A2-domain deletion mutant with an A2-domain expression vector or addition of purified A2-domain fragment itself restored procoagulant activity for the A2-domain deletion molecule.15 Although the A2-domain is essential for procoagulant activity, it is not understood how the A2 subunit contributes to tenase complex activity. Recent work using synthetic peptides showed that the amino acid region 558 to 565 within the FVIII A2 subunit represents a factor IXa interaction site.16 17
Results from site-directed mutagenesis studies of human recombinant FVIII have contributed to our understanding of its structure-function relationships. For example, missense mutations introduced at the thrombin cleavage sites showed that cleavages at R372 and R1689 are required for full functional activity18 consistent with the results from analysis of naturally occurring mutations that result in hemophilia A.19-24 To date there is only one report describing the characterization of a missense mutation within the A2-domain that resulted in a defective protein. In that situation, plasma FVIII from a patient that contained the missense mutation of isoleucine to threonine at residue 566, creating a novel N-linked glycosylation site at asparagine residue 564, was defective in procoagulant activity.25 In this report, we have studied the mechanistic basis for missense mutations within A2-domain affecting molecular interactions required for FVIII function. Site-directed mutagenesis was used to create six different mutations within the A2-domain that were previously shown to correlate with defective circulating FVIII protein in patients with severe to mild hemophilia A. Functional characterization of these mutant proteins, expressed in transiently transfected COS-1 cells, showed that I566T and S558F FVIII missense mutations have reduced specific activity that can be attributed to reduced interaction with factor IXa.
MATERIALS AND METHODS
Materials.
FVIII-deficient plasma and normal pooled human plasma were obtained from George King Biomedical Inc (Overland Park, KS). Monoclonal antibody to the heavy chain of FVIII (F8) coupled to CL4B-sepharose was a gift from Debra Pittman (Genetics Institute Inc, Cambridge, MA). ESH-4 and ESH-8 antibodies were purchased from American Diagnostica Inc (Greenwich, CT). Activated partial thromboplastin (Automated APTT reagent) was purchased from General Diagnostics Organon Teknika Corporation (Durham, NC). COAMATIC chromogenic FVIII activity assay kit was purchased from Pharmacia Hepar (Franklin, OH). Soybean trypsin inhibitor, phenylmethylsulfonylfluoride (PMSF) and aprotinin were purchased from Boehringer, Mannheim GmbH (Mannheim, Germany). Human α-thrombin and O-Phenylenediamine Dihydrochloride (OPD) and N-α-Leu-Leu-norleucinal (ALLN) were purchased from Sigma Chemical Co (St Louis, MO). [35S]-methionine (>1,000 Ci/mmol) was purchased from Amersham Life Science Inc (Arlington Heights, IL). Dulbecco's Modified Eagle's Medium (DMEM), methionine-free DMEM, Biotin N-Hydroxy Succinimide Ester, and Streptavidin-Horseradish Peroxidase Conjugate were obtained from GIBCO BRL (Gaithersburg, MD). Fetal bovine serum was purchased from PAA Laboratories Inc (Newport Beach, CA). Recombinant N-glycanase was purchased from Genzyme DIAGNOSTICS (Cambridge, MA). Patient's plasma containing FVIII variant I566T (designated as ARC-22 or JH-11725 by the Holland Laboratory, American Red Cross Blood Services, or the Center for Medical Genetics, Johns Hopkins University School of Medicine, respectively) was generously supplied by Carol Kasper (Orthopedic Hospital, Los Angeles, CA) for research purposes.
Plasmid construction.
Mutagenesis was performed within the mammalian expression vector pMT226 containing the wild-type (WT) full length FVIII cDNA. Mutant plasmids were generated through oligonucleotide site-directed mutagenesis using the gapped-heteroduplex procedure or the polymerase chain reaction as described previously.27-29Codon 527 was mutated from CGG to TGG predicting an amino acid change from arginine to tryptophan, and the resultant mutant plasmid was designated R527W. Codon 558 was mutated from TCT to either TTT or GCA predicting an amino acid change from serine to either phenylalanine or alanine, respectively, and the resultant mutant plasmids were designated S558F and S558A. Codon 566 was mutated from ATA to ACA predicting an amino acid change from isoleucine to threonine, and the resultant mutant plasmid was designated I566T. Codon 634 was mutated from GTG to either GCG or ATG predicting an amino acid change from valine to either alanine or methionine, respectively, and the resultant mutant plasmids were designated V634A and V634M. Either codon 652 or 653, which are both TTC coding phenylalanine, was deleted and the resultant 3-bp deletion mutant plasmid was designated DeltaF652/3. The mutations were confirmed by DNA sequence analysis using the dideoxy nucleotide sequencing method.30
DNA transfection and analysis.
Plasmid DNA was transfected into COS-1 cells by the diethyl aminoethyl-dextran procedure as described.27 After 40 hours the cells were fed fresh medium containing 10% heat-inactivated fetal bovine serum and samples of conditioned medium (CM) were harvested at 60 hours posttransfection for FVIII assay. Subsequently, protein synthesis and secretion were analyzed by metabolically labeling cells for 20 minutes with [35S]-methionine, followed by a chase for 4 hours in medium containing a 100-fold excess of unlabeled methionine and 0.02% aprotinin.27 Cell extracts (CE) were prepared by lysis in Nonidet P-40 lysis buffer.27 For analysis of the effect of cysteine protease inhibition, increasing amounts of ALLN were included in the chase medium. CE and CM containing labeled proteins were harvested as described previously31and immunoprecipitated with F8 antibody coupled to CL-4B sepharose. Immunoprecipitated proteins were washed with phosphate buffered saline (PBS) containing Triton X-100 and resuspended in 50 mmol/L Tris-HCl pH 7.5, 150 mmol/L NaCl, 2.5 mmol/L CaCl2, and 5% glycerol (buffer A). Immunoprecipitated proteins from conditioned medium were resuspended in buffer A and treated with or without 2 U/mL of thrombin at 37°C for 30 minutes. Samples were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions and visualized by fluorography after treatment with En3Hance (Dupont, Boston, MA).
FVIII assay.
FVIII activities were measured in a one stage clotting assay using FVIII-deficient plasma as substrate or by the COAMATIC chromogenic assay according to the manufacturer. One unit of FVIII activity is that amount measured in 1 mL of normal human pooled plasma. FVIII antigen was quantitated by a sandwich enzyme-linked immunosorbent assay (ELISA)32 using two monoclonal antibodies to the FVIII light chain. Purified recombinant FVIII protein was used as a standard.
Protein purification.
Partially purified protein was obtained from 200 mL of conditioned medium from COS-1 cells transfected with FVIII WT, S558F, S558A, I566T, and V634A by immunoaffinity chromatography as described previously.31 The bound FVIII was eluted in buffer containing 60% ethylene glycol and concentrated by dialysis against a 10% polyethylene glycol (MW 15K-20K) containing buffer33and stored at −70°C.
N-glycanase digestion of WT and I566T mutant FVIII.
Immunoprecipitated FVIII WT and I566T mutant from radiolabeled CM was resuspended with buffer A and divided into three aliquots for incubation in the absence or presence of thrombin or thrombin and subsequently N-Glycanase. Human thrombin was added to a final concentration of 5 U/mL. Recombinant N-Glycanase was added to a final concentration of 10 U/mL in the presence of 4% Nonidet P-40 and 20 mmol/L PMSF. The resulting polypeptides were separated by SDS-PAGE and visualized by autoradiography as described previously.
FVIII activity of samples from patient's plasma and conditioned medium were measured after N-Glycanase digestion. Patient's plasma was incubated with recombinant N-Glycanase (final concentration 10 U/mL) at 37°C. FVIII activity after increasing periods of time was measured by the one stage clotting assay. Normal pooled plasma was used under the same conditions as a control for this assay. For the evaluation of recombinant FVIII protein, CM was mixed with an equal volume of FVIII-deficient plasma to approximate physiological conditions. This sample was treated with recombinant N-Glycanase and FVIII activity was measured as described previously. WT FVIII conditioned medium was also mixed with FVIII-deficient plasma as a control in this assay.
Peptide inhibition of intrinsic factor Xase activity.
The synthetic peptide SVDQRGNQ, which corresponds to FVIII residues 558 to 565, and a scrambled version of this peptide, NGSQDQRV, were synthesized by the University of Michigan Protein and Carbohydrate Structure Facility (Ann Arbor, MI). Both peptides were greater than 90% pure as determined by high-liquid-performance-chromatography analysis and their identity was confirmed by mass spectrometry. The effect of synthetic peptides on intrinsic factor Xase activity was evaluated by the Factor Xa generation assay using COAMATIC chromogenic factor VIII activity assay kit. Partially purified FVIII samples were mixed with various concentrations of synthetic peptide, then factor reagent containing factor IXa, factor X, thrombin, CaCl2, and phospholipid was added. After 2 minutes incubation at 37°C, S-2765 chromogenic substrate was reacted with thrombin inhibitor I-2581, which prevents hydrolysis of S-2765 by thrombin. Factor Xa activity was determined as initial rate values by measuring optical density at 405 nm. Exponential best fit curves were determined from the independent experiments presented.
Molecular modeling.
The effects of missense mutations within a structural model for the A domains of FVIII34 were characterized by altering residue side chains using the Biopolymer module of InsightII (Molecular Simulations Inc, Cambridge, UK) and the variant molecules were reminimized using Discover (Molecular Simulations Inc).
RESULTS
CRM-Positive mutants are synthesized and secreted similarly to WT FVIII.
The profile of the mutations analyzed in this study is summarized in Table 1.12,25 35 We constructed six mutations within the A2-domain that correlate with mutations observed in five CRM-positive and one CRM-reduced hemophilia A. FVIII WT and the A2-domain mutants were compared by transient DNA transfection of the cDNA expression vectors into COS-1 monkey cells. At 60 hours after transfection, the rates of synthesis were analyzed by immunoprecipitation of CE from [35S]-methionine pulse-labeled cells. Intracellular FVIII WT was detected in its single chain form and migrated at approximately 280 kD (Fig 1, lane 1). The primary translation products for each of the A2-domain mutants were detected at 280 kD at similar levels to WT (Fig 1, lanes 2 to 7). Thus, there was no significant effect of these mutations on the rate of FVIII protein translation. Analysis of the CE after a 4-hour chase indicated that FVIII WT and all mutants disappeared from the CE at approximately similar rates (Fig 1, lanes 10 to 16).
Comparison of Phenotype of Hemophilia A Patients and Expression of Their Respective Recombinant Proteins
| Mutation . | Exon . | Classification . | Nucleotide Change . | Phenotype . | Patients' Plasma (% of normal) . | COS CM (% of WT) . | Reference . | ||
|---|---|---|---|---|---|---|---|---|---|
| Activity . | Antigen . | Activity . | Antigen . | ||||||
| R527W | 11 | CRM-pos | CGG-TGG | mild | 9.5-38 | 43-245 | 37 ± 5 | 51 ± 12 | 12, 35 |
| S558F | 11 | CRM-pos | TCT-TTT | mild | 21 | 175 | 6 ± 1 | 57 ± 9 | 12 |
| I566T | 12 | CRM-pos | ATA-ACA | severe-moderate | <1-4 | 154-200 | 4 ± 3 | 72 ± 19 | 25, 35 |
| V634A | 13 | CRM-pos | GTG-GCG | mild | 5 | 138 | 4 ± 2 | 71 ± 11 | 12 |
| V634M | 13 | CRM-pos | GTG-ATG | severe | <1 | 175 | 3 ± 3 | 104 ± 14 | 12 |
| DeltaF652/3 | 13 | CRM-red | deletion of TTC | severe | 1 | 12 | 1 ± 2 | 42 ± 3 | 12 |
| S558A | TCT-GCA | 45 ± 5 | 79 ± 13 | ||||||
| Mutation . | Exon . | Classification . | Nucleotide Change . | Phenotype . | Patients' Plasma (% of normal) . | COS CM (% of WT) . | Reference . | ||
|---|---|---|---|---|---|---|---|---|---|
| Activity . | Antigen . | Activity . | Antigen . | ||||||
| R527W | 11 | CRM-pos | CGG-TGG | mild | 9.5-38 | 43-245 | 37 ± 5 | 51 ± 12 | 12, 35 |
| S558F | 11 | CRM-pos | TCT-TTT | mild | 21 | 175 | 6 ± 1 | 57 ± 9 | 12 |
| I566T | 12 | CRM-pos | ATA-ACA | severe-moderate | <1-4 | 154-200 | 4 ± 3 | 72 ± 19 | 25, 35 |
| V634A | 13 | CRM-pos | GTG-GCG | mild | 5 | 138 | 4 ± 2 | 71 ± 11 | 12 |
| V634M | 13 | CRM-pos | GTG-ATG | severe | <1 | 175 | 3 ± 3 | 104 ± 14 | 12 |
| DeltaF652/3 | 13 | CRM-red | deletion of TTC | severe | 1 | 12 | 1 ± 2 | 42 ± 3 | 12 |
| S558A | TCT-GCA | 45 ± 5 | 79 ± 13 | ||||||
The activity and antigen in COS CM represent mean ± SD of the three independent transfections.
Synthesis of FVIII WT and mutants in COS-1 cells. WT and mutant expression plasmids were transfected into COS-1 monkey cells. At 60 hours posttransfection, cells were pulse-labeled with [35S]-methionine for 20 minutes and cell extracts (CE) were harvested. Duplicate plates were chased for 4 hours in medium containing excess unlabeled methionine and then CE were harvested. Equal volumes of CE were immunoprecipitated with anti-FVIII antibody and equal aliquots were analyzed by SDS-PAGE. Mock indicates cells that did not receive plasmid DNA. The migration of FVIII in the CE is indicated at the right as FVIII. Molecular weight size markers are shown on the left.
Synthesis of FVIII WT and mutants in COS-1 cells. WT and mutant expression plasmids were transfected into COS-1 monkey cells. At 60 hours posttransfection, cells were pulse-labeled with [35S]-methionine for 20 minutes and cell extracts (CE) were harvested. Duplicate plates were chased for 4 hours in medium containing excess unlabeled methionine and then CE were harvested. Equal volumes of CE were immunoprecipitated with anti-FVIII antibody and equal aliquots were analyzed by SDS-PAGE. Mock indicates cells that did not receive plasmid DNA. The migration of FVIII in the CE is indicated at the right as FVIII. Molecular weight size markers are shown on the left.
The secretion into the CM was analyzed by immunoprecipitation of CM from [35S]-methionine pulse-labeled transfected cells chased for 4 hours in medium containing excess unlabeled methionine. On SDS-PAGE analysis, FVIII WT was detected as a 300-kD single chain, a 200-kD heavy chain, and an 80-kD light chain (Fig 2, lane 1). The amount of secreted polypeptides from DeltaF652/3 mutant transfected cells was greatly reduced in the CM (Fig 2, lane 11), whereas the five CRM-positive mutants were secreted at levels similar to FVIII WT (Fig 2, lanes 3, 5, 7, 9, and 13).
Secretion and thrombin cleavage of WT and mutant FVIII. The secretion of each mutant was analyzed by immunoprecipitation of conditioned medium from [35S]-methionine pulse-labeled transfected cells chased for 4 hours in medium containing excess unlabeled methionine. Immunoprecipitated FVIII molecules were analyzed by SDS-PAGE before (−) and after (+) thrombin (IIa) digestion. Mock indicates cells that did not receive plasmid DNA. Molecular weight size markers are shown on the left. Single chain (Single), heavy chain (Heavy), and light chain (Light) are indicated for undigested samples. A3-C1-C2, A1, and A2 fragments are indicated for digested samples. The symbol * represents the A2 fragment with reduced mobility.
Secretion and thrombin cleavage of WT and mutant FVIII. The secretion of each mutant was analyzed by immunoprecipitation of conditioned medium from [35S]-methionine pulse-labeled transfected cells chased for 4 hours in medium containing excess unlabeled methionine. Immunoprecipitated FVIII molecules were analyzed by SDS-PAGE before (−) and after (+) thrombin (IIa) digestion. Mock indicates cells that did not receive plasmid DNA. Molecular weight size markers are shown on the left. Single chain (Single), heavy chain (Heavy), and light chain (Light) are indicated for undigested samples. A3-C1-C2, A1, and A2 fragments are indicated for digested samples. The symbol * represents the A2 fragment with reduced mobility.
The thrombin cleavage fragments for all mutants, except I566T, were indistinguishable from WT FVIII.
Immunoprecipitated FVIII molecules were treated with thrombin before analysis by SDS-PAGE. Thrombin cleavage of all mutants, except I566T, generated the light chain migrating at 73 kD and the heavy chain derived fragments corresponding to the 50-kD A1-domain and 43-kD A2-domain (Fig 2, lanes 4, 8, 10, 12, and 14) that were indistinguishable from the FVIII WT (Fig 2, lane 2). However, the A2-domain fragment from the I566T mutant showed reduced mobility at 46 kD compared with FVIII WT (Fig 2, lane 6).
Specific activity for each mutant correlated with published data for the patients' plasma-derived FVIII.
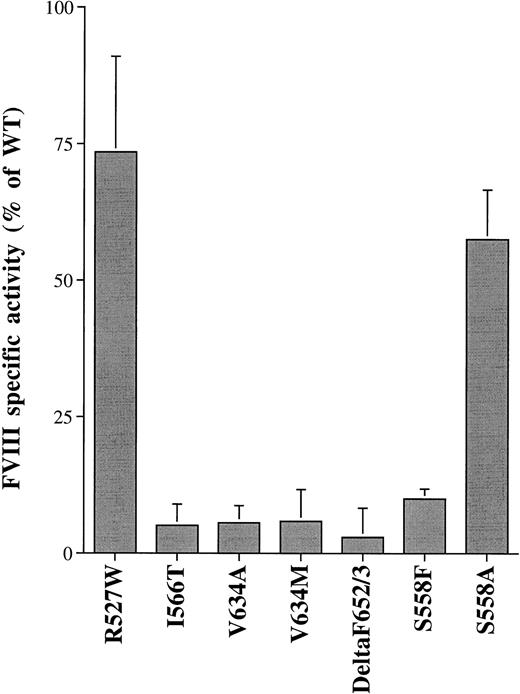
CM from cells transfected with FVIII WT or the A2-domain mutants were harvested at 60 hours posttransfection for FVIII assay. The activity and antigen in the CM of FVIII WT showed 195 mU/mL and 37 ng/mL (average of 3 different transfections), respectively. The specific activity for the WT FVIII was 5,270 U/mg, consistent with the value obtained for plasma-derived FVIII. The activities determined by the COAMATIC assay and antigen levels (represented as percent of FVIII WT) in the CM from cells transfected with the A2-domain mutants are compared with the patients' phenotype (represented as percent of normal) in Table 1. Compared with FVIII WT, the activity values obtained by the COAMATIC assay were similar to those obtained by clotting assay (data not shown). The S558F mutant CM showed a lower amount of activity than the patient's plasma-derived FVIII and the V634M mutant CM showed a slightly higher amount of activity than the patient. The other A2-domain mutants exhibited activities similar to the patients' values. Compared with antigen levels of FVIII WT in the CM or FVIII in normal plasma, the levels of FVIII antigen in the CM from most of the FVIII mutant transfected cells were lower than those obtained in the patients' plasma. Quantitation of the specific activity showed that all A2-domain mutants, except for R527W, had values less than 10% of FVIII WT (Fig 3).
The specific activity for WT and mutant FVIII. CM of WT and mutant FVIII were harvested at 60 hours posttransfection for FVIII assay. The activity and antigen in the CM were measured by COAMATIC chromogenic assay and ELISA using an anti-FVIII light chain antibody, respectively. Specific activity is expressed as percent of WT. Bars are expressed as a mean ± standard deviation (SD) of three independent transfection experiments.
The specific activity for WT and mutant FVIII. CM of WT and mutant FVIII were harvested at 60 hours posttransfection for FVIII assay. The activity and antigen in the CM were measured by COAMATIC chromogenic assay and ELISA using an anti-FVIII light chain antibody, respectively. Specific activity is expressed as percent of WT. Bars are expressed as a mean ± standard deviation (SD) of three independent transfection experiments.
Mutant S558A has increased specific activity over S558F.
As mentioned previously, the amino acid region 558 to 565 within the A2 subunit is proposed to be a factor IXa interaction site.16Because of the significant difference in the amino acid structure between serine and phenylalanine, the S558F mutant may have a reduced interaction with factor IXa. To test this possibility, a more conservative mutation of serine to alanine (S558A) was made. Analysis of pulse-chase [35S]-methionine labeled CE and CM before and after thrombin cleavage by SDS-PAGE showed that the S558A mutant was synthesized, secreted, and cleaved by thrombin similar to FVIII WT and S558F (Fig 1, lanes 8 and 17; Fig 2, lanes 15 and 16). However, its specific activity was increased sixfold over the S558F mutant (Table 1and Fig 3). The results suggest that eliminating the large side-chain of phenylalanine increased procoagulant activity.
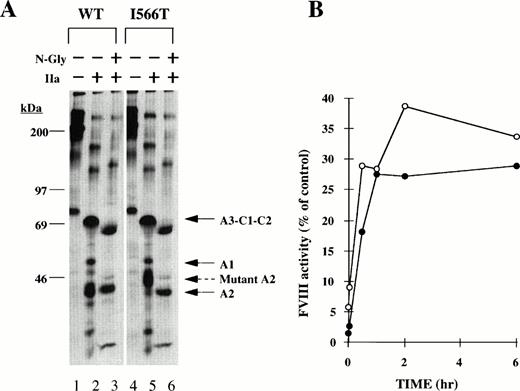
I566T creates a new N-linked glycosylation site that is used.
Substitution of isoleucine at residue 566 for threonine creates a potential new N-linked glycosylation site at asparagine 564. Analysis of plasma-derived FVIII from a patient harboring the I566T mutation suggested that this glycosylation site is used resulting in reduced FVIII activity.25 The thrombin-derived A2-domain fragment of the recombinant-derived I566T mutant had a reduced mobility (Fig 2, lane 6 and Fig 4A, lanes 2 and 5) consistent with addition of oligosaccharides to asparagine 564. To show that the reduced mobility results from additional glycosylation, immunoprecipitated WT and I566T mutant FVIII from radiolabeled CM were treated with thrombin and N-glycanase before SDS-PAGE analysis. After N-glycanase digestion the A2 fragment from I566T comigrated with the WT A2 fragment (Fig 4A, lanes 3 and 6). This result is consistent with the previous data characterizing FVIII in patient's plasma.25 To test whether N-glycanase digestion would increase FVIII activity for the I566T mutant, we analyzed the FVIII clotting activity after increasing periods of incubation with N-glycanase. N-glycanase digestion increased the activity for the I566T recombinant protein in CM to 30%, and this increase was similar to that observed for the I566T patient's plasma-derived FVIII (Fig 4B).
Effect of N-glycanase for I566T mutant FVIII. (A) Immunoprecipitated WT and I566T mutant from radiolabeled CM were treated with nothing (IIa−, N-Gly−) or thrombin (IIa+, N-Gly−) or N-glycanase after thrombin (IIa+, N-Gly+) before SDS-PAGE as described in the Materials and Methods. Molecular weight size markers are shown on the left. A3-C1-C2, A1, A2, and mutant A2 fragments are indicated at the right. (B) FVIII in conditioned medium as well as in patient's plasma were incubated with 10 U/mL of N-glycanase at 37°C. FVIII activity after increasing times was determined by one stage clotting assay. Data are plotted as the percent activity of the mutant compared with the WT-recombinant (○) or plasma-derived (•) FVIII, respectively. Data represent the average of two independent experiments.
Effect of N-glycanase for I566T mutant FVIII. (A) Immunoprecipitated WT and I566T mutant from radiolabeled CM were treated with nothing (IIa−, N-Gly−) or thrombin (IIa+, N-Gly−) or N-glycanase after thrombin (IIa+, N-Gly+) before SDS-PAGE as described in the Materials and Methods. Molecular weight size markers are shown on the left. A3-C1-C2, A1, A2, and mutant A2 fragments are indicated at the right. (B) FVIII in conditioned medium as well as in patient's plasma were incubated with 10 U/mL of N-glycanase at 37°C. FVIII activity after increasing times was determined by one stage clotting assay. Data are plotted as the percent activity of the mutant compared with the WT-recombinant (○) or plasma-derived (•) FVIII, respectively. Data represent the average of two independent experiments.
I566T and S558F FVIII are more sensitive to inhibition by a synthetic peptide corresponding to a factor IXa interaction site.
The results above show that FVIII activity increased after removal of either the large side-chain of phenylalanine for the S558F mutant or of the carbohydrate at N564 for the I566T mutant. We propose that these moieties may sterically prevent FVIII interaction with factor IXa. Previously, a synthetic peptide corresponding to FVIII residues 558 to 565 prevented Xa generation by factor IXa, presumably because of preventing factor IXa interaction.16 To measure the factor IXa interaction with the mutants, we tested the ability of the synthetic peptide 558 to 565 to inhibit FVIII activity.
Equal amounts (4 nmol/L) of partially purified FVIII samples were mixed with increasing concentrations of synthetic peptide and assayed for FVIII activity. An exponential best fit curve was determined from three independent experiments to extrapolate the concentration of peptide required for 50% inhibition of activity (IC50) for WT FVIII and for V634A mutant FVIII to be 3,340 (±80) μmol/L and 2,240 (±330) μmol/L, respectively (Fig5A). In contrast, the IC50 for the I566T and S558F mutant FVIII molecules were measured to be 800 (±130) μmol/L and 960 (±60) μmol/L, respectively. These results support that the I566T and S558F mutants are more sensitive than WT to inhibition by the peptide. The inhibition curve for S558A was shifted right and the IC50 for S558A increased to 1,690 (±300) μmol/L (Fig 5A). N-glycanase digestion did not change the inhibition profile of WT FVIII, but increased the IC50 for I566T to 1,400 (±170) μmol/L (Fig 5B). Thus I566T and S558F were inhibited at lower concentrations of peptide than WT FVIII.
Inhibition of intrinsic factor Xase activity by synthetic peptide 558 to 565. (A) Equal amounts (4 nmol/L) of partially purified FVIII samples were mixed with increasing concentrations of synthetic peptide and assayed for FVIII activity as described in the Materials and Methods. The data represent the average of three independent experiments. FVIII activity for each molecule is expressed as a percent of that obtained in the absence of the peptide. The symbols represent WT FVIII (•), S558F (▴), S558A (▵), I566T (▪) and V634A (⧫). (B) WT and I566T mutant FVIII were treated with N-glycanase for 3 hours at 37°C, then samples were tested in the peptide inhibition assay. The results represent the average of three (filled symbols) and two (open symbols) independent experiments. The symbols represent WT FVIII (•), WT FVIII with N-glycanase (○), I566T (▪) and I566T with N-glycanase (□). (C) Equal amounts (4 nmol/L) of partially purified FVIII samples were mixed with increasing concentrations of scrambled synthetic peptide and assayed for FVIII activity as described in the Materials and Methods. Two independent experiments are shown for WT FVIII (•), S558F (▴), I566T (▪) and V634A (⧫). (D) Four different concentrations of WT FVIII (4 nmol/L [•], 0.8 nmol/L [▪], 0.4 nmol/L [▴], 0.08 nmol/L [⧫]) were tested in the peptide inhibition assay. The results are the average of two independent experiments.
Inhibition of intrinsic factor Xase activity by synthetic peptide 558 to 565. (A) Equal amounts (4 nmol/L) of partially purified FVIII samples were mixed with increasing concentrations of synthetic peptide and assayed for FVIII activity as described in the Materials and Methods. The data represent the average of three independent experiments. FVIII activity for each molecule is expressed as a percent of that obtained in the absence of the peptide. The symbols represent WT FVIII (•), S558F (▴), S558A (▵), I566T (▪) and V634A (⧫). (B) WT and I566T mutant FVIII were treated with N-glycanase for 3 hours at 37°C, then samples were tested in the peptide inhibition assay. The results represent the average of three (filled symbols) and two (open symbols) independent experiments. The symbols represent WT FVIII (•), WT FVIII with N-glycanase (○), I566T (▪) and I566T with N-glycanase (□). (C) Equal amounts (4 nmol/L) of partially purified FVIII samples were mixed with increasing concentrations of scrambled synthetic peptide and assayed for FVIII activity as described in the Materials and Methods. Two independent experiments are shown for WT FVIII (•), S558F (▴), I566T (▪) and V634A (⧫). (D) Four different concentrations of WT FVIII (4 nmol/L [•], 0.8 nmol/L [▪], 0.4 nmol/L [▴], 0.08 nmol/L [⧫]) were tested in the peptide inhibition assay. The results are the average of two independent experiments.
To confirm that the effect of this peptide was specific for interaction between factor IXa and FVIII, a scrambled synthetic peptide having the same composition was used in the same assay. The scrambled peptide did not inhibit either WT or any mutant FVIII (Fig 5C). Equal antigenic amounts of each FVIII protein were used in the peptide inhibition assays. Therefore, the initial activity for the WT and each mutant was different because of differences in specific activity. Therefore, we tested if the amount of initial activity affected the peptide inhibition. The effects on increasing peptide 558 to 565 on four different concentrations of WT FVIII were measured. The inhibition profiles for all samples were indistinguishable (Fig 5D), suggesting that the results did not depend on initial activity of sample.
Intracellular accumulation of DeltaF652/3 mutant in the presence of inhibitors of intracellular degradation.
Although the DeltaF652/3 mutant was synthesized and chased from the CE similar to WT FVIII (Fig 1), it displayed significantly reduced appearance in the conditioned medium (Fig 2). Thus, this protein was either degraded within the cell or was secreted and rapidly degraded in the conditioned medium. The cysteine-protease inhibitor, ALLN, inhibits intracellular degradation and previous studies showed increased intracellular accumulation of a mutant FVIII (R2307Q) that was inefficiently secreted.29 These results support that this mutant did not accumulate in the CM because of a block in secretion with subsequent degradation within the secretory pathway and not as a consequence of instability in the CM. The effect of ALLN on the secretion of DeltaF652/3 mutant was studied by analyzing [35S]-methionine labeled FVIII protein. Addition of ALLN to the CM resulted in a greater accumulation of the DeltaF652/3 in the CE (Fig 6, lanes 6-8) compared with WT (Fig6, lanes 2-4). However, ALLN treatment did not increase the secretion of either WT or DeltaF652/3 into the CM (Fig 6, lanes 13-18). These results suggest that defective secretion with subsequent intracellular degradation is responsible for loss of the mutant FVIII from the CE, leading to the CRM-reduced phenotype.
Inhibition of intracellular degradation causes intracellular accumulation of DeltaF652/3. Parallel plates of transfected COS-1 monkey cells were labeled at 60 hours posttransfection with [35S]methionine for 30 minutes, chased for 4 hours in the absence (lanes 2, 6, 10, 13, 16, and 19) or presence of increasing amounts of ALLN (lanes 3, 4, 7, 8, 11, 12, 14, 15, 17, 18, 20, and 21). CE and conditioned medium (CM) were harvested and equal proportionate volumes of CE and CM were immunoprecipitated with anti-FVIII specific antibody for analysis by SDS-PAGE. Mock indicates cells that did not receive plasmid DNA. Molecular weight size markers are shown on the left of each CE and CM.
Inhibition of intracellular degradation causes intracellular accumulation of DeltaF652/3. Parallel plates of transfected COS-1 monkey cells were labeled at 60 hours posttransfection with [35S]methionine for 30 minutes, chased for 4 hours in the absence (lanes 2, 6, 10, 13, 16, and 19) or presence of increasing amounts of ALLN (lanes 3, 4, 7, 8, 11, 12, 14, 15, 17, 18, 20, and 21). CE and conditioned medium (CM) were harvested and equal proportionate volumes of CE and CM were immunoprecipitated with anti-FVIII specific antibody for analysis by SDS-PAGE. Mock indicates cells that did not receive plasmid DNA. Molecular weight size markers are shown on the left of each CE and CM.
DISCUSSION
The study of missense mutations in the FVIII gene that result in hemophilia A has elucidated the functional requirements for FVIII activity. Previous studies showed that the A2 subunit is essential for FVIIIa activity.8,9 13-15 In this study, we have characterized six different mutants within the A2-domain using site-directed mutagenesis. The goal of these studies was to confirm the genetic basis for the phenotype of hemophilia A and to elucidate the mechanism by which these missense mutations within the A2-domain result in hemophilia A.
We compared the phenotype of recombinant mutant FVIII proteins with their respective mutants analyzed in patients' plasma. SDS-PAGE analysis showed that there was no significant effect of these mutations on the rate of FVIII synthesis and all the CRM-positive mutants were secreted into the CM similar to WT. However, the activity in the CM for all the CRM-positive mutants was reduced and correlated with the reduced activity reported in patients' plasma. Quantitation of the antigen levels in the CM by ELISA showed that most mutants also displayed lower antigen levels in the CM compared with WT and these were lower than the values obtained from respective patients' plasma. We speculate that the synthesis and/or secretion of the FVIII mutants in vivo might be stimulated to compensate for the low plasma antigen levels. Although there was variation in the antigen levels, the specific activity of all mutants, except for the R527W mutant, were very low as predicted by the hemophilia A phenotype. Interestingly, the antigen level for the R527W was low as measured by ELISA, although SDS-PAGE analysis showed similar amounts of FVIII secretion compared with WT. It is likely that the ELISA quantitation for this mutant was reduced as a consequence of poor reactivity with the antibodies used in the ELISA. In this context, it is essential that quantitation of antigen in patients' plasma also consider the particular antibodies used in the assay. The reduced quantity of FVIII antigen measured by ELISA for the R527W mutant resulted in an apparently higher specific activity. If we assume the antigen level was similar to WT as indicated by the characterization of the secretion of the R527W mutant, then the specific activity for the R527W mutant was also significantly reduced. Therefore, the analysis of all the recombinant proteins supports that each mutation is responsible for the respective patients' phenotype, confirming the basis of the genetic defect.
How do the mutations within the A2-domain of FVIII reduce procoagulant activity? SDS-PAGE analysis showed that all mutants were susceptible to thrombin cleavage, eliminating thrombin resistance as a cause for the defect in activity. However, after thrombin cleavage, all mutants studied displayed no significant increase in activity (data not shown), indicating a functional defect in factor VIIIa. Previous studies have identified two regions within FVIII that are likely responsible for factor IXa interaction. First, peptide inhibition studies suggest that residues 558 to 565 comprise a factor IXa interaction site.16 In addition, residues 1,778 to 1,840 within the FVIII light chain were proposed to be involved in factor IXa binding because an antibody directed against this region inhibited the binding of factor IXa.36 This second factor IXa binding site has been more localized to the residues 1,811 to 1,818 by using synthetic peptides.37 Thus, present data support two interaction sites for factor IXa. It has been suggested that the binding site on the FVIII light chain is responsible for complex assembly via the first EGF-like domain of factor IXa,38 whereas the factor IXa interaction with the A2-domain might induce changes within the factor IXa active site.17 In this study we have shown that mutants I566T and S558F are defective in factor IXa interaction by an assay that measured the functional consequence of the FVIII-factor IXa interaction. We confirmed that I566T creates a new N-linked glycosylation site that is used and this prevents appearance of FVIII activity. In addition, removal of the large side-chain of phenylalanine for the S558F mutant, by mutagenesis of S558 to alanine, improved the FVIII activity. The activities of the I566T and S558F mutants were inhibited at lower concentrations of peptide 558 to 565 than WT FVIII or V634A. In our experiments, inhibition of WT FVIII required approximately 2 mmol/L peptide, compared with the value of 0.1 mmol/L previously reported.16 The greater amount of peptide required to inhibit WT FVIII in our assays may be because of the higher concentration of factor IXa (4 nmol/L) present in our assays compared with those of Fay et al16 (1 nmol/L). Either removal of the bulky phenylalanine side chain at residue 558 in mutant S558F by mutation of S558A or removal of N-linked oligosaccharides in the I566T mutant by N-glycanase increased both the specific activity of the FVIII and the IC50 of the peptide. These results support the hypothesis that the defects in the I566T and S558F FVIII molecules are caused by a reduced specific activity resulting from steric hindrance for interaction with factor IXa.
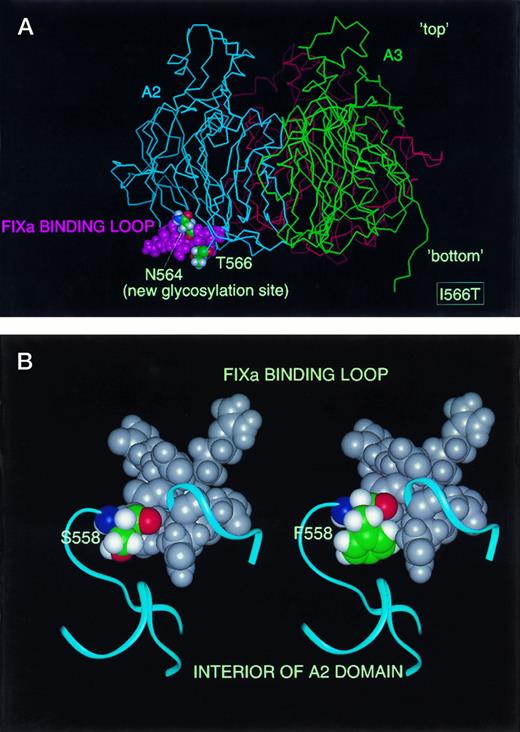
A structural model of the FVIII A domains based on the 3-angstrom structure of ceruloplasmin showed that both sequences 558 to 565 and 1,811 to 1,818 are surface exposed and oriented toward the same direction.34 Structural modeling of I566T indicates that side-chain of the new N-glycosylation site (N564) is surface exposed and lies within the proposed factor IXa binding site (Fig 7A). Modeling of S558F shows that residue 558 is surface exposed and the substitution of serine to phenylalanine introduces a bulky hydrophobic side chain, altering the conformation of the loop in that region and leading to steric strain around the proposed factor IXa binding site (Fig 7B). Thus, addition of a bulky side-chain or N-linked oligosaccharide would be expected to prevent, or severely reduce, the interaction with factor IXa.
Structural model of FVIII mutations I566T and S558F. (A) Homology model of the triplicated A domains of FVIII is shown as viewed perpendicular to threefold axis with “top” of molecule to top of Fig Red, blue, and green represent A1, A2, and A3 subunit, respectively. Binding loop of factor IXa is shown as magenta CPK spheres. N564 (new N-glycosylation site) and T566 are colored by atom (carbon, green; hydrogen, white; oxygen, red; nitrogen, blue). (B) Factor IXa binding loop 558 to 565 shown in CPK spheres: residue 558 colored by atom, 559 to 565 in gray. Blue ribbons represent the alpha-carbon trace of neighboring residues in the A2 domain. Left, WT S558; Right, variant F558 (reminimized).
Structural model of FVIII mutations I566T and S558F. (A) Homology model of the triplicated A domains of FVIII is shown as viewed perpendicular to threefold axis with “top” of molecule to top of Fig Red, blue, and green represent A1, A2, and A3 subunit, respectively. Binding loop of factor IXa is shown as magenta CPK spheres. N564 (new N-glycosylation site) and T566 are colored by atom (carbon, green; hydrogen, white; oxygen, red; nitrogen, blue). (B) Factor IXa binding loop 558 to 565 shown in CPK spheres: residue 558 colored by atom, 559 to 565 in gray. Blue ribbons represent the alpha-carbon trace of neighboring residues in the A2 domain. Left, WT S558; Right, variant F558 (reminimized).
In contrast to the missense mutations S558F and I566T, the mechanism of the molecular defect of the CRM-positive mutants R527W, V634A, and V634M remains unknown. Because these mutants were efficiently secreted as heterodimers, they are likely not significantly defective in protein folding or chain assembly. V634A was inhibited by peptide 558 to 565 similar to WT FVIII, suggesting no defect in its factor IXa interaction. Additional studies are required to elucidate the mechanism responsible for the absence of procoagulant activity for these mutants.
In contrast to the CRM-positive mutants studied, the secretion of the one CRM-reduced mutant DeltaF652/3 was reduced. According to the structural model, residues 652 and 653 are close to the A3-domain and at the interface of the A2 and A3 subunit.39 Therefore, deletion of this residue might prevent formation of the tightly packed structure of FVIII, resulting in aberrant folding and a block to transport through the secretory pathway. Previously, R2307Q mutant FVIII protein showed reduced secretion with increased intracellular degradation, although the low level of secreted protein displayed WT specific activity.29 DeltaF652/3 also displayed defective secretion; however, the low level of secreted mutant protein had, in addition, a low specific activity. Inhibition of intracellular degradation resulted in intracellular accumulation of the DeltaF652/3 mutant; however, it was not secreted, presumably as a result of failure to bypass the “quality control” machinery of the secretory pathway. Thus, even if it were possible to rescue secretion of this mutant, it would not likely improve the severity of hemophilia A caused by the DeltaF652/3 mutation because of its reduced specific activity.
ACKNOWLEDGMENT
We thank Peter Lenting for helpful comments regarding the peptide inhibition experiments, Debra Pittman for assistance in the early phase of this study, and Steven Pipe for valuable discussion throughout the course of these studies.
Supported by National Institutes of Health Grants No. HL53777 and HL52173 (R.J.K.) and the Tokyo Medical College (K.A.).
Address reprint requests to Randal J. Kaufman, PhD, Department of Biological Chemistry and the Howard Hughes Medical Institute, University of Michigan Medical Center, 4554 Medical Science Research Building II, 1150 W. Medical Center Drive, Ann Arbor, MI 48109-0650.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

![Fig. 1. Synthesis of FVIII WT and mutants in COS-1 cells. WT and mutant expression plasmids were transfected into COS-1 monkey cells. At 60 hours posttransfection, cells were pulse-labeled with [35S]-methionine for 20 minutes and cell extracts (CE) were harvested. Duplicate plates were chased for 4 hours in medium containing excess unlabeled methionine and then CE were harvested. Equal volumes of CE were immunoprecipitated with anti-FVIII antibody and equal aliquots were analyzed by SDS-PAGE. Mock indicates cells that did not receive plasmid DNA. The migration of FVIII in the CE is indicated at the right as FVIII. Molecular weight size markers are shown on the left.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/2/10.1182_blood.v91.2.538/3/m_blod4023201.jpeg?Expires=1767739070&Signature=znB2mRrAUsEAHPhezJLrM1r~aXrM9293fZCj67FZ0v~fiEpsFmdXQIH9kTyKS0IF8hbFPS7QpHoVLwWvdvJu8bq2iKJzpTe4WDVDsPHW-1KPAgJzDfgCcpOnzKBcDlHOK3JpccaY8k6yf6ESBqLkRAxM9RTZuBUz~afcyO8OYSvLb0WqdT2tJYZxfbNuJHdhYdEPpFUOzAJJ6Ypf0px~nnAxWWbrjQYoF1GY6-xO9H39a-UvqPel7MXUlyVIKZlW4uNP8A5pITO37UzflzeR-MDsLrfKZIPJvIK~qlRUvvmcvQ8O8NWqlu-mVF8ups4vHgPEsomL-lhNdZ-koOMtpQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. Secretion and thrombin cleavage of WT and mutant FVIII. The secretion of each mutant was analyzed by immunoprecipitation of conditioned medium from [35S]-methionine pulse-labeled transfected cells chased for 4 hours in medium containing excess unlabeled methionine. Immunoprecipitated FVIII molecules were analyzed by SDS-PAGE before (−) and after (+) thrombin (IIa) digestion. Mock indicates cells that did not receive plasmid DNA. Molecular weight size markers are shown on the left. Single chain (Single), heavy chain (Heavy), and light chain (Light) are indicated for undigested samples. A3-C1-C2, A1, and A2 fragments are indicated for digested samples. The symbol * represents the A2 fragment with reduced mobility.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/2/10.1182_blood.v91.2.538/3/m_blod4023202.jpeg?Expires=1767739070&Signature=AJ8KQTwVaC5tssZhBZCaVwRIPs1iTLRWCOG4DR9xsdFclLSY12Hu-BOfIueI5ZTa6N4FQajR3xH4oKOpXTbX3mBr1POZDIEJsbaOkMrbdjeULUhbrGIIHqm~r0gxMdwHFwOlcNRpv3m6xXMxr1GI-bSUyj0NdX32etRQaOyRd28cZEThIdLk7sN9FVurSCUflJDaEEfL~DZIQD3eCSqsC9Sa7x5n1~z6dD~-IV8oMbImv5Iei2t8R0JxjTBP0VgT-l8sEwef5KqYMiFd9NaQBNd32xDhK3a-xFkzMPPUe1e4ECVYjN37IXxBZP29eNohmqeUg9guFYV0aBFLy5-0Yg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Inhibition of intrinsic factor Xase activity by synthetic peptide 558 to 565. (A) Equal amounts (4 nmol/L) of partially purified FVIII samples were mixed with increasing concentrations of synthetic peptide and assayed for FVIII activity as described in the Materials and Methods. The data represent the average of three independent experiments. FVIII activity for each molecule is expressed as a percent of that obtained in the absence of the peptide. The symbols represent WT FVIII (•), S558F (▴), S558A (▵), I566T (▪) and V634A (⧫). (B) WT and I566T mutant FVIII were treated with N-glycanase for 3 hours at 37°C, then samples were tested in the peptide inhibition assay. The results represent the average of three (filled symbols) and two (open symbols) independent experiments. The symbols represent WT FVIII (•), WT FVIII with N-glycanase (○), I566T (▪) and I566T with N-glycanase (□). (C) Equal amounts (4 nmol/L) of partially purified FVIII samples were mixed with increasing concentrations of scrambled synthetic peptide and assayed for FVIII activity as described in the Materials and Methods. Two independent experiments are shown for WT FVIII (•), S558F (▴), I566T (▪) and V634A (⧫). (D) Four different concentrations of WT FVIII (4 nmol/L [•], 0.8 nmol/L [▪], 0.4 nmol/L [▴], 0.08 nmol/L [⧫]) were tested in the peptide inhibition assay. The results are the average of two independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/2/10.1182_blood.v91.2.538/3/m_blod4023205a.jpeg?Expires=1767739070&Signature=1rmZZ91yWq33-Yd8zXIZTZI6IKBRCHFDWuJQQOSsKKbvXqKNPtX20zCr0J6AgnyoyHgOS~EzKBlbtGtkB8PJbJqAhtOcVDXdvI62MDF8k29OryFcsrP3nuvX14r8WWWDH4huUAfMq123VJKQG7CKfSpGAGbHPi3mRVrkq8J6o8bKhTN52xEktd3vakZPDYCrlhYQGgRMp87opnGRLy37yA3ygAEQpAnp2bc3NlJw~oZx8xURaGhlVcKppW1xGk2GpzOi~eFvlZqj1-OyxnJjiaq4d0hdoUdq7HfL1WmyVCV4lzK0cHabtRfTCFBjQsQjU13qcYBxoiUAwfhJb5mkog__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Inhibition of intrinsic factor Xase activity by synthetic peptide 558 to 565. (A) Equal amounts (4 nmol/L) of partially purified FVIII samples were mixed with increasing concentrations of synthetic peptide and assayed for FVIII activity as described in the Materials and Methods. The data represent the average of three independent experiments. FVIII activity for each molecule is expressed as a percent of that obtained in the absence of the peptide. The symbols represent WT FVIII (•), S558F (▴), S558A (▵), I566T (▪) and V634A (⧫). (B) WT and I566T mutant FVIII were treated with N-glycanase for 3 hours at 37°C, then samples were tested in the peptide inhibition assay. The results represent the average of three (filled symbols) and two (open symbols) independent experiments. The symbols represent WT FVIII (•), WT FVIII with N-glycanase (○), I566T (▪) and I566T with N-glycanase (□). (C) Equal amounts (4 nmol/L) of partially purified FVIII samples were mixed with increasing concentrations of scrambled synthetic peptide and assayed for FVIII activity as described in the Materials and Methods. Two independent experiments are shown for WT FVIII (•), S558F (▴), I566T (▪) and V634A (⧫). (D) Four different concentrations of WT FVIII (4 nmol/L [•], 0.8 nmol/L [▪], 0.4 nmol/L [▴], 0.08 nmol/L [⧫]) were tested in the peptide inhibition assay. The results are the average of two independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/2/10.1182_blood.v91.2.538/3/m_blod4023205c.jpeg?Expires=1767739070&Signature=VKMYWXQA3JAVG7LMWvJfL~ZX2sFBT5Ds9~4KwktlwBZ6Dak~WXsiiskzHvNQd76JvaCd72gyw8KgpISpaqrBKIJkLN~KIYYympArGAi-b0Znt3H9up9KGWEAEDTMlCdzZyntsNl0UojrvsPeINfSM6ah29qG0ttXWiQYzVJw0bV38gE2ai3b0rRDtlWjjW-ZtHn13QJHDmKOwESQU~P6wqHoBd5~B-XHc6-mAtcH5STgmlOwY-SUHZH2yGwW8ym5ntReXmkKhUL53PtL5zSdgMNKdI2Uu1NtPO7xisemJJoWG-cgjOUbSt6Lh73azINs5jIcFg7Z7EvS0OhqHWwhBQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. Inhibition of intracellular degradation causes intracellular accumulation of DeltaF652/3. Parallel plates of transfected COS-1 monkey cells were labeled at 60 hours posttransfection with [35S]methionine for 30 minutes, chased for 4 hours in the absence (lanes 2, 6, 10, 13, 16, and 19) or presence of increasing amounts of ALLN (lanes 3, 4, 7, 8, 11, 12, 14, 15, 17, 18, 20, and 21). CE and conditioned medium (CM) were harvested and equal proportionate volumes of CE and CM were immunoprecipitated with anti-FVIII specific antibody for analysis by SDS-PAGE. Mock indicates cells that did not receive plasmid DNA. Molecular weight size markers are shown on the left of each CE and CM.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/2/10.1182_blood.v91.2.538/3/m_blod4023206.jpeg?Expires=1767739070&Signature=5GbF-NNP1qYZdgMhL9rI9sOBx1BKvHi8~8eEdlycjo0IUXsQtMdDsEtAi2cQMFohShRk9LnmFy02BPaV4OsNb17lnJl5EJHnIRdj2FOcpvcnKtwvhR3abCL6KfK~jr3hASWsPd91b4sVKGBZSeJwD0kc6FnkPvueplVQdoYyTj0o9M2xJ4~TsM7-GwBGARyR8rIpe05bkXGzDIWn1YlmEG7zGuUSDxJc47oJl~z6tRwvrxygGWq-fu6OikHiJVVIlceF9lDw~OcfAcIxp29n7vMOr0YKZZXHX~io0ekQFxrOzZK9EWyhHCZMl7K-3sGDBOz1dSh66oXuhk6GRa5BDA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal