Abstract
The molecular basis for heparin-induced thrombocytopenia (HIT), a relatively common complication of heparin therapy, is not yet fully understood. We found that pretreatment of platelets with AR-C66096 (formerly FPL 66096), a specific platelet adenosine diphosphate (ADP) receptor antagonist, at a concentration of 100 to 200 nmol/L that blocked ADP-dependent platelet aggregation, resulted in complete loss of platelet aggregation responses to HIT sera. AR-C66096 also totally inhibited HIT serum-induced dense granule release, as judged by measurement of adenosine triphosphate (ATP) release. Apyrase, added to platelets at a concentration that had only minor effects on thrombin- or arachidonic acid-induced aggregation, also blocked completely HIT serum-induced platelet aggregation. Furthermore, AR-C66096 inhibited platelet aggregation and ATP release induced by cross-linking FcγRIIA with specific antibodies. These data show that released ADP and the platelet ADP receptor play a pivotal role in HIT serum-induced platelet activation/aggregation. The thromboxane receptor inhibitor, Daltroban, had no effect on HIT serum-induced platelet activation whereas GPIIb-IIIa antagonists blocked platelet aggregation but had only a moderate effect on HIT serum-induced dense granule release. Pretreatment of platelets with chondroitinases but not with heparinases resulted in concentration dependent inhibition of HIT serum-induced platelet aggregation. These novel data relating to the mechanism of platelet activation induced by HIT sera suggest that the possibility should be examined that ADP receptor antagonists or compounds that inhibit ADP release may be effective as therapeutic agents for the prevention or treatment of complications associated with heparin therapy.
HEPARIN-INDUCED thrombocytopenia (HIT) is the most frequent therapeutic drug-induced immune mediated complication.1,2 Thromboembolism as a consequence of heparin administration was first described almost 40 years ago.3 Recently, several important aspects of the pathogenesis of HIT have been resolved. There is general agreement that in the majority of cases the antigen in HIT is formed by multimolecular complexes of heparin and platelet factor 4 (PF4)4-7 and that immune complexes formed from heparin-PF4 and the resulting antibodies induce an Fc-mediated platelet activation.8-11Accordingly, cross-linking of the platelet Fc receptor, FcγRIIA,12 has a crucial role in platelet activation induced by HIT antibodies. Recent reports suggested that a functional dimorphism of FcγRIIA, arginine, or histidine at position 131, which affects the avidity of the receptor for Fc binding, might be responsible for disease susceptibility.13,14 However, in their recent study Arepally et al15 found no statistically significant difference in the prevalence of each FcγRIIA isoform between HIT patients with or without thrombosis and controls or between HIT patients with thrombosis or thrombocytopenia alone. This suggests that FcγRIIA dimorphism is not responsible for disease susceptibility and the question of what kind of factors are responsible for different clinical manifestations in patients with HIT is still open. To investigate this very important question, a detailed knowledge of the mechanism of platelet activation induced by HIT antibodies is essential. Although several aspects of HIT antibodies-induced platelet activation have already been resolved, there is still no general agreement about basic questions such as whether immune complexes containing heparin, PF4, and IgG are formed in the fluid phase and then bind to platelet Fc receptors or if heparin-PF4 complexes on the platelet surface are recognized and bound by HIT antibodies; two distinct models with different consequences.16
One approach to finding factors that may be important in the mechanism of platelet activation induced by HIT antibodies is to examine whether a particular pretreatment of platelets with receptor antagonists or (where these are not available, by specific degradation) affects the platelet activating capacity of HIT antibodies. Some novel findings obtained by this method are described here that may contribute to a better understanding of platelet activation/aggregation mechanisms involved in HIT.
MATERIALS AND METHODS
Materials.
ADP receptor inhibitor AR-C66096 (2-propylthio-D-β,γ-difluoromethylene ATP, trisodium salt, formerly FPL 66096) was a kind gift from Mr R.G. Humphries, Astra Charnwood (Loughborough, UK). Apyrase (Type III), chondroitinase ABC (EC 4.2.2.4.), heparinase I (EC 4.2.2.7.), heparinase III (EC 4.2.2.8.), luciferin-luciferase, heparin (sodium salt, from porcine intestinal mucosa), and antimouse IgG, Fc-specific F(ab′)2 were from Sigma Chemical Co (St Louis, MO). Anti-FcγRIIA monoclonal antibody (MoAb) (IV.3) was from Medarex Inc (Annandale, NJ). RGDS tetrapeptide was from Bachem AG (Bubendorf, Switzerland). The low molecular mass peptidomimetic GPIIb-IIIa antagonist Ro44-9883 was a kind gift from Dr Beat Steiner, Hoffmann-La Roche (Basle, Switzerland). Low molecular mass thrombin inhibitor LY288570 was a kind gift from Dr Joseph A. Jakubowski, Lilly Research Laboratories (Indianapolis, IN). Daltroban (Boehringer Mannheim, Mannheim, Germany) was a kind gift from Dr H. Patscheke, Klinikum Karlsruhe, Germany. PF4 was purified as previously described,17 with heparin-Sepharose affinity chromatography.
Sera and platelets.
Sera from 11 patients with HIT were examined. HIT was verified in these patients, as previously described.18 Informed consent was obtained from each patient. All studies were conducted according to the principles expressed in the Declaration of Helsinki. Human platelets were isolated from buffy coats, less than 20 hours after blood collection, obtained from the Central Laboratory of the Swiss Red Cross Blood Transfusion Service. To one buffy coat was added 30 mL of 100 mmol/L citrate, pH 6.5. Platelet rich plasma and the platelet pellet were isolated by successive centrifugation steps. Platelets were washed twice with Tyrode's buffer and were finally resuspended in 20 mmol/L Hepes, 140 mmol/L NaCl, 4.5 mmol/L KCl, 5.5 mmol/L glucose, pH 7.4. The platelet count was adjusted to 400 to 800 × 109/L. Samples were kept at room temperature until used for aggregation studies. Because of the well known but poorly understood donor-related platelet variability in platelet aggregation tests with HIT sera, platelets were isolated in parallel from six buffy coats each day. The platelet suspension that aggregated most strongly with 3 HIT sera chosen randomly from the 11 HIT sera examined in this study was used for further experiments that day.
Platelet aggregation.
Platelet aggregation was monitored by light transmission with a Labintec (Monpellier, France) aggregometer with continuous stirring at 1300 rpm at 37°C. Platelets were preincubated with 2 mmol/L CaCl2 at 37°C for 10 minutes (without stirring) before starting the measurement by adding the samples for analysis. For standardization of PF4 supply and facilitating generation of heparin-PF4-complexes, 10 μg/mL PF4 and 0.5 units/mL heparin were added before adding HIT serum as an agonist. One vol HIT serum containing 1 μg/mL low molecular mass thrombin inhibitor, LY288570, was added to 20 vol of platelet suspension.
Measurement of platelet ATP release.
Determination of ATP by a luciferin/luciferase method was performed as previously described.19 Briefly, 1, 2, and 5 minutes after adding the agonists HIT serum or thrombin to activate the platelets, the reaction was terminated by mixing 5 vol of platelet suspension with 1 vol of ice-cold 600 mmol/L formaldehyde, 60 mmol/L EDTA, pH 7.4. The formaldehyde-fixed aliquots were kept on ice for 5 minutes before centrifuging in an Eppendorf centrifuge at 6000 rpm for 3 minutes at room temperature. The supernatants were analyzed for ATP. Luminescence measurements were performed with a homemade luminometer incorporating a model 1109 Photon Counter (Princeton Applied Research, Princeton, NJ).
Pretreatment of platelets with chondroitinases ABC and heparinases.
Platelets were incubated with various concentrations of enzymes, in the presence or absence of 2 mmol/L CaCl2, in Eppendorf tubes in a 37°C water bath. The samples were gently mixed every 5 minutes. After 30 minutes incubation the samples were transferred to the aggregometer cuvettes and platelet aggregation was performed as described above. Those samples that were treated with enzymes in the absence of CaCl2 were incubated with 2 mmol/L CaCl2 for 5 minutes before adding the agonists, thrombin, arachidonic acid, or HIT sera.
RESULTS
Characteristics of HIT sera examined.
Sera from 11 HIT Type II patients were examined. Summary of clinical status of the patients as well as laboratory test results are shown in Table 1. After preliminary results with randomly selected HIT sera we included sera from patients showing different clinical manifestations of HIT for further studies. Thus, patients no. 3 and 6 had thrombocytopenia only; no. 7 and 9 had thromboembolic complications without a major decrease in platelet count; no. 1, 4, and 5 had thrombocytopenia and venous thromboembolic complications and no. 2 and 11 had thrombocytopenia as well as venous and arterial thrombosis.
Summary of Clinical Presentation and Laboratory Test Results of HIT Patients
| Patient No. . | Clinical Presentation . | Platelet Count (×10−9/L) . | SRA . | HIPA . | H/PF4-ELISA . |
|---|---|---|---|---|---|
| 1 | DVT | 76 | + | + | + |
| 2 | DVT, AT | 12 | + | + | + |
| 3 | — | 40 | + | + | + |
| 4 | DVT | 28 | + | + | + |
| 5 | DVT | 83 | + | + | ND |
| 6 | — | 38 | + | + | ND |
| 7 | PE | 135 | + | + | + |
| 8 | PE | 20 | − | + | + |
| 9 | PE | 350 | + | + | + |
| 10 | PE, DVT | 8 | + | + | + |
| 11 | DVT, AT | 62 | + | + | + |
| Patient No. . | Clinical Presentation . | Platelet Count (×10−9/L) . | SRA . | HIPA . | H/PF4-ELISA . |
|---|---|---|---|---|---|
| 1 | DVT | 76 | + | + | + |
| 2 | DVT, AT | 12 | + | + | + |
| 3 | — | 40 | + | + | + |
| 4 | DVT | 28 | + | + | + |
| 5 | DVT | 83 | + | + | ND |
| 6 | — | 38 | + | + | ND |
| 7 | PE | 135 | + | + | + |
| 8 | PE | 20 | − | + | + |
| 9 | PE | 350 | + | + | + |
| 10 | PE, DVT | 8 | + | + | + |
| 11 | DVT, AT | 62 | + | + | + |
Serotonin release assay (SRA) and heparin-induced platelet activation (HIPA) test were performed as described.21 37Heparin-PF 4 ELISA (H/PF 4-ELISA; Stago, France) was performed according to the instructions of the supplier.
Abbreviations: DVT, deep vein thrombosis; AT, arterial thrombosis; PE, pulmonary embolism; +, positive result; −, negative result; ND, not determined.
Preliminary experiments showed that in the presence of 2 mmol/L CaCl2, 10 μg/mL PF4, and 0.5 U/mL heparin, 1 vol HIT serum added to 20 vol of washed platelet suspension induced clearly detectable platelet aggregation in 5 minutes. Preincubation of platelets with 2 μg/mL anti-FcγRIIA MoAb, IV.3, completely blocked the platelet aggregating effect of all HIT sera examined.
HIT serum-induced dense granule release was not prevented by inhibiting GPIIb-IIIa.
It has been shown previously that14C-serotonin release induced by HIT sera were similar when normal platelets and platelets from a patient with Glanzmann's thrombasthenia were used.20 RGDS, 500 μmol/L, or the low molecular mass peptidomimetic Ro44-9880, 1 μmol/L, which blocks the fibrinogen binding site on GPIIb-IIIa, inhibited HIT serum-induced and thrombin-induced aggregation by more than 90%, however, they inhibited ATP release by only 30% to 40% when HIT sera (no. 1 to 3; n=2), and 10% when 1 nmol/L thrombin was used as an agonist (data not shown). These data strongly suggest a GPIIb-IIIa-independent mechanism for dense granule release in HIT serum-induced platelet aggregation.
ADP receptor inhibitor inhibits HIT serum-induced platelet aggregation and dense granule release.
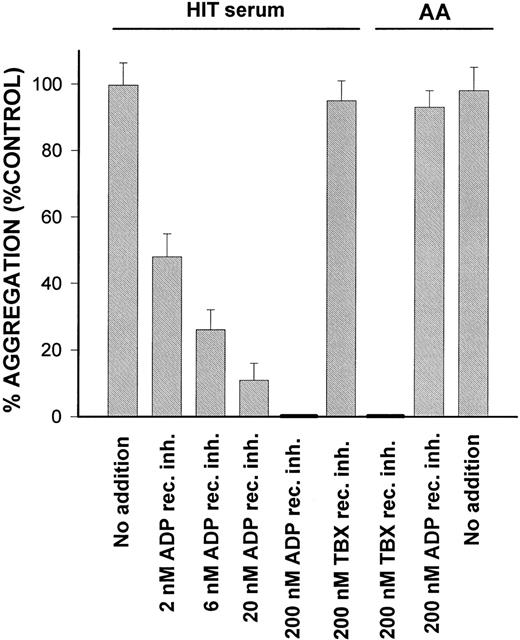
Pretreatment of platelets with the ADP receptor inhibitor AR-C66096 inhibited HIT serum-induced platelet aggregation in a concentration dependent way (Fig 1). The thromboxane receptor inhibitor Daltroban, added to platelets at a concentration that completely blocked arachidonic acid (AA)-induced aggregation, had no effect on HIT serum-induced aggregation (Fig 1).
Concentration dependent inhibition of HIT serum-induced platelet aggregation by the ADP receptor antagonist AR-C66096. Platelets, 500 × 109/L, were preincubated with various concentrations of AR-C66096 or thromboxane receptor inhibitor Daltroban at 37°C for 2 minutes before stimulation with HIT serum no. 1 or 100 nmol/L AA. HIT serum no. 1: Mean % aggregation with control buffer was 55 ± 6%. AA: Mean % aggregation with control buffer was 53 ± 4% (mean ± SD, n = 3). HIT sera no. 2 and 3 and platelet suspensions from donors other than in the case of HIT serum no.1 gave similar results.
Concentration dependent inhibition of HIT serum-induced platelet aggregation by the ADP receptor antagonist AR-C66096. Platelets, 500 × 109/L, were preincubated with various concentrations of AR-C66096 or thromboxane receptor inhibitor Daltroban at 37°C for 2 minutes before stimulation with HIT serum no. 1 or 100 nmol/L AA. HIT serum no. 1: Mean % aggregation with control buffer was 55 ± 6%. AA: Mean % aggregation with control buffer was 53 ± 4% (mean ± SD, n = 3). HIT sera no. 2 and 3 and platelet suspensions from donors other than in the case of HIT serum no.1 gave similar results.
A direct relationship was found between ATP release and platelet aggregation when the effects of different HIT sera were examined with the same platelet suspension over 5 minutes incubation time (Table 2). HIT sera 9 to 11 did not induce either ATP release or platelet aggregation. Sera 6 to 8 induced moderate to low ATP release and moderate to weak aggregation. HIT sera 1 to 5 were the most potent platelet agonists determined either by measurement of platelet aggregation or ATP release. Table 2 shows that the presence of ADP receptor inhibitor blocked completely HIT serum-induced platelet aggregation as well as ATP release (except with serum No. 7) whereas it had only little effect on thrombin-induced aggregation and ATP release. Control sera obtained from healthy volunteers did not induce platelet aggregation or ATP release under the same conditions as used with HIT sera. A platelet suspension from another donor gave similar results to those shown in Table 2 (data not shown).
Platelet Aggregation and ATP Release Induced by HIT Sera, Control Sera, and Thrombin in the Presence and Absence of ADP Receptor Inhibitor
| Agonists . | % Platelet Aggregation at 5 min (±SD; n = 3) . | ATP Release at 5 min (nmol/1010 platelets; ±SD; n = 3) . | ||
|---|---|---|---|---|
| Without ADP Rec. Inh. . | With ADP Rec. Inh. . | Without ADP Rec. Inh. . | With ADP Rec. Inh. . | |
| 2 nmol/L thrombin | 61 ± 4 | 51 ± 5 | 41 ± 6 | 32 ± 5 |
| HIT sera | ||||
| 1 | 62 ± 5 | <3 | 52 ± 4 | <1 |
| 2 | 62 ± 5 | <3 | 30 ± 4 | <1 |
| 3 | 57 ± 8 | <3 | 29 ± 4 | <1 |
| 4 | 55 ± 7 | <3 | 24 ± 4 | <1 |
| 5 | 55 ± 5 | <3 | 23 ± 5 | <1 |
| 6 | 39 ± 8 | <3 | 13 ± 2 | <1 |
| 7 | 30 ± 6 | <3 | 8 ± 3 | 3 ± 2 |
| 8 | 10 ± 4 | <3 | 2 ± 1 | <1 |
| 9, 10, 11 | <5 | <3 | <1 | <1 |
| Control sera | ||||
| 1, 2, 3 | <3 | <3 | <1 | <1 |
| Agonists . | % Platelet Aggregation at 5 min (±SD; n = 3) . | ATP Release at 5 min (nmol/1010 platelets; ±SD; n = 3) . | ||
|---|---|---|---|---|
| Without ADP Rec. Inh. . | With ADP Rec. Inh. . | Without ADP Rec. Inh. . | With ADP Rec. Inh. . | |
| 2 nmol/L thrombin | 61 ± 4 | 51 ± 5 | 41 ± 6 | 32 ± 5 |
| HIT sera | ||||
| 1 | 62 ± 5 | <3 | 52 ± 4 | <1 |
| 2 | 62 ± 5 | <3 | 30 ± 4 | <1 |
| 3 | 57 ± 8 | <3 | 29 ± 4 | <1 |
| 4 | 55 ± 7 | <3 | 24 ± 4 | <1 |
| 5 | 55 ± 5 | <3 | 23 ± 5 | <1 |
| 6 | 39 ± 8 | <3 | 13 ± 2 | <1 |
| 7 | 30 ± 6 | <3 | 8 ± 3 | 3 ± 2 |
| 8 | 10 ± 4 | <3 | 2 ± 1 | <1 |
| 9, 10, 11 | <5 | <3 | <1 | <1 |
| Control sera | ||||
| 1, 2, 3 | <3 | <3 | <1 | <1 |
Platelets were preincubated with or without 200 nmol/L ADP receptor inhibitor for 1 minute before adding the agonists. Note that HIT sera 9, 10, and 11 were also positive in SRA21 and HIPA37 tests with longer incubation times.
If released ADP plays a major role in HIT serum-induced platelet aggregation, apyrase, which breaks down ADP, should inhibit the process. Apyrase, 5 U/mL, inhibited HIT serum-induced platelet aggregation completely, whereas this concentration of apyrase had only minor effects on thrombin- and AA-induced aggregation (not shown).
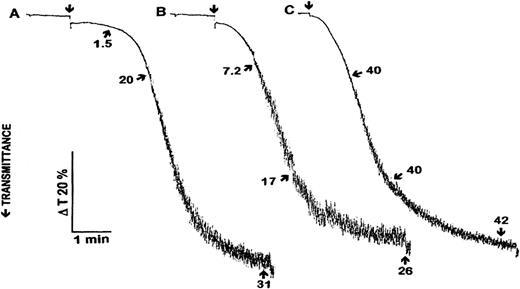
Figure 2 shows a comparison of time dependent platelet aggregation and ATP release induced by HIT sera and thrombin. HIT sera no. 3 and 4 induced approximately the same aggregation response and a similar ATP release at 5 minutes. However, there was a significant difference in the degree of aggregation and ATP release between the two sera during the first 2 minutes after adding them to platelets.
Comparison of the time course of platelet aggregation and ATP release induced by HIT sera no. 3 (A) and 4 (B) and 1 nmol/L thrombin (C). Agonists were added at time points indicated by arrows. Ten μg/mL PF4 and 0.5 U/mL heparin was added 1 minute before adding HIT sera as agonists. One, 2, and 5 minutes after adding the agonists aliquots were taken for ATP measurement. Arrows and numbers indicate released ATP (nmol/1010 platelets). The aggregation curves and ATP release data are representative of the results from three experiments.
Comparison of the time course of platelet aggregation and ATP release induced by HIT sera no. 3 (A) and 4 (B) and 1 nmol/L thrombin (C). Agonists were added at time points indicated by arrows. Ten μg/mL PF4 and 0.5 U/mL heparin was added 1 minute before adding HIT sera as agonists. One, 2, and 5 minutes after adding the agonists aliquots were taken for ATP measurement. Arrows and numbers indicate released ATP (nmol/1010 platelets). The aggregation curves and ATP release data are representative of the results from three experiments.
ADP receptor inhibitor inhibits platelet activation/aggregation and dense granule release induced by cross-linking FcγRIIA with specific antibodies.
ADP receptor inhibitor inhibited HIT serum-induced platelet aggregation, a process thought to involve FcγRIIA cross-linking, encouraged us to examine the effect of the ADP receptor inhibitor on platelet aggregation induced by cross-linking FcγRIIA. After preincubation of platelets in the presence and absence of 200 nmol/L AR-C66096, FcγRIIA cross-linking was induced by successive addition of anti-FcγRIIA MoAb, IV.3, 2 μg/mL, and antimouse IgG, Fc specific F(ab′)2, 2 to 20 μg/mL. The platelet aggregation response depended on the concentration of the cross-linker F(ab′)2, reaching maximal aggregation at 10 μg/mL cross-linker. Low and moderate aggregation responses, up to 30% to 40% aggregation, were completely abolished by pretreatment of platelets with 200 nmol/L AR-C66096 whereas strong responses (58 ± 4 % aggregation, n = 3) were inhibited 92 ± 3 % (percentage inhibition ± SD, n = 3, not shown). Similarly, pretreatment of platelets with 200 nmol/L AR-C66096 blocked or inhibited ATP-release more than 80% when the original aggregation response in the absence of AR-C66096 was moderate or strong, respectively (not shown).
Pretreatment of platelets with chondroitinases inhibits HIT serum-induced platelet aggregation.
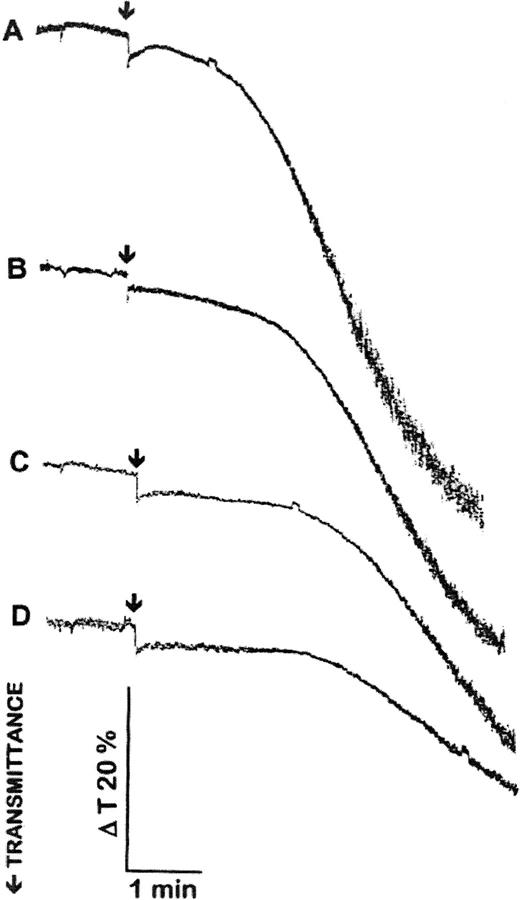
Preincubation of platelets with chondroitinase ABC resulted in concentration dependent inhibition of HIT serum-induced platelet aggregation (Fig 3). Platelets preincubated with chondroitinases ABC aggregated normally with thrombin or AA (not shown). Strong aggregation responses (such as that shown in Fig 3A) induced by HIT sera were not blocked completely by pretreatment of platelets with chondroitinases ABC, up to 1 U/mL for 30 minutes. Weak aggregation responses, induced by adding that amount of HIT sera that resulted in 10% to 20% platelet aggregation, were abolished completely by pretreatment of platelets with chondroitinases ABC (not shown). The inhibitory effect of chondroitinases ABC pretreatment was independent of the presence or absence of CaCl2 during the incubation with the enzyme. Heparinase I and III (Sigma; up to 20 U/mL and 7 U/mL, respectively) did not inhibit HIT serum-induced platelet aggregation (not shown). These results strongly suggest the involvement of a chondroitin sulfate proteoglycan in HIT serum-induced platelet activation.
Pretreatment of platelets with chondroitinase ABC inhibits HIT serum-induced platelet aggregation. Platelets, 800 × 109/L, were preincubated with 0 (A), 0.2 (B), 0.5 (C), and 1 (D) μ/mL chondroitinase ABC at 37°C for 30 minutes before stimulation with HIT sera. Agonists were added at time points indicated by arrows. A total of 10 μg/mL PF4 and 0.5 U/mL heparin was added 1 minute before adding HIT sera as agonists. The aggregation curves are representative of the results from three experiments with HIT sera 1, 3, and 4 and platelets from three different donors.
Pretreatment of platelets with chondroitinase ABC inhibits HIT serum-induced platelet aggregation. Platelets, 800 × 109/L, were preincubated with 0 (A), 0.2 (B), 0.5 (C), and 1 (D) μ/mL chondroitinase ABC at 37°C for 30 minutes before stimulation with HIT sera. Agonists were added at time points indicated by arrows. A total of 10 μg/mL PF4 and 0.5 U/mL heparin was added 1 minute before adding HIT sera as agonists. The aggregation curves are representative of the results from three experiments with HIT sera 1, 3, and 4 and platelets from three different donors.
DISCUSSION
Sera from HIT patients contain specific antibodies developed against multimolecular complexes of heparin and PF4.4-7 These antibodies induce platelet activation and aggregation via cross-linking of FcγRIIA.8-11 Dense granule release is an integral part of HIT serum-induced platelet effects and serotonin or ATP release assays are often used for laboratory diagnosis of HIT.21,22The platelet membrane receptor GPIIb-IIIa has a well documented role in both platelet aggregation and the release reaction.23 When we examined the action of GPIIb-IIIa antagonists on platelet effects induced by HIT sera, GPIIb-IIIa antagonists were found to prevent HIT serum-induced platelet aggregation, as expected. However, they inhibited HIT serum-induced dense granule release only moderately as judged by ATP release. This finding (which corroborates previous results that normal platelets and Glanzmann's thrombasthenia platelets lacking GPIIb-IIIa showed similar 14C-serotonin release induced by HIT sera20) encouraged us to look for potential platelet agonists/mechanisms, which are capable of inducing GPIIb-IIIa–independent dense granule release, and may be involved in early events of platelet activation with HIT sera. The first candidate was thromboxane A2 supposedly generated as a result of early activation of phospholipase C and arachidonic acid release. However, the thromboxane receptor inhibitor Daltroban had no effect on HIT serum-induced platelet aggregation although it clearly inhibited AA-induced platelet aggregation.
Next, we examined the possibility that ADP, released in small amounts from the dense granules as a result of HIT antibodies binding to and activating platelets, could have an important role in further activation and aggregation of platelets as in the case of collagen.24 The ADP receptor on platelets is thought to be a member of the P2Y nucleotide receptor family of G protein-coupled, seven transmembrane domain receptors.25,26The availability of a newly developed specific ADP receptor antagonist, AR-C66096,27,28 which is effective in vitro, made it possible to investigate the role of ADP and the ADP receptor in HIT serum-induced platelet activation/aggregation. Pretreatment of platelets with AR-C66096, at a concentration of 100 to 200 nmol/L that blocked ADP-dependent platelet aggregation, resulted in complete loss of platelet aggregation response to HIT sera. In addition, and more unexpected, HIT serum-induced dense granule release was also blocked by AR-C66096. These data clearly show that ADP release is the major factor responsible for HIT serum-induced platelet aggregation. This was supported by the finding that apyrase, added to platelets at a concentration that had only minor effects on thrombin- or AA-induced aggregation, blocked completely HIT serum-induced platelet aggregation. In all 11 HIT sera examined, the presence of antiheparin-PF4 IgG was shown by enzyme-linked immunosorbent assay (ELISA) and none of these sera-induced platelet aggregation or dense granule release in platelets was preincubated with anti-FcγRIIA MoAb, IV.3. This shows that antiheparin-PF4 IgG-induced FcγRIIA cross-linking is involved in the platelet effect of HIT sera examined here, in agreement with earlier reports.8-11
It was also found that AR-C66096 inhibited FcγRIIA cross-linking–induced platelet aggregation and ATP-release. A recent report by Cattaneo et al29 suggests the existence of an aggregation-independent and ADP receptor-dependent mechanism by which released ADP potentiates platelet secretion. Their finding that deficiency of ADP binding sites on platelets was associated with a secretion defect is consistent with our results that dense granule release was inhibited by the ADP receptor antagonist AR-C66096.
The recent report by Horne et al30 provided the first direct evidence for platelet binding of IgG as well as F(ab′)2 from patients with HIT. It was also shown in this study that neither addition of a 200-fold excess of normal IgG or inclusion of anti-FcγRIIA MoAb, IV.3, suppressed the binding. This suggests that HIT-IgG binding to platelets occurs via heparin-PF4 rather than FcγRIIA. However, Suh et al31 reported that IV.3 completely blocked the binding to platelets of immune complexes consisting of PF4, heparin, and immunopurified HIT IgG antibodies. So, there is no general agreement about whether immune complexes are formed in the plasma and then bind to platelets via Fc receptors or whether heparin-PF4 complexes associated with the platelet surface are recognized by HIT antibodies. We show that preincubation of platelets with chondroitinase ABC resulted in decreased reactivity to HIT sera. Since the first report,32 there is now increasing evidence that PF4 associates with platelet chondroitin sulfate proteoglycans.33-35 Although PF4 has an apparent Kd of 30 nmol/L for heparin whereas for chondroitin sulfate it is 265 nmol/L,34 a ninefold difference, this may not represent the situation in platelets. On the one hand, in a platelet proteoglycan the glycosaminoglycan chains are likely to be concentrated along a short peptide sequence of repeating serine and glycine residues that would increase the affinity to PF4 compared to free chondroitin sulfate; and on the other hand the affinity of heparin for PF4 might be decreased in the HIT antibody-heparin–PF4 complex compared to free heparin. Thus, the effect of the chondroitinases could be due to destruction of a platelet surface proteoglycan containing multiple chondroitin sulfate chains that may have a role in docking and aligning the heparin-PF4(-HIT antibody) complexes on platelets and therefore facilitating FcγRIIA clustering. That the chondroitinases do not prevent the platelet activation completely in reponse to higher doses of HIT sera may be due to the complexity of these proteoglycans and the glycosaminoglycan linkages. Such an effect of platelet membrane proteoglycans would not be unique because there is now general agreement that the affinity of fibroblast growth factors for their receptors is increased in the presence of proteoglycans and that this is probably of physiological significance.36
The data presented here suggest that enhanced susceptibility of platelets to ADP, or other differences enhancing granule release, perhaps based on elevated expression or variations in the structure of the ADP receptor on platelets or its signaling pathways, may be considered as predisposing factors for development of heparin-induced complications. The fact that ADP plays a major role in platelet activation/aggregation induced by antibodies to heparin-PF4 complexes suggests that ADP receptor antagonists inhibiting ADP release from dense granules might be effective as therapeutic agents for prevention or treatment of heparin-induced immune complications.
ACKNOWLEDGMENT
We thank Corinne Birbaum for technical assistance. We are grateful to the Central Laboratory of the Swiss Red Cross Blood Transfusion Service (Berne, Switzerland) for the supply of buffy coats.
Supported in part by a grant from the Swiss National Science Foundation (31-42336.94 to K.J.C.) and in part by the Deutsche Forschungsgemeinschaft (Grant No.1096/2-2).
Address reprint requests to Kenneth J. Clemetson, PhD, Theodor Kocher Institute, University of Berne, Freiestrasse 1, CH-3012 Berne, Switzerland.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal